GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Book a Free Consultation
Table of Contents
Overall Health Benefits of SRT2104
SRT2104 Benefits
SRT2104, a selective activator of the SIRT1 enzyme, has shown promise in improving metabolic health, cardiovascular function, and age-related conditions. It enhances mitochondrial function, reduces inflammation, and improves insulin sensitivity, making it a potential therapeutic agent for diseases like type 2 diabetes, cardiovascular diseases, and neurodegenerative disorders. Preclinical and clinical studies also suggest its role in extending lifespan, improving endothelial function, and reducing lipid accumulation, highlighting its broad potential for managing aging and associated diseases.
- Improves cardiovascular health [1-7]
- Improves lipid profile [8-10]
- Fights inflammation [11-16]
- Improves blood sugar levels [17-22]
- Improves bone health [23-27]
Key Takeaways
- Metabolic Health Improvement: SRT2104 enhances insulin sensitivity, glucose metabolism, and lipid profile, making it a promising candidate for treating type 2 diabetes and obesity-related conditions.
- Cardiovascular Protection: It improves endothelial function, reduces inflammation, and enhances mitochondrial efficiency, contributing to better cardiovascular health and reduced risk of heart diseases.
- Anti-Aging Potential: By activating SIRT1, SRT2104 promotes longevity pathways, enhances mitochondrial biogenesis, and mitigates oxidative stress, potentially extending lifespan and combating age-related decline.
- Neuroprotective Effects: Preclinical studies suggest SRT2104 may protect against neurodegenerative disorders by reducing inflammation and improving mitochondrial function in the brain.
- Safety and Tolerability: Early clinical trials indicate that SRT2104 is generally well-tolerated, with a favorable safety profile, supporting its potential for long-term use in managing chronic diseases.
What is SRT2104?
The sirtuin family of proteins (SIRT1-7) possesses a unique ability that is integral not only in human metabolism but also in the field of anti-aging and lifespan regulation. Specifically, the activation of sirtuin (silent mating type information regulation 2 homolog) 1, or SIRT1, is being studied for its role in the treatment of age-related, chronic, debilitating metabolic diseases such as diabetes, obesity, and cancer. SRT2104, a selective activator of SIRT1, has been shown to exert significant health benefits on a wide array of medical maladies.
How SRT2104 Works
The activation of sirtuin (silent mating type information regulation 2 homolog) 1, or SIRT1 by SRT2104 is thought to exert beneficial effects on different body systems such as the cardiovascular and skeletal systems. This mechanism also has positive effects on blood sugar, lipids, and inflammatory substances.
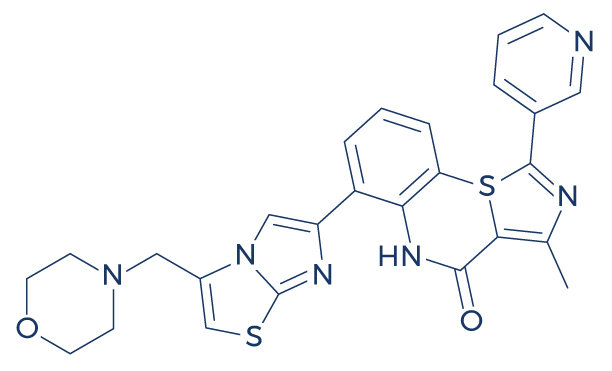
Chemical Structure of SRT2104
Research on SRT2104
A. Improves Cardiovascular Health
SRT2104 has cardioprotective effects that can help extend the lifespan of people with different heart diseases. Studies suggest that SRT2104 exerts these beneficial effects through various important mechanisms:
- In adults with type 2 diabetes mellitus, oral SRT2104 (2.0 g/day) administration for 28 days improved the function of the blood vessels of the heart and decreased blood clot formation. [1]
- In diabetic mice, SRT2104 reduced aortic endothelial dysfunction. [2]
- In both type 2 diabetes mellitus patients and healthy cigarette smokers, SRT2104 administration at 2.0 g once daily for 28 days improved cardiac blood vessel function. [3]
- In mice, administration of SRT2104 improved blood flow to the heart by inducing dilation of the blood vessels. [4]
- In mice with atherosclerosis, a condition characterized by the deposition of plaques of fatty material on the inner walls of the arteries of the heart, SRT2104 administration reduced macrophage foam cell formation – a process that can contribute to plaque build-up. [5]
- A mice study found that inhibition of SIRT1 is associated with increased blood clot formation, suggesting that SRT2104, a selective activator of SIRT1, can potentially reduce the risk of blood clots in the heart. [6]
- In healthy cigarette smokers and subjects with type 2 diabetes mellitus, administration of SRT2104 reduced arterial stiffness. [7]
B. Improves Lipid Profile
Several studies show that SRT2104 also has the ability to reduce the levels of lipids such as low-density lipoprotein (bad cholesterol) and triglycerides:
- In healthy cigarette smokers, administration of SRT2104 decreased serum total cholesterol, low‐density lipoprotein cholesterol, and triglyceride concentrations. [8]
- In elderly volunteers, oral doses of 0.5 or 2.0 g SRT2104 decreased low-density lipoprotein and triglyceride levels after 28 days of treatment. [9]
- In diabetic subjects, administration of SRT2104 at doses of 250, 500, 1,000, and 2,000 mg reduced lipid levels. [10]
C. Fights Inflammation
There’s strong scientific evidence suggesting that SRT2104 has potent anti-inflammatory properties which can be beneficial in a broad range of debilitating inflammatory conditions:
- In healthy volunteers, 2000 mg of SRT2104 reduced the LPS-induced release of inflammatory substances such as IL-6 and IL-8. [11]
- In patients with mild-to-moderate ulcerative colitis, administration of SRT2104 at doses of 50 mg or 500 mg daily, significantly reduced fecal calprotectin, a biomarker of colitis disease activity. [12-13]
- In patients with moderate-to-severe psoriasis, SRT2104 administration once daily for 12 weeks at 250, 500, and 1000 mg improved Psoriasis Area and Severity Index (PASI) scores and decreased the levels of genes associated with the disease. [14]
- In healthy volunteers, single and repeated administration of SRT2104 reduced lipopolysaccharide-induced release of the cytokines interleukin-6and interleukin-8. [15]
- A cell study also found that SIRT1 activity is decreased in lesional skin of patients with psoriasis, suggesting that SRT2104 may help reduce the risk of this autoimmune disease. [16]
D. Improves Blood Sugar Levels
Evidence also suggests that SRT2104 may help reduce elevated blood sugar levels in diabetic patients:
- In obese diabetic patients, SRT2104 administration reduced blood sugar levels, liver fat, and blood pressure. [17]
- A study found that SRT2104 exerts its anti-diabetic effects by modulating various metabolic pathways, including blood sugar metabolism. [18]
- In diet-induced obese and genetically obese mice, SRT2104 improved insulin sensitivity, reduced blood sugar levels, and increased mitochondrial capacity. [19]
- In diabetic subjects, administration of SRT2104 at doses of 250, 500, 1,000, and 2,000 mg once daily for 28 days also reduced blood sugar levels. [10]
- In diet-induced obese mice, oral treatment with SRT2104 for 12 weeks improved both metabolic function and blood sugar homeostasis. [20]
- In obese mice, SRT2104 ameliorated insulin resistance by increasing energy expenditure via enhancement of carbohydrate and fatty acid oxidation. [21]
- A study also found that SRT2104-induced activation of SIRT1 can improve insulin sensitivity, resulting in reduced blood sugar levels. [22]
E. Improves Bone Health
Numerous body of research also shows that SRT2104-induced activation of SIRT can unlock beneficial effects on bone health:
- In male mice on a standard diet, the addition of SRT2104 preserved bone and muscle mass. [23]
- In SIRT1-deficient mice, a significant reduction in cortical bone thickness is observed, suggesting that SRT2104 may help slow the progression of bone loss. [24-25]
- Mice studies have also shown that the sirtuin family of proteins can help combat osteoporosis and ovariectomy-induced bone loss. [26-27]
Associated Side Effects of SRT2104
SRT2104 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on SRT2104. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of SRT2104. Despite this, it was listed as a side effect associated with SRT2104 even though these associated side effects are very uncommon.
Side effects associated with SRT2104 may include the following:
- Diarrhea
- Dizziness
- Headache
- Hypoglycemia (low blood sugar)
- Nasopharyngitis (inflammation of the nasal passages and back of the throat)
- Nausea
- Rhinitis (characterized by a runny nose, nasal congestion, sneezing, and itching)
- Upper respiratory tract infection
SRT2104 Psoriasis Treatment
Background and Mechanism of Action
SRT2104 is a small molecule activator of SIRT1, a protein that regulates various cellular functions, including inflammation and metabolic processes. Studies have shown that SRT2104 exerts its effects in a dose-dependent manner, targeting immune signaling pathways, particularly those involving IL-17 and TNF-α, which are critical in psoriasis. By modulating these pathways in a dose-dependent manner, SRT2104 promotes anti-inflammatory effects and improves keratinocyte differentiation, essential for maintaining healthy skin structure and reducing psoriasis symptoms.
Clinical Efficacy and Findings
In a clinical trial involving patients with moderate-to-severe psoriasis, SRT2104 demonstrated promising results. Clinical research revealed that 35% of treated patients achieved good to excellent improvement in skin biopsy samples, indicating a potential therapeutic effect. Although these improvements were not consistently mirrored in PASI scores, clinical research underscores the importance of the compound’s ability to modulate gene expression and signaling pathways. Continued clinical research is crucial to fully understand SRT2104’s role in addressing the disease’s underlying mechanisms and improving treatment outcomes.
Safety and Future Directions
SRT2104 was generally well-tolerated, with most adverse events being mild to moderate, such as headaches and dizziness. Its dose-dependent pharmacokinetics and favorable safety profile make it a promising candidate for further research. By targeting the SIRT1 pathway, SRT2104 has the potential to regulate inflammation and cellular dysfunction through its effects on gene expression, offering a novel approach to psoriasis treatment. Continued investigation into its impact on gene expression will be essential to confirm its clinical efficacy and further understand its role in modulating gene expression to address the disease at a molecular level.
SRT2104 Crohns Disease
Mechanism of Action and Rationale
SRT2104 is a selective activator of SIRT1, a protein that regulates cellular stress responses, inflammation, and metabolism. In Crohn’s disease, chronic inflammation of the gastrointestinal tract disrupts immune and epithelial functions, leading to severe complications. Clinical studies utilizing magnetic resonance imaging have demonstrated the potential of SRT2104 in reducing intestinal inflammation and improving mucosal integrity. By targeting SIRT1 activation, SRT2104 helps reduce pro-inflammatory cytokine production and restore immune balance, with magnetic resonance imaging offering a valuable tool to assess its therapeutic effects. This innovative approach underscores the importance of magnetic resonance imaging in tracking disease progression and evaluating treatment efficacy while highlighting SRT2104’s role in addressing Crohn’s disease’s underlying mechanisms. Further research and magnetic resonance imaging studies are needed to confirm these promising findings.
Clinical Evaluation and Findings
Although SRT2104 has demonstrated anti-inflammatory effects in preclinical research, its clinical evaluation in inflammatory bowel diseases such as Crohn’s remains limited. Trials in related conditions like ulcerative colitis showed modest benefits, including reduced inflammatory markers and cytokine modulation. These findings suggest that SRT2104 may have potential in Crohn’s disease, but efficacy and dosing must be optimized to overcome variability in response and ensure clinical significance.
Safety and Future Prospects
SRT2104 has been generally well-tolerated in trials, with most adverse events being mild to moderate, and was tested against a matching placebo. Its favorable safety profile, combined with its ability to modulate inflammation and metabolic pathways, makes it a promising candidate for further investigation in Crohn’s disease. Future studies should focus on refining dosing strategies, assessing long-term outcomes, and identifying patient populations most likely to benefit from SIRT1-targeted therapies.
SRT2104 Availability
Current Availability
SRT2104, a selective activator of SIRT1, remains primarily an investigational compound and is not widely available for clinical use. It has been studied in various clinical trials targeting conditions such as psoriasis, ulcerative colitis, and metabolic disorders, but it has not yet received regulatory approval for any specific indication. Consequently, access to SRT2104 is limited to research settings and specialized clinical studies, where it may hold potential to promote aging resistance and address age-related diseases.
Challenges to Commercialization
One of the main hurdles to SRT2104’s broader availability is the need for further robust clinical data demonstrating its efficacy and safety across multiple therapeutic areas. Although it has shown promise in preclinical and early-phase trials, inconsistencies in clinical outcomes and variability in patient responses have delayed its progress toward regulatory approval. Additionally, the pharmaceutical landscape for SIRT1 activators has been competitive, with developers requiring compelling evidence to justify large-scale production and commercialization.
Future Prospects
Despite its current limitations, SRT2104 holds potential as a therapeutic agent, especially for conditions involving inflammation, metabolic dysregulation, and aging-related diseases. Ongoing and future clinical trials, including those with placebo groups, will be critical in determining its viability for market introduction. If these studies provide clear evidence of clinical benefits, SRT2104 could eventually become available as a novel therapeutic option, paving the way for SIRT1-targeted therapies in modern medicine.
SIRT1 Activators
SIRT1 Activators Overview
SIRT1 activators are compounds designed to enhance the activity of Sirtuin 1 (SIRT1), a protein involved in regulating cellular metabolism, stress responses, and aging. SIRT1 deacetylates both histone and non-histone proteins, influencing key pathways such as glucose homeostasis, mitochondrial function, and inflammation. Naturally occurring compounds like resveratrol, found in red wine, were among the first discovered activators. These compounds mimic the effects of calorie restriction, offering potential benefits in managing age-related diseases, including diabetes, cardiovascular disorders, and neurodegenerative conditions, through multiple mechanisms.
Therapeutic Potential
The activation of SIRT1 has shown promise in preclinical and early clinical studies for treating metabolic and inflammatory diseases. Synthetic SIRT1 activators, such as SRT2104 and SRT1720, have demonstrated benefits in improving insulin sensitivity, reducing inflammation, and enhancing mitochondrial function in animal models. These compounds are being explored for their potential to treat diseases like type 2 diabetes, psoriasis, and osteoporosis, addressing multiple pharmacodynamic endpoints. By targeting SIRT1, researchers aim to harness its role in cellular repair and metabolism to slow aging-related decline and improve overall health.
Challenges and Future Directions
Despite their potential, the development of SIRT1 activators as therapeutic agents has faced challenges. Early clinical trial results have shown mixed efficacy, and some compounds have been associated with side effects or limited bioavailability. For instance, the discontinuation of certain SIRT1-based drugs like SRT501, following issues identified in a clinical trial, highlighted the need for improved formulations and delivery methods. Nonetheless, ongoing clinical trials continue to explore SIRT1 activation as a promising approach, aiming to optimize potency, safety, and efficacy, with potential applications in improving metabolic health and cognitive function in age-related conditions.
SIRT1 Activator Supplement
Overview of SIRT1 Activator Supplements
SIRT1 activator supplements are designed to enhance the activity of the SIRT1 protein, a key regulator of cellular metabolism, aging, and stress responses. These supplements often include naturally occurring compounds like resveratrol, a polyphenol found in red wine, and other plant-based ingredients believed to mimic the benefits of calorie restriction. Clinical studies have shown varying results, with some suggesting modest benefits in metabolic health and inflammation, but further research is needed to confirm their efficacy compared to a placebo group, ensuring measurable improvements. By activating SIRT1, these supplements aim to support metabolic health, reduce inflammation, and promote longevity, with ongoing studies continuing to compare outcomes against a placebo group for validation.
Potential Benefits of SIRT1 Activators
Research suggests that SIRT1 activators may improve mitochondrial function, enhance insulin sensitivity, and reduce markers of inflammation, making them attractive for addressing metabolic disorders, age-related diseases, and general wellness. While preclinical findings are promising, a clinical trial is essential to confirm these benefits in diverse populations and to understand their long-term safety. A clinical trial investigating the effects of these activators on metabolic health could provide valuable insights into their mechanisms of action. Some studies indicate that these supplements can help support cardiovascular health and protect against neurodegenerative diseases, but large-scale clinical trials are necessary to validate these effects. Further, conducting a well-structured clinical trial could determine optimal dosing and minimize potential risks. However, their exact efficacy in humans remains a topic of ongoing investigation, underscoring the need for more robust clinical trial data to fully establish their therapeutic potential.
Considerations and Usage
While SIRT1 activator supplements are widely available, their benefits may vary based on formulation, dosage, and individual health conditions. Neuronal survival is one area where SIRT1 activation shows promise, but clinical study is crucial to verify the effectiveness and safety of these supplements, especially since they are not as strictly regulated as pharmaceuticals. Users should consult healthcare professionals before starting these supplements, particularly if they are managing chronic conditions or taking medications. As more clinical studies are conducted, advancements may lead to supplements that better support neuronal survival and promote overall health and longevity, with evidence-based guidance improving their targeted use.
FAQ
What is the rarest form of psoriasis?
The rarest form of psoriasis is erythrodermic psoriasis, a severe and potentially life-threatening condition characterized by widespread redness, scaling, and shedding of the skin. A clinical study is essential to understand this rare condition’s underlying mechanisms and potential treatments. Further clinical study efforts may help improve outcomes for those affected by erythrodermic psoriasis.
What is the life expectancy for psoriasis?
Psoriasis itself does not typically affect life expectancy, but severe cases, particularly those associated with comorbidities like cardiovascular disease or psoriatic arthritis, may slightly increase the risk of reduced lifespan when compared to outcomes in a matched placebo group in clinical studies.
What infection triggers psoriasis?
Streptococcal throat infections, particularly those caused by group A Streptococcus, can trigger or worsen psoriasis, especially guttate psoriasis, potentially affecting pathways related to endothelial function in chronic inflammatory responses.
What is the most successful treatment for psoriasis?
The most successful treatment for psoriasis varies by individual but often includes biologic therapies targeting specific immune pathways, such as TNF-alpha inhibitors or IL-17 and IL-23 inhibitors, which address the key mechanism driving inflammation and have shown high efficacy and safety in managing moderate to severe cases.
What is the new treatment for inverse psoriasis?
A new treatment for inverse psoriasis includes biologic therapies like IL-17 inhibitors (e.g., secukinumab or ixekizumab), which target specific immune pathways to reduce inflammation and symptoms effectively, while ongoing research explores their neuroprotective properties in related inflammatory conditions.
What is the most aggressive treatment for psoriasis?
The most aggressive treatments for psoriasis are systemic therapies such as biologics (e.g., infliximab or adalimumab) and oral medications (e.g., methotrexate or cyclosporine), which target the immune system to control severe cases and can be safely administered under medical supervision to minimize potential side effects.
What is the new treatment for psoriasis arthritis?
The new treatments for psoriatic arthritis include IL-23 inhibitors (e.g., guselkumab and risankizumab) and Janus kinase (JAK) inhibitors (e.g., upadacitinib), which work through various mechanisms to target specific pathways, reducing inflammation and preventing joint damage effectively.
What are the three types of Crohn's disease?
The three main types of Crohn’s disease are ileitis (affecting the ileum), colitis (affecting the colon), and ileocolitis (affecting both the ileum and colon), with a higher prevalence and more severe symptoms often observed in elderly individuals.
What are 4 symptoms of Crohn's disease?
Four common symptoms of Crohn’s disease are abdominal pain, diarrhea, weight loss, and fatigue, which may also be associated with changes in subcutaneous fat distribution in some individuals.
What are the symptoms of terminal ileitis Crohn's disease?
Symptoms of terminal ileitis Crohn’s disease include abdominal pain, diarrhea, weight loss, fever, and fatigue, often accompanied by nausea, malnutrition, and potential disruptions in lipid metabolism.
What disease is associated with Crohn's disease?
Crohn’s disease is often associated with other autoimmune conditions, such as ulcerative colitis, rheumatoid arthritis, and ankylosing spondylitis, which can sometimes impact serum cholesterol levels due to chronic inflammation.
What supplements activate SIRT1?
Supplements like resveratrol, NAD+ precursors (such as nicotinamide riboside and nicotinamide mononucleotide), and pterostilbene are known to activate SIRT1 and have shown efficacy in promoting cellular health and supporting metabolic processes.
What is the best SIRT1 activator?
Resveratrol is often considered one of the best and most studied SIRT1 activators due to its potent effects on enhancing SIRT1 activity and promoting autophagy, which supports cellular health and longevity.
What is a SIRT1 activator?
Resveratrol is often considered one of the best and most studied SIRT1 activators due to its potent effects on enhancing SIRT1 activity and regulating energy homeostasis. By activating SIRT1, resveratrol helps improve energy homeostasis in cells, supporting processes like mitochondrial function and metabolic efficiency. Its role in maintaining energy homeostasis makes it a promising candidate for addressing metabolic disorders and age-related diseases, further highlighting the importance of targeting energy homeostasis through therapeutic interventions like SIRT1 activators.
What increases SIRT1?
Calorie restriction, exercise, and certain compounds like resveratrol, NAD+ precursors, and SIRT1 activators can increase SIRT1 activity, with some effects being more pronounced at higher doses.
What supplements increase SIRT1?
Supplements such as resveratrol, NAD+ precursors (like nicotinamide riboside and nicotinamide mononucleotide), and pterostilbene are known to increase SIRT1 activity, as supported by findings from various literature sources.
How to increase SIRT1 naturally?
SIRT1 can be increased naturally through practices like intermittent fasting, regular exercise, and calorie restriction, all of which stimulate the body’s metabolic response. Consuming foods rich in polyphenols, such as grapes, berries, and dark chocolate, enhances the antioxidant response, while ensuring adequate sleep supports the body’s restorative response. Together, these lifestyle adjustments create a comprehensive response to promote SIRT1 activity.
What are the benefits of Senolytic supplements?
Senolytic supplements are believed to promote healthy aging by targeting and clearing senescent cells, which may reduce inflammation, improve tissue function, and potentially delay age-related diseases.
What activates SIRT1?
SIRT1 is activated by factors like caloric restriction, resveratrol, certain polyphenols, and NAD+ precursors, which enhance its role in metabolism, aging, and cellular repair.
Reference
Available from https://openheart.bmj.com/content/4/2/e000647
Cardiometabolic effects of a novel SIRT1 activator, SRT2104, in people with type 2 diabetes mellitus
This study explored the cardiometabolic effects of SRT2104, a SIRT1 activator, in individuals with type 2 diabetes mellitus (T2DM). In a randomized, double-blind, placebo-controlled trial, participants received SRT2104 or a placebo for 28 days. The results showed no significant impact on lipid profiles, platelet-monocyte activation, or forearm vasodilatation. However, SRT2104 treatment led to a reduction in plasminogen activator inhibitor type 1 and a slight weight loss (−0.93 kg), but also a rise in glycated hemoglobin, indicating worsened glycemic control. These findings suggest that while SRT2104 may induce weight loss, it may also have negative effects on blood sugar regulation.
You can read the abstract of the article at https://openheart.bmj.com/content/4/2/e000647.
Wu H, Wu J, Zhou S, et al. SRT2104 attenuates diabetes-induced aortic endothelial dysfunction via inhibition of P53. J Endocrinol. 2018;237(1):1-14.
SRT2104 attenuates diabetes-induced aortic endothelial dysfunction via inhibition of P53
This study investigates the role of SIRT1 activation, specifically by the novel activator SRT2104, in protecting against diabetes-induced aortic endothelial dysfunction. In diabetic mice, SRT2104 reversed increased aortic contractility, oxidative stress, inflammation, and P53 hyperacetylation, all of which were associated with reduced SIRT1 levels. In endothelial cells, SRT2104 and P53 silencing similarly reduced oxidative stress and inflammation, indicating that P53 deacetylation plays a key role in SRT2104’s protective effects. Additionally, activating P53 with nutlin3a negated the protective effects of SRT2104, highlighting P53’s pathogenic role in endothelial dysfunction in diabetes.
You can read the full article at https://joe.bioscientifica.com/view/journals/joe/237/1/JOE-17-0672.xml.
Available from https://clinicaltrials.gov/ct2/show/NCT01031108
A Clinical Trial to Assess the Safety of Oral SRT2104 and Its Effects on Vascular Dysfunction in Otherwise Healthy Cigarette Smokers and Subjects With Type 2 Diabetes Mellitus
This study aims to assess the safety and tolerability of SRT2104 (2.0 g daily for 28 days) and its effects on vasomotor and fibrinolytic dysfunction in patients with type 2 diabetes mellitus and healthy cigarette smokers. It evaluates SRT2104’s impact on platelet activation markers, inflammatory markers, oxidative stress, and glucose control biomarkers, as well as its pharmacokinetics after single and repeated dosing. The findings will provide insights into SRT2104’s potential for addressing vascular and metabolic dysfunctions and its role in SIRT1 activation.
You can read the full article at https://clinicaltrials.gov/ct2/show/NCT01031108.
Available from https://www.pnas.org/content/104/37/14855?ijkey=9b8d33a7d87b2cbaecdb95665dc7751bf27fee94&keytype2=tf_ipsecsha
SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase
This study reveals that SIRT1 protein deacetylase plays a crucial role in promoting endothelium-dependent vasodilation by deacetylating endothelial nitric oxide synthase (eNOS), which enhances eNOS activity and nitric oxide (NO) production. SIRT1 and eNOS interact directly in endothelial cells, with specific lysine residues on eNOS mediating this effect. Inhibition of SIRT1 reduces endothelial NO and vasodilation, demonstrating its significance in endothelial cells, while caloric restriction in mice induces eNOS deacetylation in these cells. These findings link the vascular benefits of caloric restriction to SIRT1’s regulation of eNOS, connecting metabolic and vascular health.
You can read the full article at https://www.pnas.org/content/104/37/14855?ijkey=9b8d33a7d87b2cbaecdb95665dc7751bf27fee94&keytype2=tf_ipsecsha.
Stein S, Lohmann C, Schäfer N, et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31(18):2301-9.
SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis
This study demonstrates that SIRT1, a class III deacetylase, protects against atherosclerosis by reducing macrophage foam cell formation. In mice with partial or bone marrow-specific SIRT1 deletion, macrophages accumulated more oxidized LDL (oxLDL), promoting foam cell formation and atherogenesis. SIRT1 achieves this protective effect by suppressing NF-κB signaling, which reduces the expression of the oxLDL receptor LOX-1. These findings highlight SIRT1 as a potential therapeutic target for atherosclerosis through its role in modulating macrophage function and foam cell formation.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC2938465/.
Breitenstein A, Stein S, Holy EW, et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc Res. 2011;89(2):464-72.
Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells
This study shows that Sirt1 suppresses tissue factor (TF) expression and arterial thrombus formation by inhibiting NFκB/p65 activation in endothelial cells. Sirt1 inhibition increases TF activity and accelerates thrombus formation in vivo, demonstrating its critical role in regulating thrombosis-related processes within these cells. These findings suggest that targeting Sirt1 in endothelial cells could be a potential therapeutic strategy for preventing thrombosis.
You can read the full article at https://academic.oup.com/cardiovascres/article/89/2/464/325074?login=false.
Available from https://openheart.bmj.com/content/3/1/e000402
Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus
This study explored the effects of the SIRT1 activator SRT2104 on arterial stiffness in healthy smokers and individuals with type 2 diabetes. SRT2104 treatment reduced augmentation pressure and showed a trend toward improving other stiffness indices, although no significant changes were observed in pulse wave velocity or blood pressure. The findings suggest a potential benefit of SRT2104 in reducing arterial stiffness, but the small sample size limits definitive conclusions.
You can read the full article at https://openheart.bmj.com/content/3/1/e000402.
Venkatasubramanian S, Noh RM, Daga S, et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2013;2(3):e000042.
Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers
This study evaluated the effects of the SIRT1 activator SRT2104 on cardiovascular function in healthy cigarette smokers. Over 28 days, SRT2104 improved the lipid profile, reducing total cholesterol, LDL cholesterol, and triglycerides, but did not significantly affect vascular or platelet function. The treatment was well tolerated and appeared safe, suggesting potential benefits for lipid management without notable changes in blood flow or fibrinolytic activity.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3698759/.
Libri V, Brown AP, Gambarota G, et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS ONE. 2012;7(12):e51395.
A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers
This study evaluated the safety and effects of SRT2104, a SIRT1 activator, in elderly volunteers over 28 days. SRT2104 was well tolerated, improved lipid profiles by reducing cholesterol, LDL, and triglycerides, and showed trends toward enhanced mitochondrial oxidative capacity without significant changes in glucose tolerance. The development of this compound revealed a 15–20 hour half-life with less-than-proportional dose exposure. The findings suggest SRT2104 has biological effects consistent with SIRT1 activation and supports its further development as a potential therapeutic for age-related conditions, highlighting the importance of continued development in this area.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3527451/.
Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br J ClinPharmacol. 2014;78:69–77.
A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes
This study evaluated the tolerability, pharmacokinetics, and effects of SRT2104 on glycemic control in adults with type 2 diabetes. SRT2104 was well tolerated, but its pharmacokinetics showed significant variability and were not dose-proportional. Treatment did not consistently improve fasting or postprandial glucose or insulin levels but was associated with improved lipid profiles. The lack of glycemic improvements may be attributed to the variable pharmacokinetics of SRT2104.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4168381/.
van der Meer AJ, Scicluna BP, Moerland PD, Lin J, Jacobson EW, Vlasuk GP, van der Poll T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Critical care medicine. 2015;43:e199–202.
The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Critical care medicine
This study investigated the effects of the SIRT1 activator SRT2104 on inflammation and coagulation in healthy humans exposed to lipopolysaccharide. Pretreatment with SRT2104 reduced pro-inflammatory cytokines (interleukin-6 and interleukin-8), C-reactive protein, and coagulation activation (lower prothrombin fragment F1+2 levels) but did not affect endothelial or fibrinolytic system activation. These findings provide the first evidence of SRT2104’s anti-inflammatory and anticoagulant effects in humans, consistent with SIRT1 activation.
You can read the abstract of the article at https://journals.lww.com/ccmjournal/abstract/2015/06000/the_selective_sirtuin_1_activator_srt2104_reduces.38.aspx.
Sands BE, Joshi S, Haddad J, Freudenberg JM, Oommen DE, Hoffmann E, McCallum SW, Jacobson E. Assessing Colonic Exposure, Safety, and Clinical Activity of SRT2104, a Novel Oral SIRT1 Activator, in Patients with Mild to Moderate Ulcerative Colitis. Inflammatory bowel diseases. 2016;22:607–614.
Assessing Colonic Exposure, Safety, and Clinical Activity of SRT2104, a Novel Oral SIRT1 Activator, in Patients with Mild to Moderate Ulcerative Colitis
This study evaluated the SIRT1 activator SRT2104 in patients with mild to moderate ulcerative colitis (UC). Despite high colonic drug exposure, SRT2104 did not significantly improve clinical or endoscopic outcomes, with few patients achieving remission and persistently elevated fecal calprotectin levels. Adverse events were common, including one severe UC flare, and variability in patient exposure may have influenced therapeutic responses. These findings suggest that, despite promising preclinical data, SRT2104 is not a viable therapeutic option for UC, warranting further studies to explore the impact of drug exposure on its efficacy.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4885523/.
Available from https://clinicaltrials.gov/ct2/history/NCT01453491?V_13=View
A Phase 1b Study to Assess the Safety and Anti-inflammatory Effects of Two Different Doses of SRT2104 in Patients With Ulcerative Colitis
This study aimed to evaluate the safety and tolerability of two oral doses of SRT2104 in ulcerative colitis patients, measure drug levels in blood and colon/rectal tissues, assess its anti-inflammatory effects after 8 weeks of treatment, and identify any changes in specific biomarkers associated with disease risk or progression.
You can read the full article at https://clinicaltrials.gov/ct2/history/NCT01453491?V_13=View.
Krueger JG, Suarez-Farinas M, Cueto I, Khacherian A, Matheson R, Parish LC, Leonardi C, Shortino D, Gupta A, Haddad J, Vlasuk GP, Jacobson EW. A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis. PLoS One. 2015;10:e0142081.
A Randomized, Placebo-Controlled Study of SRT2104, a SIRT1 Activator, in Patients with Moderate to Severe Psoriasis
This study evaluated the SIRT1 activator SRT2104 in 40 patients with moderate-to-severe psoriasis, showing that 35% of patients achieved significant histological improvement, linked to modulation of IL-17 and TNF-α pathways, despite inconsistent PASI results. Adverse events, mostly mild or moderate, were reported in 69% of participants, with common issues including headache and dizziness. Drug exposure increased dose-dependently but exhibited high variability, emphasizing the need for optimized dosing strategies. The findings suggest potential for SIRT1 activators in psoriasis treatment and highlight the importance of further research to better understand the relationship between therapeutic effects and exposure variability.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4640558/.
Van der meer AJ, Scicluna BP, Moerland PD, et al. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit Care Med. 2015;43(6):e199-202.
The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans
This study found that SRT2104, a SIRT1 activator, reduced inflammation and coagulation in healthy humans following lipopolysaccharide injection by attenuating cytokines (interleukin-6 and interleukin-8), reducing C-reactive protein levels, and lowering coagulation markers like prothrombin fragment F1+2. The treatment did not affect vascular endothelium activation or fibrinolysis, demonstrating anti-inflammatory and anticoagulant effects linked to SIRT1 activation.
You can read the abstract of the article at https://journals.lww.com/ccmjournal/abstract/2015/06000/the_selective_sirtuin_1_activator_srt2104_reduces.38.aspx.
Becatti M, Barygina V, Emmi G, et al. SIRT1 activity is decreased in lesional psoriatic skin. Intern Emerg Med. 2016;11(6):891-3.
Available from http://blogs.nature.com/spoonful/2011/11/small_resveratrol_trial_shows_1.html
Small resveratrol trial shows metabolic benefits, and might give hope to drug developers
A small study found that resveratrol, a compound in red wine, may positively impact metabolism in obese individuals by activating the sirtuin-1 gene. In a trial with 11 obese men taking resVida, a resveratrol supplement, participants experienced reduced liver fat, lower blood glucose, and a 4% decrease in systolic blood pressure, despite no weight loss. Protein analysis suggested improved mitochondrial function and increased sirtuin-1 levels, mimicking the benefits of exercise and calorie restriction, offering renewed hope for its therapeutic potential after previous drug trials faced setbacks.
You can read the full article at http://blogs.nature.com/spoonful/2011/11/small_resveratrol_trial_shows_1.html.
Available from https://medkoo.com/products/5855
Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712-6.
Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes
Calorie restriction extends lifespan and improves glucose homeostasis, partly through SIRT1, an NAD+-dependent deacetylase. Resveratrol, a SIRT1 activator, mimics these effects but with limited potency. Researchers have identified small molecule SIRT1 activators 1,000 times more potent than resveratrol, which bind an allosteric site and enhance enzyme activity. In obese mice and rats, these activators improved insulin sensitivity, lowered blood glucose, and increased mitochondrial capacity, highlighting SIRT1 activation as a promising therapeutic strategy for age-related diseases like type 2 diabetes.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC2753457/.
Qi Y, Davis ML, Hirsch ML, Cote AM, Lainez EO, Johnson MO, Gagne DJ, Vlasuk GP, Ellis JL. Activation of sirtuin1 (SIRT1) by the novel small molecule SRT2104 promotes body weight loss, increases exercise capacity and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2010;59(Suppl. 1) 390-PP.
Qi Y, Davis ML, Lainez EO, Cote AM, Johnson MO, Gagne DJ, Vlasuk GP, Ellis JL, Suri V. SRT2104, a novel small molecule SIRT1 activator ameliorates insulin resistance and promotes glucose utilization measured under a hyperinsulinemic-euglycemic clamp by enhancing both glycolysis and carbohydrate oxidation in mice fed a high fat diet. Diabetes. 2011;60(Suppl. 1) 1007-P.
Elliott P J, Jirousek M. Sirtuins: novel targets for metabolic disease. CurrOpinInvestig Drugs. 2008;9(4):371-8.
Sirtuins: novel targets for metabolic disease
Sirtuins are enzymes that regulate nutrient sensing, metabolism, and metabolic diseases. Activation of SIRT1 enhances proteins like PGC-1α, reducing glucose levels, improving insulin sensitivity, boosting mitochondrial function, decreasing adiposity, and improving exercise tolerance. SRT-501, a bioavailable resveratrol formulation, is the first SIRT1 activator shown to be safe and well-tolerated in humans, with clinical trials underway for type 2 diabetes treatment.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/18393104/.
Mercken EM, Mitchell SJ, Martin-Montalvo A, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13(5):787–796. doi:10.1111/acel.12220.
SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass
SRT2104, a synthetic SIRT1 activator, extends lifespan and improves health in mice on a standard diet, enhancing motor coordination, bone density, insulin sensitivity, and reducing inflammation. Short-term treatment also preserves bone and muscle mass during atrophy, further demonstrating its benefits. These findings highlight the potential of SIRT1 activators to improve motor coordination and mitigate age-related diseases in mammals, suggesting therapeutic promise for humans.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4172519/.
Cohen-kfir E, Artsi H, Levin A, et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152(12):4514-24.
Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor
Sirt1, a key regulator in metabolism and aging, also plays a critical role in bone health. Sirt1 haplo-insufficient female mice exhibit reduced bone mass due to decreased bone formation and increased marrow adipogenesis. Sirt1 negatively regulates Sost, the gene encoding sclerostin—a major inhibitor of bone formation—by deacetylating histone 3 at its promoter. Suppressing sclerostin restores bone formation markers and mineralization, suggesting Sirt1 as a potential target for anabolic osteoporosis therapies.
You can read the abstract of the article at https://academic.oup.com/endo/article-abstract/152/12/4514/2457299?redirectedFrom=fulltext&login=false.
Edwards JR, Perrien DS, Fleming N, et al. Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling. J Bone Miner Res. 2013;28(4):960-9.
Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling
SirT1 plays a critical role in bone remodeling by repressing NF-κB signaling in osteoclasts and osteoblasts, linking aging to bone loss. Deleting SirT1 in these cells leads to decreased bone mass due to increased resorption and reduced formation. In osteoclasts, SirT1 deficiency enhances osteoclastogenesis by increasing acetylation of Lysine 310 in NF-κB, while in osteoblasts, it impairs differentiation. Both effects can be mitigated by inhibiting NF-κB. Elevated NF-κB activity in these cells further disrupts bone remodeling, highlighting SirT1 as a key genetic determinant of bone mass via its regulation of NF-κB and bone cell function.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1002/jbmr.1824.
Artsi H, Cohen-kfir E, Gurt I, et al. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology. 2014;155(9):3508-15.
The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice
Estrogen deficiency-induced bone loss is linked to increased sclerostin expression, a negative regulator of bone formation. Sirt1 inhibits sclerostin by deacetylating histone 3 at the sost gene promoter. In ovariectomized mice, oral administration of the Sirt1 activator SRT3025 reversed bone loss, improved bone microarchitecture and strength, decreased sclerostin expression, and increased bone formation markers. In vitro, SRT3025 downregulated sclerostin and activated β-catenin. These findings highlight Sirt1 activation as a promising approach for anabolic osteoporosis therapies while potentially addressing other age-related conditions.
You can read the full article at https://academic.oup.com/endo/article/155/9/3508/2423228?login=false.
Zainabadi K, Liu CJ, Caldwell ALM, Guarente L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS ONE. 2017;12(9):e0185236.
SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis
Pharmacological activation of SIRT1 with SRT1720 significantly improves bone mass in ovariectomized female and aged male mice, models for postmenopausal and aging-related osteoporosis. Calorie restriction also upregulates Sirt1 expression in bone tissue and enhances bone mass, while SIRT1 knockout mice exhibit low bone mass. These findings establish SIRT1 as a positive regulator of bone mass and highlight its potential as a therapeutic target for osteoporosis.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5609767/.
Other Peptides
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

