GENEMEDICS APP
GENEMEDICS NUTRITION
NMN (Nicotinamide Mononucleotide)
Author: Dr. George Shanlikian, M.D. | Last Updated: November 20th, 2024
- Home
- >
- Health Library
- >
- NMN (Nicotinamide Mononucleotide)
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of NMN (Nicotinamide Mononucleotide)
- Key Takeaways
- What is NMN?
- How NMN Works
- Chemical Structure of NMN
- Research on NMN
- Nicotinamide Mononucleotide Side Effects
- What is NMN Supplement (Nicotinamide Mononucleotide Supplement)?
- Nicotinamide Mononucleotide vs Nicotinamide Riboside (NMN vs NR)?
- What is NMN Powder?
- What is NMN Sublingual?
- NMN Bodybuilding
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of NMN (Nicotinamide Mononucleotide)
- Key Takeaways
- What is NMN?
- How NMN Works
- Chemical Structure of NMN
- Research on NMN
- Nicotinamide Mononucleotide Side Effects
- What is NMN Supplement (Nicotinamide Mononucleotide Supplement)?
- Nicotinamide Mononucleotide vs Nicotinamide Riboside (NMN vs NR)?
- What is NMN Powder?
- What is NMN Sublingual?
- NMN Bodybuilding
- FAQ
- Reference
Overall Health Benefits of NMN (Nicotinamide Mononucleotide)
Nicotinamide mononucleotide (NMN) supports overall health by extending lifespan, producing anti-aging effects, enhancing cognitive and cardiovascular function, improving metabolic health, boosting immunity, and promoting organ health, including the liver, kidneys, and eyes. It also helps combat inflammation, cancer, diabetes symptoms, and supports fertility, energy levels, and weight management.
- Extends lifespan [1-12]
- Produces anti-aging effects [13-25]
- Improves cognitive function [26-40]
- Lowers the risk of cardiovascular disease [41-55]
- Fights cancer [56-63]
- Improves blood sugar levels and treats diabetes symptoms [16, 64-74]
- Fights inflammation [75-78]
- Improves fertility [79-85]
- Improves eye health [86-100]
- Boosts immune function [14, 101-110]
- Increases energy levels [111-117]
- Promotes weight loss [118-120]
- Treats stroke [121-124]
- Improves liver health [125-132]
- Improves kidney health [133-137]
Key Takeaways
- NAD+ Booster: NMN is a precursor to nicotinamide adenine dinucleotide (NAD+), a vital coenzyme in cellular energy production. NAD+ levels decline with age, leading to reduced cellular function. Supplementing with NMN can help restore NAD+ levels, supporting overall health and potentially slowing aspects of aging.
- Anti-Aging Potential: Research suggests NMN may help mitigate age-related decline by promoting cellular repair, enhancing mitochondrial function, and improving metabolic processes. These benefits have made NMN a popular supplement in anti-aging and longevity circles.
- Metabolic Health Benefits: NMN supplementation has been associated with improvements in glucose metabolism, insulin sensitivity, and overall metabolic health, which may benefit conditions like obesity, type 2 diabetes, and other metabolic disorders.
- Cardiovascular and Neuroprotection: NMN shows promise in protecting cardiovascular health by supporting blood vessel function. Additionally, some studies suggest it may offer neuroprotective effects, helping to maintain cognitive function and potentially reduce the risk of neurodegenerative diseases.
- Safety and Dosage: NMN is generally considered safe at typical dosages used in studies (ranging from 250-500 mg daily). However, research is ongoing, and while early results are promising, long-term effects and optimal dosage need further exploration.
What is NMN?
NMN (Nicotinamide mononucleotide) is a natural molecule produced by the body and is classified as a nucleotide. Nucleotides are involved in a wide array of important bodily functions, including as the building blocks of DNA. Within the cells, NMN is converted into another molecule called nicotinamide adenine dinucleotide (NAD+). NAD+ plays an integral role in energy production and regulation of vital cellular processes such as DNA repair, immune function, conversion of food into a usable form of energy called adenosine triphosphate (ATP), and regulation of circadian rhythm. In simple terms, NMN is the raw material and NAD+ is the refined version that the body can actually use to perform essential biological processes. In addition, the amount of NAD+ that the body can produce greatly depends on the available NMN.
NAD+ is not very bioavailable. This means that ingesting it directly will not achieve its therapeutic or desired effects. Therefore, one of the most effective ways of boosting NAD+ levels is through NMN supplementation.
How NMN Works
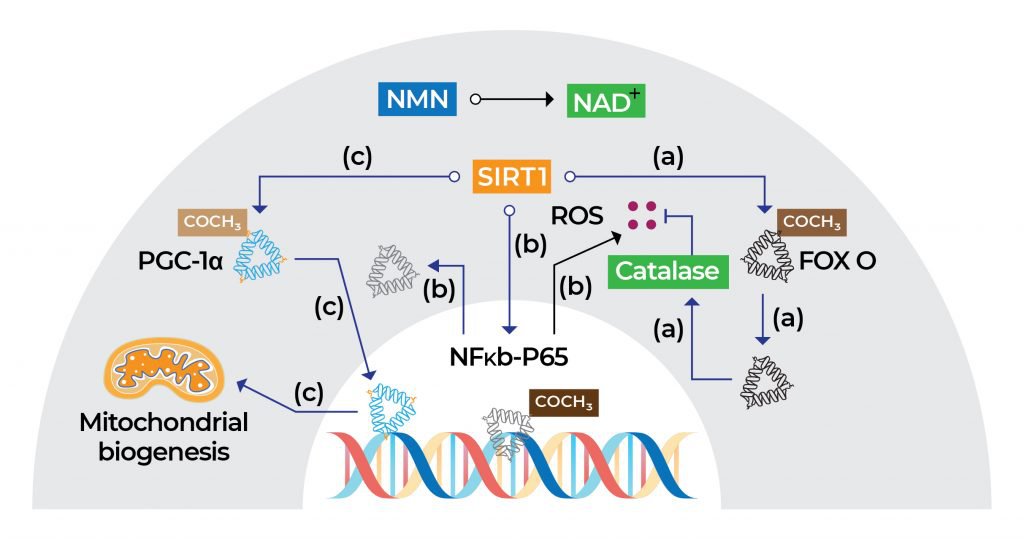
The health benefits of NMN can be attributed to its ability to boost NAD+ levels. Once NMN is converted into NAD+, activation of the sirtuin 1 (SIRT1) function in the nucleus of cells happens. SIRT1 is an enzyme that helps regulate proteins involved in cellular metabolism and processes associated with longevity, inflammation, and stress. In addition, the NMN-mediated increase in NAD+ levels counteracts age-related mitochondrial deterioration by promoting mitochondrial biogenesis, a process by which cells increase mitochondrial numbers.
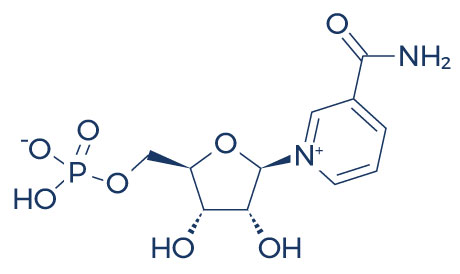
Chemical Structure of NMN
Research on NMN
A. Extends Lifespan

Within the cells, NMN is converted into NAD+ which plays an integral role in energy production and regulation of vital cellular processes. By boosting NAD+ levels, NMN can contribute to a longer lifespan. Studies show that people with higher NAD+ levels have a longer lifespan compared to those with lower NAD+ levels. [1-5]
Another mechanism that increases longevity is through increasing sirtuin (SIRT) activity which is associated with stable telomeres (located at chromosomes ends). This in turn helps attenuate the age-related telomere shortening which is linked to a shorter lifespan. [6] NMN boosts NAD+ levels which cause activation of SIRT, resulting in stable and longer telomeres. This process helps extends lifespan.
In addition, the NMN-mediated increase in NAD+ levels promotes mitochondrial biogenesis via SIRT1 activation. Mitochondrial biogenesis is characterized by the production of new mitochondria (the powerhouse of cells) and is essential for a longer lifespan since mitochondrial dysfunction is linked to various age-related diseases and a shorter lifespan. [7-8]
The longevity effects of NMN are backed by a number of studies:
- In mice, NMN maintained telomere length, reduced the DNA damage response, improved mitochondrial function, and rescued liver fibrosis (scarring) in a partially SIRT1-dependent manner. [9]
- Increasing sirtuin activity is known to stabilize telomeres and attenuate age-related telomere shortening. [10] Since the NMN-mediated increase in NAD+ activates SIRT1, it can help achieve chromosome stability and longer telomeres.
- In a rodent model of decompensated hemorrhagic shock, rats that received NMN had decreased inflammation, improved cellular metabolism, and increased survival. [11]
- In mice with progressive neurodegeneration, the addition of NMN in the drinking water of the subjects normalized neuromuscular function, delayed memory loss, and extended lifespan. [12]
B.Produces Anti-Aging Effects
Mitochondrial aging contributes to cellular senescence (also known as biological aging), increased inflammation, decreased stem cell activity, reduced healing rate, and a decline in tissue and organ function. [13] Interestingly, studies show that the NMN-mediated increase in NAD+ levels produces anti-aging effects such as increasing mitochondrial numbers, amelioration of mitochondrial dysfunction, and promotion of chromosome stability via activation of sirtuin 1 (SIRT1), stimulation of DNA repair, and maintaining telomere length:
- The administration of NMN via injections in elderly mice reversed age-related mitochondrial deterioration. It was observed that declining NAD+ levels were associated with interruptions in the normal signaling between the cell’s nucleus and mitochondria and that raising NAD+ levels via NMN administration restored the communication between these cellular structures. [14]
- In elderly mice, treatment with NMN improved blood flow and increased endurance via the promotion of SIRT1-dependent increases in capillary density. [15]
- In mice, the administration of NMN prevented age-related weight gain and improved physical activity, energy metabolism, lipid profiles, and insulin sensitivity. [16]
- In healthy men, the single oral administration of NMN was safe and effectively metabolized without any adverse effects, indicating a potential therapeutic strategy to mitigate disorders related to aging. [17-18]
- NMN effectively mitigated the age-associated physiological decline in the lungs of old mice and bleomycin-induced pulmonary fibrosis in young mice. [19]
- In aged mice, NMN treatment promoted mitochondrial rejuvenation and decreased inflammation. [20]
- In pre-aging male mice, oral short-term administration of NMN significantly increased telomere length. [21]
- Elevating NAD+ levels has been shown to slow down various mechanisms associated with aging such as decreased energy production in the mitochondria, oxidative stress, DNA damage, cognitive impairment, and inflammation. [22-23]
- NMN has been shown to slow down age-related changes in the skin by restoring skin homeostasis, protecting against oxidative stress, increasing mitochondrial efficiency, and reducing excess melanin production. [24-25]
C.Improves Cognitive Function

A decline in NAD+ levels is associated with brain disorders such as Alzheimer’s disease, Parkinson’s disease, and other conditions that cause cognitive impairment. [26] By boosting NAD+ levels, NMN can lower the risk for these medical conditions. Another interesting mechanism is that the NMN-mediated increase in NAD+ levels can decrease the production of reactive oxygen species (ROS), which are linked to various brain disorders. Moreover, NMN can also help reverse the age-related cognitive decline by mitigating mitochondrial dysfunction.
A number of studies demonstrate the beneficial effects of NMN on cognitive function:
- In an Alzheimer’s disease-relevant murine model, NMN treatment restored mitochondrial respiratory function in the brain. [27]
- In an animal model of Alzheimer’s disease, NMN treatment significantly decreased the production of β-amyloid (abnormal protein structures), loss of nerve signaling, and inflammatory response. [28]
- In a rat model of vascular cognitive impairment, NMN protected against cognitive decline. [29]
- In older rats, NMN treatment at a dose of 100 mg/kg alleviated aging-induced memory impairment via modulation of mitochondrial function and apoptosis (programmed cell death) in the brain. [30]
- In D-galactose-induced aging rat models, the combination of NMN and lycopene improved the ability of spatial location learning and memory. [31]
- In rats, NMN ameliorated neuronal damage and cognitive impairment caused by severe hypoglycemia (low blood sugar levels). [32]
- In rats, NMN protected against diabetes-induced memory deficits by preserving mitochondrial oxidative phosphorylation (OXPHOS) function and preventing neuronal loss. [33]
- In the brain cells of aged rats, NMN treatment increased the formation of new blood vessels and decreased the production of oxidative stress. [34]
- In a rat model of Alzheimer’s disease, NMN protected against β-amyloid oligomer-induced cognitive impairment and neuronal death. [35]
- In aged mice, NMN supplementation improved cognitive function by ameliorating age-related cerebromicrovascular dysfunction. [36]
- Studies found that NMN can help improve cognitive function by promoting the renewal of neural stem/progenitor cells (NSPCs) via SIRT1, SIRT2, and SIRT6. [37-38]
- In old mice, short-term NMN supplementation improved the sensory processing aspect of some aversive stimuli, suggesting that the treatment can treat cognitive impairments and enhance the quality of life in old age. [39]
- In a cellular model of Parkinson’s disease (PD), ameliorated mitochondrial inhibitor-induced impairments of energy metabolism and inhibited death of brain cells. [40]
D.Lowers the Risk of Cardiovascular Disease

The NMN-mediated increase in NAD+ levels activates SIRT1, which in turn increases the production of cardioprotective molecules, such as MnSOD (antioxidants), Trx1 (antioxidants), and Bcl-xL (anti-apoptotic). [41] In addition, SIRT1 activation can also help protect the heart from inflammation and oxidative stress.
Compelling evidence supports the cardioprotective effects of NMN:
- In mice, NMN protected against heart injury caused by insufficient blood flow (ischemia). [42]
- A study suggests that NMN exerts its cardioprotective effects by generating adenosine triphosphate via glucose breakdown. [43]
- In mice with ischemia, NMN (62.5mg/kg) dramatically ameliorated injury and significantly improved the neurological outcome. [44]
- In mice, NMN treatment prevented post-ischemic depletion of mitochondrial NAD+, suppressed mitochondrial fragmentation, and reduced oxidative stress via SIRT3-dependent mechanisms. [45-46]
- In the heart cells of mice, short-term administration of NMN preserved mitochondrial ultrastructure, reduced oxidative stress, and prevented cell death in the heart. [47]
- Studies reported that NMN administration in patients with intractable cardiac diseases such as heart failure with preserved ejection fraction may produce beneficial effects. [48-49]
- In rats, NMN attenuated doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation, and programmed cell death. [50]
- In aged male rats, NMN counteracted damage to the heart muscle by activating SIRT3/FOXO1 and reducing programmed cell death. [51]
- In mice with heart scarring, NMN administration via injections reduced scarring by suppressing oxidative stress and Smad3 acetylation in a NAD+/SIRT1-dependent manner. [52]
- In aged mice, NMN administration increased NAD+ levels and protected against ischemic heart injury. [53-55]
E.Fights Cancer
Mitochondrial respiration malfunction and increased glucose uptake are mechanisms observed in cancer cells. [56] The NMN-mediated increase in NAD+ levels has been shown to increase mitochondrial respiration and reduce glucose (blood sugar) uptake, indicating that NMN may help combat cancer. Another important mechanism is that NMN increases NAD+ levels which in turn activates SIRT1 and SIRT6, both of which inhibit the growth and spread of tumors.
A number of studies support the anti-cancer properties of NMN:
- The NMN-mediated increase in NAD+ levels is associated with cell cycle arrest and programmed cell death of malignant cells, enhanced efficacy of chemotherapeutic drugs and radiation therapy, and prevention of cancer cell progression. [57-61]
- NMN has been shown to combat cancer by boosting cellular energy and enhancing DNA repair activity. [62]
- NMN has also been shown to enhance colorectal cancer cell-kill by the chemotherapeutic drug Tiazofurin. [63]
F.Improves Blood Sugar Levels and Treats Diabetes Symptoms
NMN has the ability to improve the body’s response to the hormone insulin, which helps blood sugar enter the cells. This process is called insulin sensitivity. With increased insulin sensitivity, blood sugar stays at healthy levels.
The blood sugar-lowering effects of NMN and its benefits on diabetes symptoms are backed by a number of studies:In mice, the administration of NMN prevented age-related weight gain and improved physical activity, energy metabolism, lipid profiles, and insulin sensitivity. [16]
- In prediabetic women, NMN supplementation at 250 mg/day increased muscle insulin sensitivity. [64]
- In obese mice, increased NAD+ levels induced by NMN improved blood glucose and lipid homeostasis by increasing the activity of SIRT1 and SIRT3. [65-66]
- In old mice with type 2 diabetes, NMN improved glucose intolerance and lipid profiles. [67]
- In mice, NMN treatment ameliorated NAD+ deficiency and improved insulin secretion. [68]
- In mice fed with fructose, a type of sugar, administration of NMN restored insulin secretion by correcting inflammation of the islet of the pancreas (responsible for insulin production). [69]
- Studies found that NMN supplementation for 12 months decreased insulin resistance in mice. [70-71]
- In mice fed with a high-fat diet, NMN administered via intravenous injections improved glucose tolerance. [72]
- A cell study found that NMN can stimulate insulin secretion. [73]
- In lean type 2 diabetic patients with secondary failure to sulphonylureas (anti-diabetic medication), NMN improved insulin secretion and metabolic control. [74]
G.Fights Inflammation
NMN has the potential to suppress inflammaging, which is the age-related increase in inflammation. Specifically, NMN has been found to suppress cyclooxygenase-2 (COX-2), an enzyme that synthesizes the proinflammatory mediators known as prostaglandins. With this effect, NMN can help treat and ward off a wide array of inflammatory conditions.
A convincing number of studies support the anti-inflammatory effects of NMN:
- In mice, NMN inhibited lipopolysaccharide (LPS)-induced inflammation and oxidative stress via suppression of COX-2. [75]
- In aging mice, NMN reduced inflammatory markers such as tumor necrosis factor alpha (TNF-α). [76]
- In mice with inflammation of the abdomen due to blood infection, NMN prevented clinical deterioration and improved survival. [77]
- A cell study found that NMN inhibited endothelial inflammation and improved the function of nitric oxide (a substance that widens the blood vessels). [78]
H. Improves Fertility
NMN has the capacity to improve male and female fertility. It does this by improving the quality of both the egg cell and sperm cell. This in turn ensures successful fertilization and pregnancy. In addition, NMN can also help reverse some of the effects of aging on the reproductive system.
The beneficial effects of NMN on male and female reproductive health are backed by a number of studies:
- In animal subjects, NMN supplementation protected egg cell quality against environmental pollutant-induced deterioration, contributing to improved fertility. [79]
- In aged animals, treatment with the NAD+ metabolic precursor NMN rejuvenated egg cell quality, leading to the restoration of fertility. [80-81]
- NMN supplementation improved the quality of porcine egg cells under heat stress by restoring cell division. [82]
- Supplementation of NMN improved the quality of postovulatory aged porcine egg cells. [83]
- In female mice, NMN supplementation improved egg cell quality by restoring mitochondrial structures. [84]
- In streptozotocin-induced diabetic mice, NMN treatment significantly increased the area and diameter of seminiferous tubules and the number of spermatogenic cells and sperms. [85]
I. Improves Eye Health
Restoration of NAD+ through NMN supplementation can help protect photoreceptors (special cells in the retina that converts light into signals that are sent to the brain) against light-induced retinal damage. [86-87] The exact mechanism of NMN-induced eye protection can be attributed to SIRT1 activation since it is essential in the development and survival of the retina. Alterations in SIRT1 activity have been linked to various eye conditions such as aged retina, diabetic retinopathy, light-induced retinal degeneration, and oxygen-induced retinal damage. [88-93]
Studies show that NMN supplementation is essential for eye health:
- NMN treatment increased NAD+ levels and improved cell viability, reduced programmed cell death, and decreased lactate dehydrogenase (LDH) release in corneal epithelial cells. [94]
- In high-glucose-treated human corneal epithelial cells, NMN increased cell viability by reversing cell damage, reducing programmed cell death, increasing cell migration, and restoring the structures of corneal cells. [95]
- A study reported that NMN supplementation can treat glaucoma and age-related macular degeneration by correcting NAD+ pool depletion and mitochondrial dysfunction. [96]
- In a mouse model of retinal ischemia-reperfusion injury (cellular dysfunction and death after the restoration of blood flow to tissues with previously impaired blood circulation), NMN injection significantly suppressed retinal functional damage and inflammation and protected against oxidative stress-induced cell death. [97]
- In a photoreceptor degenerative model of retinal detachment, NMN administration exerted neuroprotective effects on photoreceptors and against oxidative injury. [98]
- In mice, NMN effectively prevented ultraviolet B light-induced tissue damage and endothelial cell death in the mouse cornea. [99]
- In mice with corneal injury, the replenishment of NMN or NAD+ slowed down corneal nerve fiber degeneration by restoring the activation levels of SIRT1. [100]
J. Boosts Immune Function
The age-related shortening of telomeres adversely affects immune function, thus, increasing the risk of severe infection, inflammatory conditions, and chronic diseases. [101-103] Interestingly, NMN boosts NAD+ levels which in turn activates SIRT1. As a result, the telomeres lengthen and become more stable. Moreover, NMN has anti-inflammatory effects and the ability to regulate the activity of certain cells of the immune system.
A good deal of evidence supports the immune-boosting effects of NMN:
- Treatment of 24-month-old mice with NMN for 1 week significantly reduced the levels of inflammatory markers such as TNFα and IL-6 in the skeletal muscle. [14]
- In young and older mice, NMN augmented the cytotoxic activity of natural killer cells of the immune system. [104]
- The NMN-mediated increase in NAD+ levels can help improve immune function by promoting cell survival, DNA repair, and enhanced intercellular communication. [105-106]
- Restoring normal NAD+ levels via NMN can decrease the severity of immune reactions in patients with COVID-19 infection. [107]
- An increase in NAD+ levels was associated with significant immunomodulatory effects such as modulation of cytokine action, regulation of the intercellular adhesion molecules, blockage of mast cell degranulation, and inhibition of protease release from leukocytes. [108-110]
K. Increases Energy Levels
Sirtuins play a critical role in regulating various cellular functions including energy metabolism, stress resistance, and circadian rhythm neuronal function – all of which are essential for increasing energy levels. [111-112] Since NMN activates SIRT1 by increasing NAD+ levels, it may help boost energy levels and reduce fatigue. Moreover, NAD+ is essential for the production of adenosine triphosphate (ATP), which is needed by the cells to perform various biological functions.
An increasing number of studies support the beneficial effects of NMN on energy levels and medical conditions that cause fatigue:
- In older adults, NMN intake in the afternoon for 12 weeks effectively improved sleep quality, fatigue, and physical performance as evidenced by improved lower limb function and reduced drowsiness. [113]
- In amateur runners, NMN supplementation for 6 weeks increased the aerobic capacity during exercise training via enhanced O2 utilization of the skeletal muscle. [114]
- In healthy young and elderly mice, NMN supplementation at 500 mg/kg/d with exercise training increased endurance performance. [115-116]
- Raising intracellular NAD+ levels through NMN supplementation can improve the quality of life of patients with chronic fatigue syndrome by improving neurological function, promoting energy production, and lowering fatigue. [117]
L. Promotes Weight Loss
NMN can help promote weight loss via different mechanisms such as increased energy expenditure and enhanced insulin sensitivity. Increased energy expenditure prevents excess fat storage. With enhanced insulin sensitivity, the body responds well to the effects of insulin which in turn prevents high blood sugar (hyperglycemia) which is associated with increased adiposity.
Evidence suggests that NMN is beneficial for achieving a healthier weight because of its fat-burning properties:
- In healthy individuals, the intravenous administration of NMN significantly reduced blood triglyceride (TG) levels and fat accumulation in the liver. [118]
- In mice with severe insulin resistance, NMN treatment reduced visceral adipose tissue (VAT) and adiponectin (a hormone produced by fat cells). [119]
- In obese female mice, NMN reduced adiposity and improved glucose and markers of mitochondrial function. [120]
M. Treats Stroke
A stroke occurs when the blood supply to the brain is cut off. NMN has the ability to widen the blood vessels which can help restore blood flow to the brain. In addition, the anti-inflammatory effects of NMN can help relieve brain swelling associated with stroke.
A number of studies suggest that NMN treatment is beneficial in treating the symptoms of stroke and improving recovery outcomes:
- In mice with brain injury caused by stroke, NMN treatment for 7 days markedly promoted the recovery of body weight and neurological function via suppression of brain inflammation and oxidative stress. [121]
- In a rodent model of hemorrhagic shock due to stroke, NMN significantly improved survival after resuscitation. [122]
- NAD replenishment with NMN protected blood-brain barrier integrity and attenuated brain changes caused by significant bleeding. [123]
- NMN treatment attenuated traumatic brain injury in mice via restoration of NAD+ levels. [124]
N. Improves Liver Health
NMN boosts NAD+ levels resulting in SIRT1 activation. This process is essential in liver health as SIRT1 activation improves cholesterol, fat, and lipid transport as well as fatty acid homeostasis in the liver. [125-127]
Studies show that NMN can improve liver function and protect against liver disease:
- NMN treatment can help protect against liver injury by raising NAD+ levels. [128]
- An increase in NAD+ has been shown to protect against aging-induced non-alcoholic fatty liver disease-like liver dysfunction in mice. [129]
- Increased NAD+ levels can prevent the progression of non-alcoholic fatty liver disease by influencing the oxidative stress response, programmed cell death, and inflammatory response. [130]
- In mouse models of liver cirrhosis (scarring), NMN treatment inhibited the production of substances that cause liver inflammation and scarring. [131]
- In aged mice, NMN administration protected against oxidative stress-induced liver injury. [132]
O. Improves Kidney Health
The anti-aging effects of NMN can also help address the age-related decline in kidney function. Reduced levels of NAD+ are associated with reduced sirtuin activity which in turn causes deterioration in the overall function of the kidneys. The ability of NMN to boost NAD+ levels activates SIRT1 which can possibly mitigate the negative effects of aging on the kidneys.
Evidence suggests that NMN can help address kidney problems associated with aging and certain medical conditions:
- In old mice with acute kidney injury, NMN supplementation improved kidney function via restoration of renal SIRT1 activity and NAD+ content. [133]
- In human kidney cells, NMN suppressed DNA damage and senescence induced by hydrogen peroxide and hypoxia (low oxygen). [134]
- In mice, short-term NMN treatment ameliorated adriamycin-induced kidney damage by increasing SIRT1. [135]
- In mice with kidney complications due to diabetes, NMN treatment increased kidney concentrations of NAD+ and SIRT1, improved survival rates, and alleviated kidney scarring. [136-137]
Nicotinamide Mononucleotide Side Effects
NMN side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on NMN. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of NMN. Despite this, it was listed as a side effect associated with NMN even though these associated side effects are very uncommon.
Side effects associated with NMN may include the following:
- Abdominal distension
- Abdominal pain
- Belching
- Diarrhea
- Fatigue
- Fever
- Flatus
- Joint pain
- Muscle pain
- Sense of hunger
What is NMN Supplement (Nicotinamide Mononucleotide Supplement)?
Nicotinamide Mononucleotide (NMN) is a molecule that occurs naturally in the body and plays a crucial role in cellular metabolism. It is a precursor to nicotinamide adenine dinucleotide (NAD+), a vital coenzyme involved in numerous biological processes, including energy production, DNA repair, and cellular aging. NMN supplements aim to boost NAD+ levels, which tend to decline with age, potentially supporting overall health and longevity.
Research into NMN supplements has been promising, suggesting they may have various health benefits. Studies in animals have indicated that NMN can improve metabolic health, enhance physical activity, and slow down certain aspects of aging. In humans, preliminary research suggests that NMN supplementation may help improve insulin sensitivity, increase muscle strength, and support cardiovascular health, although more extensive clinical trials are needed to fully understand its effects.
Despite the potential benefits, NMN supplements should be approached with caution. The supplement industry is not strictly regulated, so the quality and effectiveness of NMN products can vary. It’s important to consult with a healthcare professional before starting any new supplement regimen, especially if you have underlying health conditions or are taking other medications.
Nicotinamide Mononucleotide vs Nicotinamide Riboside (NMN vs NR)?
Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR) are both compounds that play a role in the production of NAD+ (nicotinamide adenine dinucleotide), a vital coenzyme involved in numerous cellular processes, including energy metabolism and DNA repair. NMN is a direct precursor to NAD+, meaning it is converted into NAD+ more directly within cells. This pathway potentially makes NMN a more efficient option for boosting NAD+ levels.
On the other hand, Nicotinamide Riboside (NR) is a slightly different compound that also contributes to NAD+ synthesis but through a more indirect route. NR is first converted into nicotinamide mononucleotide (NMN) before being transformed into NAD+. This extra step may influence its effectiveness compared to NMN. However, research suggests that NR is still highly effective in increasing NAD+ levels and has demonstrated various health benefits in studies.
Both NMN and NR have shown promise in preclinical and clinical studies for their potential anti-aging effects, including improving metabolic health and enhancing physical endurance. While both compounds seem to offer similar benefits, the choice between NMN and NR might come down to individual preferences or specific health goals. Ongoing research continues to explore their comparative efficacy and optimal use.
What is NMN Powder?
NMN powder is a dietary supplement derived from nicotinamide mononucleotide (NMN), a naturally occurring compound in the body that plays a crucial role in cellular metabolism. NMN is a precursor to nicotinamide adenine dinucleotide (NAD+), a coenzyme essential for energy production, DNA repair, and various metabolic processes. As we age, NAD+ levels decline, which can impact overall health and vitality.
Supplementing with NMN powder is believed to help boost NAD+ levels, potentially counteracting some effects of aging and supporting cellular function. Research into NMN’s benefits is ongoing, but preliminary studies suggest that it may enhance physical endurance, improve cognitive function, and promote healthier aging by improving cellular energy production and repair mechanisms.
NMN powder is typically taken as a dietary supplement in capsule or powdered form. While promising, it’s important to approach NMN with a balanced perspective, as more research is needed to fully understand its long-term effects and benefits. Consulting with a healthcare provider before starting any new supplement regimen is recommended to ensure it aligns with individual health needs and goals.
What is NMN Sublingual?
NMN sublingual refers to nicotinamide mononucleotide (NMN) delivered via a sublingual method, meaning it is taken under the tongue. NMN is a compound that plays a crucial role in the production of NAD+ (nicotinamide adenine dinucleotide), a coenzyme involved in various biological processes, including energy metabolism and cellular repair. By delivering NMN directly under the tongue, the supplement can be absorbed more rapidly into the bloodstream, bypassing the digestive system and potentially increasing its effectiveness.
The sublingual form of NMN is designed to offer faster absorption and higher bioavailability compared to oral tablets or capsules. This method leverages the rich blood supply under the tongue, which allows for quicker entry into the systemic circulation. Consequently, users might experience more immediate effects and enhanced benefits related to NMN’s role in promoting cellular health and combating age-related decline.
Many proponents of NMN sublingual supplements believe they can contribute to improved energy levels, cognitive function, and overall vitality. Research into NMN’s potential benefits is ongoing, but preliminary studies suggest that enhancing NAD+ levels may have positive effects on aging and various health conditions. As with any supplement, it is important to consult with a healthcare provider before starting NMN sublingual to ensure it is appropriate for individual health needs and conditions.
NMN Bodybuilding
Nicotinamide Mononucleotide (NMN) has gained attention in the bodybuilding community for its potential benefits in enhancing physical performance and recovery. NMN is a precursor to Nicotinamide Adenine Dinucleotide (NAD+), a vital coenzyme involved in cellular energy production and metabolism. By boosting NAD+ levels, NMN may improve muscle endurance, reduce fatigue, and promote more efficient recovery after intense workouts.
Research into NMN’s impact on bodybuilding is still emerging, but some studies suggest it could help mitigate age-related declines in muscle function and strength. As we age, NAD+ levels naturally decrease, which can contribute to decreased muscle mass and performance. Supplementing with NMN might counteract these effects, helping bodybuilders maintain their muscle mass and strength over time.
Additionally, NMN’s potential anti-inflammatory and antioxidant properties could offer further advantages for bodybuilders. Reducing oxidative stress and inflammation can help in preventing exercise-induced muscle damage and speeding up recovery. While more research is needed to fully understand NMN’s effects, its role in supporting cellular health makes it an intriguing option for those looking to enhance their bodybuilding regimen.
FAQ
What does nicotinamide mononucleotide do?
Nicotinamide Mononucleotide (NMN) is a precursor to Nicotinamide Adenine Dinucleotide (NAD+), a crucial coenzyme involved in energy production, DNA repair, and cellular metabolism. In various animal models, studies show that by increasing NAD+ levels, NMN helps enhance cellular function and supports a healthy plasma lipid profile. Research involving animal models suggests that NMN supports metabolic processes, improves overall energy levels, and contributes to a healthy plasma lipid profile. These findings from animal models highlight the potential of NMN in promoting better cellular health and maintaining a healthy plasma lipid profile.
What are the benefits of taking NMN?
NMN supplementation may offer several benefits, including improved cellular energy production, enhanced physical performance, better recovery from exercise, and potential anti-aging effects. By increasing NAD+ levels, NMN supports metabolic health, muscle function, and overall vitality. In terms of dosage, it is important to consider the mg kg measurement to ensure optimal results. Research often uses mg kg to determine effective dosages for NMN supplementation. This helps in understanding how NMN impacts various aspects of health on a mg kg basis.
Is NMN just niacin?
No, NMN is not just niacin. Niacin (vitamin B3) is a precursor to NAD+, but NMN is a more direct precursor in the NAD+ synthesis pathway. NMN is converted into NAD+ more efficiently than niacin or niacinamide, which can positively impact sleep quality, especially with long term administration. This efficiency makes NMN a more targeted supplement for boosting NAD+ levels and potentially improving sleep quality, as evidenced by human clinical studies. Enhanced NAD+ levels through NMN, particularly with long term administration, might also contribute to better overall sleep quality, according to human clinical research. Long term administration of NMN could be a key factor in sustaining these benefits, supported by ongoing human clinical trials.
Is NMN worth taking?
The worth of NMN largely depends on individual health goals and needs. For those seeking to enhance cellular energy, support aging processes, or improve physical performance, NMN might be beneficial. Additionally, NMN could potentially have an impact on sleep quality, which is a crucial factor in overall health. For example, NMN might influence the function of skeletal muscle, which could in turn affect physical performance. However, its effectiveness in improving sleep quality and its role in skeletal muscle health can vary depending on long term administration, and ongoing research is needed to fully understand its benefits and long-term safety. Long term administration of NMN could provide more stable benefits, but careful consideration is required when evaluating its long-term administration and effects.
What are the downsides of NMN?
Potential downsides of NMN include gastrointestinal discomfort, such as nausea or diarrhea. Human clinical trials are crucial for determining these effects in real-world scenarios, particularly in relation to mitochondrial oxidative metabolism. Long-term effects, especially on mitochondrial oxidative metabolism, are still not well established, and the supplement might interact with certain medications or conditions. Human clinical trials are ongoing to better understand these risks, including the impact on mitochondrial oxidative metabolism. It’s important to monitor for any adverse reactions and consult a healthcare provider, as insights from human clinical trials can guide safe usage.
Does NMN actually work?
Preliminary research and anecdotal evidence suggest that NMN can effectively boost NAD+ levels in the human body and support various aspects of health, including energy metabolism in skeletal muscle and aging. NAD decline is a crucial factor in age-related health issues, and NMN supplementation may help address this. Adenine dinucleotide levels are integral to this process, as NAD+ is derived from adenine dinucleotide. However, more comprehensive clinical trials are needed to confirm its efficacy and safety across different populations within the human body, including effects on skeletal muscle health. The potential benefits for skeletal muscle function and overall well-being, especially in the context of NAD decline, make it an area of interest for further investigation. Understanding how NMN counters NAD decline, including its role in adenine dinucleotide levels, could unlock important insights into aging and metabolic health.
Who should be taking NMN?
NMN may be beneficial for individuals looking to improve cellular energy, support aging processes, or enhance physical performance through pathways related to nicotinic acid and body temperature regulation. It also has anti-aging properties that make it appealing for those aiming to maintain youthfulness by optimizing body temperature. It might also be considered by those with specific health conditions that impact NAD+ levels, where nicotinic acid and body temperature regulation could play a role. Additionally, the anti-aging properties of NMN have been linked to supporting overall vitality. Always consult with a healthcare provider to determine if NMN and related compounds like nicotinic acid are appropriate for you, especially if you are interested in its anti-aging properties.
What is nicotinamide mononucleotide good for?
Nicotinamide Mononucleotide (NMN) is good for boosting NAD+ levels, which supports cellular energy production, metabolic health, DNA repair, and potentially slows down some aspects of aging and age-related diseases. Caloric restriction is known to be beneficial in promoting longevity, and when combined with NMN, the effects could be more pronounced. Nicotinic acid can also aid in improving exercise performance and recovery, especially when aligned with caloric restriction practices. Additionally, nicotinic acid contributes to overall metabolic processes that enhance the body’s resilience against age-related diseases. Integrating nicotinic acid alongside NMN may further amplify the benefits for cellular health, energy production, and help in managing age-related diseases, particularly when combined with caloric restriction approaches.
What are the risks of taking NMN?
The risks of taking NMN are generally low but may include gastrointestinal issues such as nausea or diarrhea. Age-associated weight gain is another factor that might interact with supplement efficacy, although further studies are needed. NMN plays a role in maintaining cell membranes, but since it is a relatively new supplement, long-term risks related to age-associated weight gain and other factors are not well understood. Consulting a healthcare professional is recommended to assess any personal risks, especially if age-associated weight gain is a concern and the health of your cell membranes is a factor.
Is NMN just vitamin B3?
No, NMN is not just vitamin B3. While vitamin B3 (niacin) is a precursor to NAD+, NMN is a more direct precursor and is converted into NAD+ more efficiently. NMN is particularly relevant for addressing metabolic disorders and body weight management, as it provides a more targeted approach to increasing NAD+ levels compared to vitamin B3. Additionally, NMN’s efficiency in boosting NAD+ levels can play a crucial role in mitigating the impact of metabolic disorders and managing body weight. By focusing on NMN, there’s potential for better management of metabolic disorders, body weight, and overall health through enhanced NAD+ production.
What does NMN do for the body?
NMN boosts NAD+ levels, which supports various bodily functions, including energy production, cellular repair, and metabolism. As one of the popular dietary supplements, this can lead to improved physical performance, enhanced recovery, and potentially slowing some aspects of aging, which may also have an impact on body weight. Many studies use animal models to explore the effects of dietary supplements like NMN for these benefits, especially when focusing on maintaining a healthy body weight. Incorporating dietary supplements such as NMN can be a strategic choice for those looking to optimize their health, body weight, and longevity. Research involving animal models continues to shed light on how these supplements can influence overall well-being.
Is it worth taking NMN supplement?
For individuals aiming to enhance their cellular health, boost energy levels, or support aging processes through neuronal DNA repair, oral administration of NMN supplements may be worth considering. However, it’s important to evaluate personal health goals and consult a healthcare provider to determine if oral administration of NMN is a suitable option for promoting neuronal DNA repair. Animal models can provide valuable insights into the effects of NMN on neuronal DNA repair. Ultimately, oral administration should be discussed with a healthcare professional before beginning supplementation to ensure it aligns with your goals for neuronal DNA repair. Additionally, research involving animal models can help further understand the potential benefits and risks associated with NMN supplementation.
Is it safe to take NMN daily?
Daily NMN supplementation via oral administration is generally considered safe for most people when taken in recommended doses. However, long-term safety data on oral administration is limited, so it’s important to monitor for any adverse effects and consult a healthcare provider for personalized advice regarding oral administration. Ensuring cell survival is crucial when considering any supplementation, as this can impact overall health. Additionally, while NMN can support cellular processes, its effects on cell survival over the long term remain an area of ongoing research.
Does NMN have side effects?
NMN appears to be usually well-tolerated, but potential side effects may include gastrointestinal issues such as nausea or diarrhea. As with any supplement, it’s important to start with a lower dose to assess tolerance and consult a healthcare professional if any adverse effects occur. Animal models have shown that NMN is generally safe, but if NMN appears to cause discomfort, it’s crucial to seek medical advice. Studies using animal models can provide insights into potential issues, but individual responses may vary.
Are there any side effects of NMN?
Possible side effects of NMN can include gastrointestinal discomfort such as nausea or diarrhea. Because NMN is still relatively new as a supplement, its long-term effects and safety are not fully established. Human trials are essential for gaining a clearer understanding of NMN’s effects. However, it is often discussed in the context of healthy aging, as it may play a role in promoting overall health. Continued research, including human trials, is needed to understand its impact on healthy aging fully and to ensure it is safe for long-term use.
Who should not take nicotinamide?
Individuals with certain health conditions, allergies, or those on specific medications should avoid nicotinamide or NMN supplements. It’s essential to consult a healthcare provider to ensure that the oral administration of the supplement is safe and appropriate for your individual health situation. In some cases, clinical trials may indicate that the oral administration of nicotinamide or NMN is not recommended. Always verify with your healthcare provider to determine if oral administration aligns with your health needs, and consider reviewing any relevant clinical trials for additional information.
How does NMN make you feel?
Many people report feeling increased energy and improved physical performance with NMN supplementation. However, individual responses can vary, and some may experience mild gastrointestinal discomfort. It’s worth noting that NMN, like many other substances, is subject to scrutiny and regulation, but it is not yet classified as a heavily regulated therapeutic drug. Monitoring how NMN affects you personally is important, as the effects and regulations can differ. Be aware that while NMN shows promise, it is not a heavily regulated therapeutic drug and should be used with informed caution.
Is nicotinamide mononucleotide the same as nicotinamide?
No, nicotinamide mononucleotide (NMN) is not the same as nicotinamide. While both are involved in NAD+ production, NMN, a naturally occurring molecule, is a direct precursor to NAD+ and is converted into it more efficiently. In contrast, nicotinamide, another naturally occurring molecule, is another form of vitamin B3. Thus, NMN, a naturally occurring molecule, plays a more direct role in NAD+ synthesis.
Should I be taking NMN or NAD?
NMN is a precursor to NAD+ and is often preferred for supplementation because it can be converted into NAD+ more efficiently. Direct NAD+ supplements are also available, but NMN may provide a more effective means of boosting NAD+ levels, which could be beneficial for managing Alzheimer’s disease. Research into NMN and its effects on NAD+ levels is ongoing, and its potential impact on conditions such as Alzheimer’s disease is an area of active investigation. As we continue to understand the role of NAD+ in cognitive health, NMN supplementation might become an important factor in the prevention and management of Alzheimer’s disease.
Can I take niacinamide instead of NMN?
Niacinamide (a form of vitamin B3) can contribute to NAD+ production but is less direct compared to NMN. NMN, at varying mg kg dosages, is more efficiently converted into NAD+ and may offer more targeted benefits for boosting NAD+ levels. When considering supplements, it’s important to note the mg kg amounts used in studies to assess their impact on NAD+ production.
Which is better NR or NMN?
Both Nicotinamide Riboside (NR) and NMN are effective in boosting NAD+ levels in healthy adults, but NMN is a more direct precursor in the NAD+ synthesis pathway for healthy adults. Substantial clinical investigations suggest that the choice between NR and NMN can depend on personal preference and individual response in healthy adults. Moreover, substantial clinical investigations continue to explore their long-term benefits, ensuring that individuals can make informed decisions. Ongoing substantial clinical investigations further confirm that both NR and NMN have significant roles in promoting NAD+ levels.
Why does David Sinclair take NMN instead of NR?
David Sinclair, a prominent researcher in aging, prefers NMN because it is a more direct precursor to NAD+ and may be more efficient at increasing NAD+ levels in mammalian cells compared to Nicotinamide Riboside (NR). His choice reflects ongoing research into the most effective methods for boosting NAD+ in mammalian cells. This research is crucial for understanding how these methods impact NAD+ levels in mammalian cells and overall aging processes.
Should you take NR and NMN together?
Combining NR and NMN may offer synergistic effects for increasing NAD+ levels, but this approach is not widely studied. It’s important to consult with a healthcare provider before combining supplements to ensure safety and efficacy, especially if you have concerns about blood pressure. Monitoring blood pressure is crucial when trying new supplements, as it can affect your overall health. Always discuss potential impacts on blood pressure with your healthcare provider to avoid any adverse effects.
Is NMN converted to NR?
NMN is not directly converted to Nicotinamide Riboside (NR). Instead, NMN is converted into NAD+ through its own pathway, which is influenced by various clinical parameters, including the role of the nicotinamide mononucleotide transporter. NR is another precursor to NAD+, but NMN and NR operate through different metabolic routes, with each pathway potentially influenced by the nicotinamide mononucleotide transporter and other distinct clinical parameters. Understanding the role of the nicotinamide mononucleotide transporter is key to differentiating the metabolic effects of NMN and NR.
What is NMN powder used for?
NMN powder is used as a supplement to increase NAD+ levels in the body, which can support energy production, metabolism, and overall cellular health. By influencing altered NAD+ metabolism, it’s often taken to potentially improve physical performance, support aging processes, and enhance recovery. The potential benefits of NMN powder are linked to its role in managing altered NAD+ metabolism, which can positively impact various aspects of health and well-being.
What does NMN do to the body?
NMN boosts NAD+ levels, which supports cellular energy production, metabolism, DNA repair, and overall health. Cell and animal studies have shown that this can lead to improved physical performance, enhanced recovery from exercise, and potentially slow some aspects of aging. Furthermore, ongoing cell and animal studies continue to explore how NMN’s effects might be further optimized. The promising results from these cell and animal studies highlight NMN’s potential benefits for overall well-being and longevity.
Should NMN be taken sublingually?
Sublingual administration of NMN, a form of nad precursors, may improve absorption compared to oral ingestion, as it allows the supplement to enter the bloodstream directly through the tissues under the tongue. However, the best method of absorption for nad precursors can vary based on individual factors, as highlighted in animal studies. While animal studies suggest that sublingual administration of nad precursors might be more effective, individual variations can still influence the outcome. Therefore, while animal studies provide useful insights, personal response to different methods of NMN absorption may differ.
What is the best way to absorb NMN?
The best way to absorb NMN may depend on the formulation. Sublingual forms of NMN might offer better absorption compared to oral tablets or capsules. It’s also important to choose high-quality supplements from reputable sources, especially when considering the effects on skeletal muscle aging. Addressing skeletal muscle aging through effective NMN absorption could significantly benefit overall health. Additionally, selecting the right NMN supplement is crucial for managing skeletal muscle aging effectively.
What are the side effects of NMN sublingual?
Side effects of sublingual NMN are generally similar to those of oral forms and may include mild gastrointestinal discomfort or irritation. As with any supplement, monitoring for adverse effects and consulting a healthcare provider is advised. It’s important to note that NMN, a type of nad precursors, plays a role in cell growth and maintaining healthy cell function. Monitoring the impact on cell growth, as well as any other side effects, can help ensure the supplement, along with other nad precursors, is used safely. Additionally, understanding how nad precursors affect your overall health can be beneficial in making informed decisions about their use.
Which form of NMN is best for absorption?
Sublingual NMN, a type of NAD precursor, is often considered to have better absorption compared to oral forms because it bypasses the digestive system and enters the bloodstream directly. However, individual responses to NAD precursors can vary, and the best form may depend on personal preference and efficacy. Choosing the right NAD precursor can be crucial for optimizing results.
Does NMN help with bodybuilding?
NMN may help with bodybuilding by improving cellular energy production, reducing fatigue, and enhancing recovery. Increased NAD+ levels can support better performance, muscle repair, and muscle insulin sensitivity, although results can vary and more research is needed. Additionally, improving muscle insulin sensitivity through NMN could contribute to overall fitness and well-being.
Does NMN increase testosterone?
There is limited evidence to suggest that NAD precursors, like NMN, directly increase testosterone levels. NAD precursors primarily boost NAD+ levels, which supports overall cellular health and metabolism. For specific effects on testosterone, other supplements or interventions might be more relevant.
Reference
Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004 May 14;117(4):495-502. doi: 10.1016/s0092-8674(04)00416-7. PMID: 15137942.
Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans
NAD+ is essential for life in all organisms, both as a coenzyme for oxidoreductases and as a source of ADPribosyl groups used in various reactions, including those that retard aging in experimental systems. Nicotinic acid and nicotinamide were defined as the vitamin precursors of NAD+ in Elvehjem’s classic discoveries of the 1930s. The accepted view of eukaryotic NAD+ biosynthesis, that all anabolism flows through nicotinic acid mononucleotide, was challenged experimentally and revealed that nicotinamide riboside is an unanticipated NAD+ precursor in yeast. Nicotinamide riboside kinases from yeast and humans essential for this pathway were identified and found to be highly specific for phosphorylation of nicotinamide riboside and the cancer drug tiazofurin. Nicotinamide riboside was discovered as a nutrient in milk, suggesting that nicotinamide riboside is a useful compound for elevation of NAD+ levels in humans.
You can read the full article at https://www.cell.com/cell/fulltext/S0092-8674(04)00416-7?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867404004167%3Fshowall%3Dtrue.
Yang, N. C., Cho, Y. H., & Lee, I. (2019). The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations. International journal of molecular sciences, 21(1), 142. https://doi.org/10.3390/ijms21010142.
The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations
Calorie restriction prolongs lifespan by boosting intracellular nicotinamide adenine dinucleotide (NAD+) levels, enhancing sirtuin activity. While nicotinic acid (NA) can elevate NAD+, its calorie restriction mimetic (CRM) potential remains uncertain. This study investigated NA’s effect on human Hs68 cells and C. elegans lifespan, revealing its ability to increase NAD+ levels in both but extend lifespan only in C. elegans. Notably, NA’s efficacy depended on intracellular NAD+ levels being below the sirtuin-saturating concentration, suggesting that its CRM potential is limited to individuals with lower NAD+ levels.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6982340/.
Hashimoto, T., Horikawa, M., Nomura, T., & Sakamoto, K. (2010). Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology, 11(1), 31–43. https://doi.org/10.1007/s10522-009-9225-3.
Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16
The role of sir2 (sirtuin), an NAD-dependent deacetylase, in lifespan extension through caloric restriction is well-established, yet its activation mechanism remains unclear. In nematodes, caloric restriction-induced lifespan extension relies on the sir2 ortholog sir-2.1 but occurs independently of the transcription factor DAF-16. Investigating the link between NAD and DAF-16, we found that supplementing Caenorhabditis elegans medium with NAD extended lifespan in a sir-2.1-dependent manner, but this effect was abolished in daf-16-RNAi nematodes, suggesting NAD-dependent longevity requires daf-16. NAD activation of daf-16 was also evident through sod-3 expression and enhanced oxidative-stress resistance and adiposity, indicating a distinct signaling pathway from caloric restriction-mediated lifespan extension, potentially involving parts of the insulin-like signaling pathway.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s10522-009-9225-3.
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell metabolism, 27(3), 529–547. https://doi.org/10.1016/j.cmet.2018.02.011.
Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence
Nicotinamide adenine dinucleotide (NAD), essential for redox reactions, has emerged as a crucial signaling molecule, regulating numerous processes from energy metabolism to cell survival. Its levels fluctuate with factors like diet, exercise, and circadian rhythms, declining with age, leading to metabolic changes and heightened disease risk. Restoring NAD+ levels in aging or diseased individuals holds potential for improving health and extending lifespan. Thus, there is ongoing research to identify safe and effective NAD-boosting compounds to enhance the body’s resilience.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6342515/.
Yaku, K., Okabe, K., & Nakagawa, T. (2018). NAD metabolism: Implications in aging and longevity. Ageing research reviews, 47, 1–17. https://doi.org/10.1016/j.arr.2018.05.006.
NAD metabolism: Implications in aging and longevity
Nicotinamide adenine dinucleotide (NAD) serves as a crucial co-factor in a myriad of physiological functions, encompassing metabolism, protein modification, and DNA repair. Maintaining a delicate balance between NAD synthesis and breakdown is vital for regulating its levels in living organisms. Recent studies have highlighted age-related declines in NAD levels, correlating with the onset of aging-related ailments like metabolic disorders, neurodegenerative conditions, and cancer. Conversely, interventions that bolster NAD metabolism, such as dietary supplementation with NAD precursors, have demonstrated protective effects against aging and associated diseases, even extending lifespan in various organisms. This review underscores the pivotal role of NAD metabolism in aging and longevity, summarizing the functions of key enzymes involved in NAD synthesis and degradation, alongside discussing the challenges and prospects in this burgeoning research domain.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1568163718300060?via%3Dihub.
Palacios, J. A., Herranz, D., De Bonis, M. L., Velasco, S., Serrano, M., & Blasco, M. A. (2010). SIRT1 contributes to telomere maintenance and augments global homologous recombination. The Journal of cell biology, 191(7), 1299–1313. https://doi.org/10.1083/jcb.201005160.
SIRT1 contributes to telomere maintenance and augments global homologous recombination
The yeast Sir2 deacetylase, a key player in the silent information regulator (SIR) complex, regulates telomere length and subtelomeric DNA through histone deacetylation. While the functions of its mammalian orthologue, SIRT1, at telomeres are less understood, our study utilizing SIRT1-deficient and SIRT1(super) mouse models reveals that SIRT1 positively regulates telomere length in vivo and mitigates age-related telomere shortening, dependent on telomerase activity. Chromatin immunoprecipitation assays demonstrate SIRT1’s interaction with telomeric repeats in vivo, while SIRT1 overexpression enhances genome-wide homologous recombination, impacting telomeres, centromeres, and chromosome arms. These findings elucidate SIRT1’s role in telomere biology and DNA repair, shedding light on its protective mechanisms against DNA damage and age-related conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3010065/.
Wang, Y., Oxer, D., & Hekimi, S. (2015). Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nature communications, 6, 6393. https://doi.org/10.1038/ncomms7393.
Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis
In this study, a mouse model with controllable interruption and restoration of ubiquinone (UQ) biosynthesis sheds light on its roles in mitochondrial function, oxidative stress, and lifespan. While global UQ loss leads to mitochondrial dysfunction, disease development, and shortened lifespan, UQ’s in vivo antioxidant role is questioned, and its necessity for electron transport is found to be lower than expected, even in crucial mitochondria-rich tissues. Surprisingly, severely compromised mitochondrial function in the heart due to UQ depletion does not immediately impair organ function. Remarkably, partial restoration of UQ levels and mitochondrial function reverses severe disease phenotypes and extends lifespan, suggesting that irreversible aging-associated degeneration may not solely stem from gradual mitochondrial dysfunction observed in aging.
You can read the abstract of the article at https://www.nature.com/articles/ncomms7393.
Lanza, I. R., & Nair, K. S. (2010). Mitochondrial function as a determinant of life span. Pflugers Archiv: European journal of physiology, 459(2), 277–289. https://doi.org/10.1007/s00424-009-0724-5.
Mitochondrial function as a determinant of life span
Human life expectancy has steadily risen due to advancements in nutrition, vaccination, disease treatment, and prevention, yet maximal lifespan remains largely unchanged. While caloric restriction extends lifespan in various species by improving mitochondrial function, its practical application in humans is challenging. Physical activity shows promise for enhancing healthy life expectancy but has uncertain effects on maximal lifespan. In Caenorhabditis elegans, longevity correlates with activity levels, possibly through mitochondrial maintenance. In humans, age-related declines in muscle mitochondrial function are linked to decreased physical ability, but long-term aerobic exercise can mitigate these declines. Despite this, the impact of exercise on maximal lifespan remains unclear, suggesting mitochondrial health may be a key factor in regulating lifespan.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2801852/.
Amano, H., Chaudhury, A., Rodriguez-Aguayo, C., Lu, L., Akhanov, V., Catic, A., Popov, Y. V., Verdin, E., Johnson, H., Stossi, F., Sinclair, D. A., Nakamaru-Ogiso, E., Lopez-Berestein, G., Chang, J. T., Neilson, J. R., Meeker, A., Finegold, M., Baur, J. A., & Sahin, E. (2019). Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell metabolism, 29(6), 1274–1290.e9. https://doi.org/10.1016/j.cmet.2019.03.001.
Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease
Telomere shortening, linked to stem cell decline and premature aging, triggers a p53-mediated repression of all seven sirtuins in the livers of telomerase-deficient mice. P53 regulates non-mitochondrial sirtuins post-transcriptionally via microRNAs, while mitochondrial sirtuins are transcriptionally controlled by peroxisome proliferator-activated receptor gamma co-activator 1 alpha-/beta. Administration of the NAD(+) precursor nicotinamide mononucleotide preserves telomere length, reduces DNA damage response and p53 activity, enhances mitochondrial function, and alleviates liver fibrosis, partially through Sirt1. These findings suggest that targeting sirtuins, particularly Sirt1, may counteract telomere-related disorders and preserve telomere stability.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6657508/.
Sims, C. A., Guan, Y., Mukherjee, S., Singh, K., Botolin, P., Davila, A., Jr, & Baur, J. A. (2018). Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI insight, 3(17), e120182. https://doi.org/10.1172/jci.insight.120182.
Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock
Hemorrhagic shock induces nicotinamide adenine dinucleotide (NAD) depletion and metabolic disruptions that persist despite blood volume restoration. We proposed that nicotinamide mononucleotide (NMN), a precursor to NAD, could bolster cellular energetics and resilience during shock. In a rodent hemorrhagic shock model, NMN administration reduced lactic acidosis and serum IL-6 levels, indicative of improved survival. NMN elevated NAD levels, preserved mitochondrial function in liver and kidney tissues, and protected hepatocytes from cytokine-induced damage. Notably, NMN enhanced shock tolerance by 25% and significantly improved post-resuscitation survival, highlighting its potential as a therapeutic adjunct for managing hemorrhagic shock.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6171817/.
Fang, E. F., Kassahun, H., Croteau, D. L., Scheibye-Knudsen, M., Marosi, K., Lu, H., Shamanna, R. A., Kalyanasundaram, S., Bollineni, R. C., Wilson, M. A., Iser, W. B., Wollman, B. N., Morevati, M., Li, J., Kerr, J. S., Lu, Q., Waltz, T. B., Tian, J., Sinclair, D. A., Mattson, M. P., … Bohr, V. A. (2016). NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell metabolism, 24(4), 566–581. https://doi.org/10.1016/j.cmet.2016.09.004.
NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair
Ataxia telangiectasia (A-T) is a rare genetic disorder marked by progressive neurodegeneration and cerebellar ataxia, primarily linked to ATM gene defects. The exact mechanism behind cerebellar atrophy in A-T remains elusive. This study highlights increased PARylation, reduced NAD+, and mitochondrial dysfunction in ATM-deficient neurons, mice, and worms. Intriguingly, interventions boosting intracellular NAD+ levels ameliorate A-T neuropathology, normalize neuromuscular function, and extend lifespan across animal models. Moreover, these treatments enhance neuronal DNA repair, improve mitochondrial quality via mitophagy, and bridge aging theories involving DNA damage and mitochondrial dysfunction, offering promising therapeutic avenues for A-T and potentially other age-related conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5777858/.
Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693.
NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice
In this study, the role of nicotinamide adenine dinucleotide (NAD(+)) in modulating muscle stem cell (MuSC) senescence is elucidated, highlighting its significance in tissue maintenance and regeneration. Treatment with nicotinamide riboside (NR), a precursor of NAD(+), induces mitochondrial activity and rejuvenates MuSCs in aged mice by triggering the mitochondrial unfolded protein response and prohibitin protein synthesis. Moreover, NR prevents MuSC senescence in a mouse model of muscular dystrophy and delays senescence in neural and melanocyte stem cells while increasing mouse lifespan. These findings suggest that strategies preserving cellular NAD(+) levels hold potential to reprogram dysfunctional stem cells and extend mammalian lifespan.
You can read the full article at https://www.science.org/doi/10.1126/science.aaf2693?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Sun, N., Youle, R. J., & Finkel, T. (2016). The Mitochondrial Basis of Aging. Molecular cell, 61(5), 654–666. https://doi.org/10.1016/j.molcel.2016.01.028.
The Mitochondrial Basis of Aging
This review examines the role of mitochondrial dysfunction in the aging process and its association with age-related diseases. It discusses how declining mitochondrial function contributes to cellular senescence, chronic inflammation, and the reduction in stem cell activity during aging. The review also explores signaling pathways like the mitochondrial unfolded protein response and mitophagy, highlighting their potential roles in regulating longevity. Overall, the evidence suggests that enhancing mitochondrial quality and function could have broad beneficial effects on aging-related processes and diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4779179/.
Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., White, J. P., Teodoro, J. S., Wrann, C. D., Hubbard, B. P., Mercken, E. M., Palmeira, C. M., de Cabo, R., Rolo, A. P., Turner, N., Bell, E. L., & Sinclair, D. A. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037.
Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging
Since the incorporation of mitochondria into eukaryotic cells, coordination between nuclear and mitochondrial genomes has been crucial for oxidative phosphorylation (OXPHOS) system function. Mitochondrial dysfunction is a hallmark of aging, with the loss of mitochondrial-encoded OXPHOS subunits being a specific feature. This decline is attributed to an alternate pathway of nuclear-mitochondrial communication induced by decreased nuclear NAD(+) and HIF-1α accumulation, reminiscent of Warburg reprogramming. SIRT1 deletion accelerates this process, while increasing NAD(+) levels in old mice restores mitochondrial function to that of younger mice in a SIRT1-dependent manner, indicating a reversible aspect to age-related mitochondrial decline.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4076149/.
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., … Sinclair, D. A. (2018). Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 173(1), 74–89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging
Understanding the mechanisms underlying age-related decline in capillary density and blood flow is crucial for enhancing human health and longevity. NAD precursors have shown promise in reversing aspects of aging by activating sirtuin deacylases, particularly SIRT1-SIRT7, which mediate the benefits of exercise and dietary restriction. Research indicates that SIRT1 in endothelial cells plays a pivotal role in mediating pro-angiogenic signals from myocytes. Treatment with nicotinamide mononucleotide (NMN), an NAD+ booster, enhances blood flow and endurance in elderly mice by promoting SIRT1-dependent increases in capillary density, an effect further potentiated by exercise or hydrogen sulfide (H2S) supplementation, a dietary restriction mimetic. These findings offer insights into improving organ and tissue perfusion, enhancing human performance, and restoring mobility in the aging population.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5884172/.
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., Redpath, P., Migaud, M. E., Apte, R. S., Uchida, K., Yoshino, J., & Imai, S. I. (2016). Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell metabolism, 24(6), 795–806. https://doi.org/10.1016/j.cmet.2016.09.013.
Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice
As NAD+ availability diminishes with age and in certain disease states, nicotinamide mononucleotide (NMN), a critical NAD+ precursor, emerges as a promising intervention. Administered orally over a 12-month period to aging wild-type C57BL/6N mice, NMN effectively boosts NAD+ synthesis in tissues and significantly mitigates age-related physiological decline. The study reveals that NMN administration suppresses age-associated weight gain, enhances energy metabolism, improves insulin sensitivity, lipid profile, and physical activity, and ameliorates eye function and other age-related pathologies without observable toxicity. Furthermore, NMN prevents age-induced gene expression changes in metabolic organs, enhances mitochondrial oxidative metabolism, and mitigates mitonuclear protein imbalance in skeletal muscle, showcasing its potential as a preventive and therapeutic strategy against aging-related ailments in humans.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5668137/.
Irie, J., Inagaki, E., Fujita, M., Nakaya, H., Mitsuishi, M., Yamaguchi, S., Yamashita, K., Shigaki, S., Ono, T., Yukioka, H., Okano, H., Nabeshima, Y. I., Imai, S. I., Yasui, M., Tsubota, K., & Itoh, H. (2020). Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocrine journal, 67(2), 153–160. https://doi.org/10.1507/endocrj.EJ19-0313.
Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men
Recent investigations highlight the role of declining cellular nicotinamide adenine dinucleotide (NAD+) levels in aging-related disorders, with therapeutic approaches aimed at boosting NAD+ showing promise in animal models. To assess the safety of nicotinamide mononucleotide (NMN) in humans, we conducted a clinical trial involving 10 healthy men. Single oral administrations of 100, 250, and 500 mg NMN were administered, with clinical parameters, pharmacokinetics of NMN metabolites, ophthalmic examination, and sleep quality assessed. Results revealed no significant clinical symptoms or changes in vital signs, except for minor alterations within normal ranges in laboratory analyses, and no differences in ophthalmic or sleep quality assessments before and after intervention. Importantly, NMN was effectively metabolized without causing adverse effects, suggesting its feasibility as a potential therapeutic avenue to combat aging-related disorders in humans.
You can read the full article at https://www.jstage.jst.go.jp/article/endocrj/67/2/67_EJ19-0313/_article.
Okabe, K., Yaku, K., Uchida, Y., Fukamizu, Y., Sato, T., Sakurai, T., Tobe, K., & Nakagawa, T. (2022). Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Frontiers in nutrition, 9, 868640. https://doi.org/10.3389/fnut.2022.868640.
Nicotinamide mononucleotide (NMN), an orally bioavailable precursor to NAD+, has shown promising effects against aging and aging-related diseases in animal models by boosting NAD+ levels, a vital redox cofactor regulating numerous metabolic enzymes and biological processes. While previous mouse models demonstrated NMN’s efficacy in mitigating various age-related conditions, evidence of its effects in humans remains limited. In a placebo-controlled, randomized, double-blind trial involving thirty healthy subjects, oral NMN supplementation at 250 mg/day for 12 weeks exhibited no adverse effects and significantly increased NAD+ levels in whole blood. The rise in NAD+ levels correlated with pulse rate, suggesting NMN as a safe and effective strategy to enhance NAD+ levels in humans.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9036060/.
Fang, T., Yang, J., Liu, L., Xiao, H., & Wei, X. (2021). Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells. MedComm, 2(2), 279–287. https://doi.org/10.1002/mco2.62.
Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells
Investigating the mechanisms underlying alveolar epithelial cell (ACE) senescence is crucial for understanding respiratory system functionality and defense decline with age. NAD+ depletion during aging contributes to this process. Supplementation with NAD+ intermediates such as nicotinamide mononucleotide (NMN) activates sirtuin deacylases, reduces intracellular oxidative stress, and enhances mitochondrial function, thereby reversing cell senescence. Our study demonstrated that NMN effectively attenuates age-related physiological decline in lung function in mice and mitigates bleomycin-induced pulmonary fibrosis. Moreover, NMN treatment of primary ACEs in vitro markedly alleviates cellular senescence. These findings hold significant promise for enhancing respiratory function and reducing the burden of respiratory diseases in the elderly.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8491199/.
Kiss, T., Nyúl-Tóth, Á., Balasubramanian, P., Tarantini, S., Ahire, C., Yabluchanskiy, A., Csipo, T., Farkas, E., Wren, J. D., Garman, L., Csiszar, A., & Ungvari, Z. (2020). Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. GeroScience, 42(2), 527–546. https://doi.org/10.1007/s11357-020-00165-5.
Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects
Age-related changes in the neurovascular unit contribute significantly to vascular cognitive impairment (VCI), characterized by impaired neurovascular coupling, dysregulated cerebral blood flow, and increased neuroinflammation. Decreased NAD+ availability is implicated in age-related neurovascular dysfunction. Our recent research shows that replenishing cellular NAD+ levels in aged mice using nicotinamide mononucleotide (NMN) rescues neurovascular function, enhances cerebral blood flow, and boosts cognitive performance. Transcriptome analysis reveals that NMN treatment restores the expression of 204 genes in the aged neurovascular unit towards youthful levels, primarily mediated by SIRT1 activation. Pathway analysis suggests that NMN exerts neurovascular protection through mitochondrial rejuvenation and modulation of anti-inflammatory and anti-apoptotic pathways. These findings underscore the potential of NMN in mitigating age-related neurovascular decline and improving cerebral blood flow by rejuvenating mitochondrial function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7206476/.
Niu, K. M., Bao, T., Gao, L., Ru, M., Li, Y., Jiang, L., Ye, C., Wang, S., & Wu, X. (2021). The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase. Frontiers in nutrition, 8, 756243. https://doi.org/10.3389/fnut.2021.756243.
The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase
Aging brings about changes in the gut microbiota and associated metabolomes, prompting interest in beta-nicotinamide mononucleotide (NMN) to slow down this process. We examined the effects of short-term oral NMN administration on the fecal microbiota and metabolomes of pre-aging male mice and assessed telomere length in peripheral blood mononuclear cells (PBMC) of both mice and human volunteers. NMN administration did not affect body weight or feed intake significantly in mice, while metabolomics revealed alterations in serum metabolites, with 34 potential biomarkers identified. NMN reduced fecal bacterial diversity, favoring certain bacteria associated with nicotinamide metabolism. This reshaped microbiota correlated with improved immune function and metabolism. Importantly, NMN supplementation led to significant telomere elongation in both mice and humans, suggesting its potential in retarding aging. Further research is needed to elucidate the underlying mechanisms and validate NMN’s effects through comprehensive clinical trials.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8667784/.
Nadeeshani, H., Li, J., Ying, T., Zhang, B., & Lu, J. (2021). Nicotinamide mononucleotide (NMN) as an anti-aging health product – Promises and safety concerns. Journal of advanced research, 37, 267–278. https://doi.org/10.1016/j.jare.2021.08.003.
Nicotinamide mononucleotide (NMN) as an anti-aging health product – Promises and safety concerns
With the global elderly population steadily increasing, there’s a growing demand for anti-aging health products aimed at ensuring longevity and addressing age-related complications. Nicotinamide mononucleotide (NMN) has emerged as a notable contender in this arena, garnering attention from both consumers and the scientific community. This review aims to provide an overview of NMN’s promises and safety concerns as an anti-aging health product. Declining levels of nicotinamide adenine dinucleotide (NAD+) with aging are associated with various adverse effects including reduced energy production, oxidative stress, DNA damage, cognitive decline, and inflammation. NMN, as a precursor to NAD+, holds promise in counteracting these effects by boosting NAD+ levels. While several in vivo studies have shown positive therapeutic effects of NMN supplementation on age-induced complications, only a limited number of safety assessments, including one preclinical and one clinical study, have been conducted. With numerous NMN-based anti-aging products flooding the market, rigorous clinical investigations are urgently required to ascertain both the effectiveness and safety of NMN supplementation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9039735/.
Soma, M., & Lalam, S. K. (2022). The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Molecular biology reports, 49(10), 9737–9748. https://doi.org/10.1007/s11033-022-07459-1.
The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions
Recent attention has been drawn to the biosynthesis and regulation of nicotinamide adenine dinucleotide (NAD+), with a systemic decline in NAD+ levels associated with aging across multiple tissues. NAD+ influences various cellular processes crucial for homeostasis, including metabolic pathways, DNA repair, and immune cell activity. Age-related declines in NAD+ levels have been linked to several age-related disorders, but restoring NAD+ levels has shown promise in delaying or reversing these conditions. Studies in mice and humans have explored targeting NAD+ metabolism with NAD+ intermediates, particularly nicotinamide mononucleotide (NMN), which demonstrates therapeutic potential in age-related chronic conditions like diabetes, cardiovascular issues, and cognitive impairment. Further human interventions are needed to understand the long-term effects of NMN supplementation, emphasizing the importance of NAD+ in human aging and survival, its biosynthesis from precursors, key clinical trial findings, and NMN’s role in various health conditions.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s11033-022-07459-1.
Brito, S., Baek, J. M., Cha, B., Heo, H., Lee, S. H., Lei, L., Jung, S. Y., Lee, S. M., Lee, S. H., Kwak, B. M., Chae, S., Lee, M. G., & Bin, B. H. (2022). Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. Journal of dermatological science, 106(3), 159–169. https://doi.org/10.1016/j.jdermsci.2022.05.002.
Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling
The study investigated the effects of nicotinamide mononucleotide (NMN) on melanogenesis. NMN treatment significantly reduced melanin production in aged melanocytes, with no apparent effects on young melanocytes. Genome-wide analysis revealed downregulation of melanogenesis-related signaling pathways in aged melanocytes treated with NMN. Additionally, NMN downregulated expression of melanogenesis-related proteins and reduced cAMP/Wnt signaling. The study suggests that NMN could be a safe and effective anti-melanogenic agent for aging-related hyperpigmentation therapy.
You can read the abstract of the article at https://www.jdsjournal.com/article/S0923-1811(22)00122-0/abstract.
Oblong J. E. (2014). The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA repair, 23, 59–63. https://doi.org/10.1016/j.dnarep.2014.04.005.
The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging
Daily exposure of human skin to environmental factors, particularly solar radiation, triggers molecular responses leading to cellular dysfunction and accelerating skin aging. Increased oxidative stress disrupts cellular bioenergetics, evidenced by depleted NAD+ and ATP levels, both acutely and chronically, due to mitochondrial damage. Nicotinamide, a precursor to NAD+, has been implicated in maintaining skin homeostasis, treating inflammatory conditions, and preventing photoaging and cancer. Nicotinamide treatment is thought to restore NAD+ levels, thus mitigating cellular bioenergetics dysfunction by protecting against oxidative stress and enhancing mitochondrial efficiency through sirtuin-dependent mitophagy. Recent research indicates dynamic regulation of NAD+ pools, influencing cellular metabolism, mitochondrial efficiency, and circadian rhythmicity. Understanding these processes is crucial for developing interventions to preserve skin homeostasis and efficient cellular bioenergetics amidst UV-induced oxidative stress.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S1568786414001165?via%3Dihub.
Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693.
NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice
We highlight the critical role of cellular nicotinamide adenine dinucleotide (NAD(+)) levels and their impact on mitochondrial activity in modulating adult stem cell (SC) senescence. Treatment with the NAD(+) precursor nicotinamide riboside (NR) rejuvenates aged muscle SCs (MuSCs) in mice by inducing the mitochondrial unfolded protein response and synthesis of prohibitin proteins. NR also prevents MuSC senescence in a mouse model of muscular dystrophy, delays senescence of neural SCs and melanocyte SCs, and increases mouse lifespan. Strategies aimed at preserving cellular NAD(+) levels may offer a means to reprogram dysfunctional SCs and enhance lifespan in mammals.
You can read the full article at https://www.science.org/doi/10.1126/science.aaf2693?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Long, A. N., Owens, K., Schlappal, A. E., Kristian, T., Fishman, P. S., & Schuh, R. A. (2015). Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC neurology, 15, 19. https://doi.org/10.1186/s12883-015-0272-x.
Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model
Mitochondrial dysfunction is a prominent feature of neurodegenerative disorders like Alzheimer’s disease (AD), characterized by impaired electron transport chain and ATP production due to NAD depletion. Administering nicotinamide mononucleotide (NMN), an NAD precursor, to AD-Tg mice restored mitochondrial respiratory function and altered levels of NAD-dependent substrates (SIRT1 and CD38) and fission/fusion proteins (DRP1, OPA1, and MFN2). This study provides novel insights into the potential therapeutic role of NMN in preserving mitochondrial function and ameliorating NAD catabolism in neurodegenerative diseases like AD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358858/.
Yao, Z., Yang, W., Gao, Z., & Jia, P. (2017). Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neuroscience letters, 647, 133–140. https://doi.org/10.1016/j.neulet.2017.03.027.
Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease
Nicotinamide mononucleotide (NMN), a precursor of NAD+, has shown potential therapeutic effects in Alzheimer’s disease (AD) by reducing Aβ toxicity. In a study on APPswe/PS1dE9 (AD-Tg) mice, NMN improved cognitive impairments, decreased β-amyloid production and plaque burden, preserved synaptic integrity, and reduced inflammation. Mechanistically, NMN inhibited JNK activation and modulated amyloid precursor protein processing, suggesting its multifaceted role in mitigating AD pathology.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S030439401730246X?via%3Dihub.
Yu, M., Zheng, X., Cheng, F., Shao, B., Zhuge, Q., & Jin, K. (2022). Metformin, Rapamycin, or Nicotinamide Mononucleotide Pretreatment Attenuate Cognitive Impairment After Cerebral Hypoperfusion by Inhibiting Microglial Phagocytosis. Frontiers in neurology, 13, 903565. https://doi.org/10.3389/fneur.2022.903565.
Metformin, Rapamycin, or Nicotinamide Mononucleotide Pretreatment Attenuate Cognitive Impairment After Cerebral Hypoperfusion by Inhibiting Microglial Phagocytosis
Vascular cognitive impairment (VCI) presents a significant challenge in elderly populations, lacking specific therapies. Investigating the potential of anti-aging drugs like metformin, rapamycin, and nicotinamide mononucleotide (NMN) in a rat model of VCI induced by bilateral common carotid artery occlusion (BCCAO), we found that pretreatment with these drugs significantly improved cognitive function and white matter integrity, while modulating microglial response and phagocytosis. These findings suggest a promising avenue for VCI treatment through the manipulation of microglial polarization and inhibition of phagocytosis by these anti-aging drugs.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9234123/.
Hosseini, L., Farokhi-Sisakht, F., Badalzadeh, R., Khabbaz, A., Mahmoudi, J., & Sadigh-Eteghad, S. (2019). Nicotinamide Mononucleotide and Melatonin Alleviate Aging-induced Cognitive Impairment via Modulation of Mitochondrial Function and Apoptosis in the Prefrontal Cortex and Hippocampus. Neuroscience, 423, 29–37. https://doi.org/10.1016/j.neuroscience.2019.09.037.
Nicotinamide Mononucleotide and Melatonin Alleviate Aging-induced Cognitive Impairment via Modulation of Mitochondrial Function and Apoptosis in the Prefrontal Cortex and Hippocampus
This study investigates the effects of melatonin and nicotinamide mononucleotide (NMN) on cognitive function, mitochondrial health, and apoptosis in aged rats. Administered separately or together, both compounds improved memory, reduced mitochondrial dysfunction, and decreased apoptosis in the prefrontal cortex and hippocampus. Combined treatment showed the most significant neuroprotective effects, suggesting enhanced cognitive benefits and mitochondrial support.
You can read the abstract of the article at https://www.ibroneuroscience.org/article/S0306-4522(19)30682-7/abstract.
Liu, X., Dilxat, T., Shi, Q., Qiu, T., & Lin, J. (2022). The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene, 822, 146348. https://doi.org/10.1016/j.gene.2022.146348.
The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling
This study evaluates the anti-aging effects of combining nicotinamide mononucleotide (NMN) and lycopene (Lyco) in aging rats and senescent PC12 cells. The combination improved spatial learning and memory, reduced oxidative stress, and mitigated cellular senescence more effectively than either compound alone. NMN + Lyco also down-regulated senescence-related genes and activated the Keap1-Nrf2 signaling pathway, suggesting enhanced protection against aging and cognitive decline.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0378111922001676?via%3Dihub.
Wang, X., Hu, X., Zhang, L., Xu, X., & Sakurai, T. (2020). Nicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in rats. Brain research bulletin, 160, 98–106. https://doi.org/10.1016/j.brainresbull.2020.04.022.
Nicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in ratsNicotinamide mononucleotide administration after sever hypoglycemia improves neuronal survival and cognitive function in rats
This study investigates the potential of nicotinamide mononucleotide (NMN) to mitigate brain injury and cognitive impairment following severe hypoglycemia in a rat model. NMN treatment significantly reduced neuronal death, restored hippocampal synaptic plasticity, and improved spatial learning and memory. Mechanistically, NMN decreased reactive oxygen species, suppressed PARP-1 activation, and restored NAD+ and ATP levels in the hippocampus. These results suggest NMN may be a promising therapy for preventing brain injury related to severe hypoglycemia.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0361923020301453?via%3Dihub.
Chandrasekaran, K., Choi, J., Arvas, M. I., Salimian, M., Singh, S., Xu, S., Gullapalli, R. P., Kristian, T., & Russell, J. W. (2020). Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons. International journal of molecular sciences, 21(11), 3756. https://doi.org/10.3390/ijms21113756.
Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons
This study examines the effects of nicotinamide mononucleotide (NMN) on diabetes-induced memory deficits in rats. NMN administration restored decreased brain NAD+ levels, normalized hippocampal metabolites, preserved neuronal volume, and improved memory despite no significant impact on glucose or lipid levels. NMN treatment also activated the SIRT1 pathway, prevented mitochondrial dysfunction, and mitigated cognitive decline associated with diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7313029/.
Kiss, T., Balasubramanian, P., Valcarcel-Ares, M. N., Tarantini, S., Yabluchanskiy, A., Csipo, T., Lipecz, A., Reglodi, D., Zhang, X. A., Bari, F., Farkas, E., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. GeroScience, 41(5), 619–630. https://doi.org/10.1007/s11357-019-00074-2.
Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment
This study explores how nicotinamide mononucleotide (NMN), an NAD+ precursor, affects age-related impairments in endothelial angiogenesis. NMN treatment improved angiogenic processes and reduced oxidative stress in aged cerebromicrovascular endothelial cells (CMVECs), reversing age-related deficiencies. The beneficial effects of NMN were linked to SIRT1 activation, suggesting that NAD+ depletion and SIRT1 dysregulation contribute to impaired angiogenesis and vascular cognitive impairment in aging. The findings support the potential use of NAD+ boosters to enhance vascular health in aging.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6885080/.
Wang, X., Hu, X., Yang, Y., Takata, T., & Sakurai, T. (2016). Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain research, 1643, 1–9. https://doi.org/10.1016/j.brainres.2016.04.060.
Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death
This study evaluates the effects of nicotinamide mononucleotide (NMN) on Alzheimer’s disease (AD) by addressing amyloid-β (Aβ) oligomer-induced neuronal death and cognitive decline. NMN treatment improved cognitive function, reduced neuronal cell death, and restored NAD+ and ATP levels in AD models. It also mitigated oxidative stress and preserved synaptic function. These findings suggest NMN may offer a promising therapeutic approach for AD by enhancing brain energy metabolism and protecting neurons.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0006899316303018?via%3Dihub.
Tarantini, S., Valcarcel-Ares, M. N., Toth, P., Yabluchanskiy, A., Tucsek, Z., Kiss, T., Hertelendy, P., Kinter, M., Ballabh, P., Süle, Z., Farkas, E., Baur, J. A., Sinclair, D. A., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox biology, 24, 101192. https://doi.org/10.1016/j.redox.2019.101192.
Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice
This study investigates the role of nicotinamide mononucleotide (NMN) in restoring neurovascular coupling (NVC) responses in aging mice, addressing impaired cerebral blood flow and cognitive decline. NMN supplementation improved NVC responses, enhanced endothelial vasodilation, and boosted spatial memory and gait coordination. These effects were linked to improved mitochondrial function and reduced oxidative stress. The findings suggest NMN may be a promising intervention for age-related neurovascular dysfunction and cognitive impairment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6477631/.
Stein, L. R., & Imai, S. (2014). Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO journal, 33(12), 1321–1340. https://doi.org/10.1002/embj.201386917.
Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging
This study reveals that aging reduces NAD+ levels and Nampt, an enzyme crucial for NAD+ production, in hippocampal neural stem/progenitor cells (NSPCs). Nampt depletion leads to decreased NSPC proliferation and reduced oligodendrogenesis, which can be partially reversed by boosting NAD+ levels. The findings suggest that Nampt-mediated NAD+ biosynthesis is essential for maintaining NSPC function and may contribute to age-related declines in neural regeneration.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4194122/.
Zhao, Y., Guan, Y. F., Zhou, X. M., Li, G. Q., Li, Z. Y., Zhou, C. C., Wang, P., & Miao, C. Y. (2015). Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke, 46(7), 1966–1974. https://doi.org/10.1161/STROKEAHA.115.009216.
Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade
This study investigates the role of the Nampt-NAD cascade in brain regeneration following ischemic stroke. Nampt transgenic mice exhibited enhanced neural stem cell activation, improved recovery, and better survival compared to wild-type mice, while enzymatic-dead Nampt mice did not show these benefits. Nicotinamide mononucleotide (NMN) and NAD+ promoted neural stem cell proliferation and differentiation, with effects mediated through various sirtuins. These findings suggest that targeting the Nampt-NAD pathway could be a promising approach for improving long-term recovery after stroke.
You can read the full article at https://www.ahajournals.org/doi/10.1161/STROKEAHA.115.009216?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Johnson, S., Wozniak, D. F., & Imai, S. (2018). CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ aging and mechanisms of disease, 4, 10. https://doi.org/10.1038/s41514-018-0029-z.
CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves
This study explores how aging affects cognitive function, specifically focusing on NAD+ levels in the hippocampus. While old mice showed minimal spatial learning/memory decline, they exhibited increased sensitivity to aversive stimuli. Nicotinamide mononucleotide (NMN) supplementation improved this hypersensitivity. The research identified reduced expression of Cask in the hippocampus, linked to NAD+ reduction and cognitive changes, and suggested that boosting NAD+ with NMN could mitigate age-related cognitive dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224504/.
Lu, L., Tang, L., Wei, W., Hong, Y., Chen, H., Ying, W., & Chen, S. (2014). Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Experimental and therapeutic medicine, 8(3), 943–950. https://doi.org/10.3892/etm.2014.1842.
Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease
This study investigated nicotinamide mononucleotide (NMN) as a potential treatment for Parkinson’s disease (PD) by using a rotenone-treated PC12 cell model. NMN significantly improved cell survival, reduced apoptosis, and restored NAD+ and ATP levels, suggesting it alleviates energy metabolism impairments and cell death associated with PD. These findings indicate that NMN could be a promising therapeutic option for PD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4113526/.
Hsu, C. P., Zhai, P., Yamamoto, T., Maejima, Y., Matsushima, S., Hariharan, N., Shao, D., Takagi, H., Oka, S., & Sadoshima, J. (2010). Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation, 122(21), 2170–2182. https://doi.org/10.1161/CIRCULATIONAHA.110.958033.
Silent information regulator 1 protects the heart from ischemia/reperfusion
Sirt1, a histone deacetylase, protects the heart from myocardial ischemia/reperfusion (I/R) injury by upregulating antioxidant proteins and downregulating proapoptotic factors. In mice with cardiac-specific Sirt1 overexpression, myocardial infarction size and cell death were significantly reduced, and functional recovery was improved compared to controls. Sirt1 enhances the transcriptional activity of FoxO1, which helps mitigate oxidative stress and supports cardiac cell survival.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3003297/.
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972. https://doi.org/10.1371/journal.pone.0098972.
Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion
Nicotinamide phosphoribosyltransferase (Nampt) and Sirt1 play crucial roles in protecting the heart against ischemia/reperfusion (I/R) injury. Ischemic preconditioning (IPC) enhances Nampt levels, and this protection is diminished in Nampt-deficient mice. Nicotinamide mononucleotide (NMN), a Nampt product, mimics IPC’s protective effects by increasing NAD+ and activating Sirt1, reducing heart injury during I/R. Caloric restriction (CR) also upregulates Nampt and protects the heart through the Nampt-Sirt1 pathway. Thus, both NMN and CR provide cardiac protection by activating Sirt1 and mimicking IPC effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4048236/.
Nadtochiy, S. M., Wang, Y. T., Nehrke, K., Munger, J., & Brookes, P. S. (2018). Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH. Journal of molecular and cellular cardiology, 121, 155–162. https://doi.org/10.1016/j.yjmcc.2018.06.007.
Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH
Nicotinamide mononucleotide (NMN) protects the heart from ischemia-reperfusion (IR) injury by stimulating glycolysis, which enhances ATP production and acidosis during reperfusion. Although NMN’s cardioprotective effects are independent of SIRT1, they are diminished in Sirt3-deficient hearts, likely due to higher baseline injury. NMN induces glycolysis in cardiomyocytes, evidenced by increased lactate and acidification, and its benefits are negated without glucose or with non-glycolytic fuels. NMN also provides protection when administered at reperfusion, partly overlapping with mechanisms of acidosis-induced protection.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6103815/.
Park, J. H., Long, A., Owens, K., & Kristian, T. (2016). Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiology of disease, 95, 102–110. https://doi.org/10.1016/j.nbd.2016.07.018.
Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia
Nicotinamide mononucleotide (NMN) protects against ischemic brain damage by boosting NAD+ levels and preventing poly-ADP-ribosylation (PAR) and NAD+ depletion. In a mouse model of transient forebrain ischemia, NMN treatment improved neurological outcomes and reduced CA1 neuronal injury, without affecting reperfusion conditions. These results indicate NMN’s strong neuroprotective potential through enhancement of NAD+ biosynthesis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5580241/.
Martin, A. S., Abraham, D. M., Hershberger, K. A., Bhatt, D. P., Mao, L., Cui, H., Liu, J., Liu, X., Muehlbauer, M. J., Grimsrud, P. A., Locasale, J. W., Payne, R. M., & Hirschey, M. D. (2017). Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI insight, 2(14), e93885. https://doi.org/10.1172/jci.insight.93885.
Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model
Supplementing with nicotinamide mononucleotide (NMN) significantly improves cardiac function in Friedreich’s ataxia mice by restoring near-normal levels of cardiac performance. This effect is dependent on the activation of the mitochondrial NAD+-dependent protein deacetylase SIRT3, as NMN’s benefits are lost in mice lacking SIRT3. The study highlights NMN’s potential therapeutic role and suggests further exploration of NMN or SIRT3 activators for treating cardiac dysfunction in Friedreich’s ataxia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5518566/.
Klimova, N., Fearnow, A., Long, A., & Kristian, T. (2020). NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Experimental neurology, 325, 113144. https://doi.org/10.1016/j.expneurol.2019.113144.
NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms
Global cerebral ischemia depletes brain NAD+, causing mitochondrial dysfunction and cell death. Administering nicotinamide mononucleotide (NMN) effectively replenishes NAD+ levels, protects against ischemic brain damage, and prevents mitochondrial fragmentation. This protection involves normalization of mitochondrial NAD+, reduction of protein acetylation and ROS levels, and inhibition of mitochondrial fission, all dependent on SIRT3 activity. NMN’s ability to restore these mitochondrial functions highlights its potential as a therapeutic approach for acute brain injuries and neurodegenerative diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8328278/.
Zhang, R., Shen, Y., Zhou, L., Sangwung, P., Fujioka, H., Zhang, L., & Liao, X. (2017). Short-term administration of Nicotinamide Mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure. Journal of molecular and cellular cardiology, 112, 64–73. https://doi.org/10.1016/j.yjmcc.2017.09.001.
Short-term administration of Nicotinamide Mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure
Heart failure is linked to mitochondrial dysfunction, and reduced NAD+ levels and NAD+-mediated deacetylase activity are implicated. In a cardiac-specific KLF4-deficient mouse model, which shows mitochondrial protein hyperacetylation and reduced Sirt3 and NAD+ levels, short-term NMN administration protected against pressure overload-induced heart failure. NMN preserved mitochondrial structure, reduced ROS, and improved fatty acid oxidation in cardiomyocytes, highlighting its potential as a therapeutic agent for cardiac diseases linked to mitochondrial dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6257991/.
Abdellatif, M., Sedej, S., & Kroemer, G. (2021). NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation, 144(22), 1795–1817. https://doi.org/10.1161/CIRCULATIONAHA.121.056589.
NAD+ Metabolism in Cardiac Health, Aging, and Disease
NAD+ is crucial for energy, redox balance, DNA repair, and protein deacetylation, and its levels decline with aging and cardiovascular risk factors. Enhancing NAD+ through pharmacological inhibition of NAD+ degrading enzymes, supplementation with NAD+ precursors, or transgenic overexpression has shown promise in improving metabolic health and treating cardiovascular diseases in preclinical models. This review discusses the potential of NAD+ replenishment for treating heart failure and other cardiac conditions, emphasizing the need for further research and optimized clinical trials to explore the benefits of NAD+ elevation in patients.
You can read the full article at https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.056589?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Abdellatif, M., Trummer-Herbst, V., Koser, F., Durand, S., Adão, R., Vasques-Nóvoa, F., Freundt, J. K., Voglhuber, J., Pricolo, M. R., Kasa, M., Türk, C., Aprahamian, F., Herrero-Galán, E., Hofer, S. J., Pendl, T., Rech, L., Kargl, J., Anto-Michel, N., Ljubojevic-Holzer, S., Schipke, J., … Sedej, S. (2021). Nicotinamide for the treatment of heart failure with preserved ejection fraction. Science translational medicine, 13(580), eabd7064. https://doi.org/10.1126/scitranslmed.abd7064.
Nicotinamide for the treatment of heart failure with preserved ejection fraction
Diastolic dysfunction in heart failure with preserved ejection fraction (HFpEF) is linked to NAD+ deficits. Oral nicotinamide supplementation improved diastolic dysfunction in animal models by enhancing myocardial bioenergetics and alleviating systemic comorbidities. Nicotinamide also improved cardiomyocyte function by increasing protein deacetylation. Long-term human studies found that high intake of NAD+ precursors was associated with lower blood pressure and reduced cardiac mortality, suggesting that NAD+ precursors could be effective in treating HFpEF.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7611499/.
Wan, Y., He, B., Zhu, D., Wang, L., Huang, R., Zhu, J., Wang, C., & Gao, F. (2021). Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Archives of biochemistry and biophysics, 712, 109050. https://doi.org/10.1016/j.abb.2021.109050.
Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats
Nicotinamide mononucleotide (NMN) protects against doxorubicin (DOX)-induced cardiotoxicity in rats by mitigating cardiac dysfunction and injury. NMN reduces inflammation by inhibiting NLRP3 inflammasome activation, enhances antioxidant defenses by increasing glutathione and superoxide dismutase levels, and decreases oxidative stress markers. Additionally, NMN prevents cardiomyocyte apoptosis and cardiac fibrosis, suggesting it could be a valuable strategy for mitigating DOX-related cardiac damage.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S000398612100299X?via%3Dihub.
Jafari-Azad, A., Hosseini, L., Rajabi, M., Høilund-Carlsen, P. F., Vafaee, M. S., Feyzizadeh, S., & Badalzadeh, R. (2021). Nicotinamide mononucleotide and melatonin counteract myocardial ischemia-reperfusion injury by activating SIRT3/FOXO1 and reducing apoptosis in aged male rats. Molecular biology reports, 48(4), 3089–3096. https://doi.org/10.1007/s11033-021-06351-8.
Nicotinamide mononucleotide and melatonin counteract myocardial ischemia-reperfusion injury by activating SIRT3/FOXO1 and reducing apoptosis in aged male rats
Combining nicotinamide mononucleotide (NMN) and melatonin effectively protects the aged heart from ischemia/reperfusion (IR) injury by reducing infarct size and apoptosis. Both NMN and melatonin individually upregulate anti-apoptotic markers and SIRT3, while downregulating pro-apoptotic genes. The combination therapy enhances these protective effects more than either treatment alone, suggesting a synergistic benefit through activation of the SIRT3/FOXO1 pathway.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s11033-021-06351-8.
Wu, K., Li, B., Lin, Q., Xu, W., Zuo, W., Li, J., Liu, N., Tu, T., Zhang, B., Xiao, Y., & Liu, Q. (2021). Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation. Life sciences, 274, 119299. https://doi.org/10.1016/j.lfs.2021.119299.
Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation
Nicotinamide mononucleotide (NMN) alleviates cardiac fibrosis and dysfunction induced by isoproterenol in mice and inhibits fibroblast activation stimulated by TGF-β in vitro. NMN’s protective effects are linked to restoring the NAD+/SIRT1 axis, reducing oxidative stress, and decreasing Smad3 acetylation, although these effects are partially reversed by the SIRT1 inhibitor sirtinol.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0024320521002848?via%3Dihub.
Yamamoto, T., Byun, J., Zhai, P., Ikeda, Y., Oka, S., & Sadoshima, J. (2014). Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PloS one, 9(6), e98972. https://doi.org/10.1371/journal.pone.0098972.
Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion
Nicotinamide phosphoribosyltransferase (Nampt) and Sirt1 protect the heart against ischemia/reperfusion (I/R) injury, with ischemic preconditioning (IPC) and caloric restriction (CR) enhancing Nampt activity and Sirt1 activation. Nicotinamide mononucleotide (NMN) mimics IPC effects by increasing NAD+ levels and activating Sirt1, which reduces heart injury from I/R. NMN’s cardioprotective effects are diminished in Sirt1 knockout mice, indicating the importance of the Nampt-Sirt1 pathway in these protective mechanisms.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4048236/.
Kiss, T., Giles, C. B., Tarantini, S., Yabluchanskiy, A., Balasubramanian, P., Gautam, T., Csipo, T., Nyúl-Tóth, Á., Lipecz, A., Szabo, C., Farkas, E., Wren, J. D., Csiszar, A., & Ungvari, Z. (2019). Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. GeroScience, 41(4), 419–439. https://doi.org/10.1007/s11357-019-00095-x.
Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects
Vascular aging is linked to NAD+ depletion, and restoring NAD+ levels with nicotinamide mononucleotide (NMN) in aged mice improves vascular function and reduces oxidative stress. NMN treatment also restores a youthful miRNA expression profile in the aorta, suggesting that NAD+ boosts might counteract age-related vascular changes by modulating miRNA expression, with potential benefits for preventing and treating age-related vascular diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6815288/.
Whitson, J. A., Bitto, A., Zhang, H., Sweetwyne, M. T., Coig, R., Bhayana, S., Shankland, E. G., Wang, L., Bammler, T. K., Mills, K. F., Imai, S. I., Conley, K. E., Marcinek, D. J., & Rabinovitch, P. S. (2020). SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging cell, 19(10), e13213. https://doi.org/10.1111/acel.13213.
SS-31 and NMN: Two paths to improve metabolism and function in aged hearts
In old mouse hearts, the mitochondrial-targeted drugs SS-31 and NMN each improved specific aspects of cardiac function: SS-31 partially restored diastolic function, while NMN fully reversed systolic dysfunction. Combined treatment with both drugs led to enhanced NAD+ turnover and greater steady-state NAD(H) levels, normalized energy dynamics under higher workloads, and achieved the most significant rejuvenation of cardiac function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7576234/.
Wu, L. E., Gomes, A. P., & Sinclair, D. A. (2014). Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer cell, 25(1), 12–19. https://doi.org/10.1016/j.ccr.2013.12.005.
Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis
Cancer risk increases with age due to the gradual decline in oxidative metabolism, which may serve as an early driver of tumorigenesis. This decline affects sirtuins, NAD+-dependent deacylases crucial for managing metabolic responses, suggesting that targeting sirtuins could help counteract age-related metabolic changes and reduce cancer risk.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3970212/.
Lee MK, Cheong HS, Koh Y, Ahn KS, Yoon SS, Shin HD. Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population. Genet Test Mol Biomarkers. 2016;20:696–701.
Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population
The study explored the role of PARP15 gene polymorphisms in acute myeloid leukemia (AML) risk and chemotherapy outcomes. Analysis of 344 individuals revealed that certain PARP15 polymorphisms were linked to improved overall survival in AML patients. This suggests PARP15 may be a potential therapeutic target, though further research with larger sample sizes is needed for validation.
You can read the abstract of the article at https://www.liebertpub.com/doi/10.1089/gtmb.2016.0007?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed.
Dollerup O.L., Christensen B., Svart M., Schmidt M.S., Sulek K., Ringgaard S., Stødkilde-Jørgensen H., Møller N., Brenner C., Treebak J.T., Jessen N. A randomized placebo-controlled clinical trial of nicotinamideriboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018;108:343–353.
A randomized placebo-controlled clinical trial of nicotinamideriboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects
A 12-week clinical trial of nicotinamide riboside (NR) supplementation in obese, insulin-resistant men found that while NR was safe and did not cause serious adverse effects, it did not improve insulin sensitivity, glucose metabolism, or body composition. The study involved 40 participants receiving NR or a placebo, and results showed no significant benefits in metabolic parameters despite the safety of the supplement.A 12-week clinical trial of nicotinamide riboside (NR) supplementation in obese, insulin-resistant men found that while NR was safe and did not cause serious adverse effects, it did not improve insulin sensitivity, glucose metabolism, or body composition. The study involved 40 participants receiving NR or a placebo, and results showed no significant benefits in metabolic parameters despite the safety of the supplement.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0002916522029409?via%3Dihub.
Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., Chonchol M., Seals D.R. Chronic nicotinamideriboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018;9:1286.
Chronic nicotinamideriboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults
A 12-week clinical trial showed that chronic supplementation with the NAD+ precursor nicotinamide riboside (NR) is well tolerated and enhances NAD+ metabolism in healthy middle-aged and older adults. The study suggests that NR may improve physiological functions, including blood pressure and arterial stiffness, warranting further investigation into its potential cardiovascular benefits.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5876407/.
Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD Metabolism in Cancer Therapeutics. Front Oncol. 2018;8:622. Published 2018 Dec 12. doi:10.3389/fonc.2018.00622.
NAD Metabolism in Cancer Therapeutics
Cancer cells preferentially use anaerobic glycolysis, known as the Warburg effect, to support rapid growth, a process fueled by increased levels of nicotinamide adenine dinucleotide (NAD). NAD, which enhances glycolysis and supports various metabolic pathways, is often elevated in cancer cells due to increased nicotinamide phosphoribosyltransferase (Nampt) activity. Inhibitors targeting Nampt deplete NAD, disrupt cancer cell metabolism, and suppress proliferation. NAD also influences cancer progression through its role in DNA repair and gene regulation, making NAD metabolism a promising target for cancer therapies.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6315198/.
Available from https://www.biorxiv.org/content/10.1101/2020.03.21.001123v1.
Potentiating the anti-tumor response of tumor infiltrated T cells by NAD+ supplementation
Low NAD+ levels impair tumor-infiltrating T lymphocytes (TILs), but NAD+ supplementation can significantly boost the effectiveness of CAR-T cell therapies and improve survival in cancer models by enhancing T cell activation and mitochondrial energy production.
You can read the abstract of the article at https://www.biorxiv.org/content/10.1101/2020.03.21.001123v1.
Fania, L., Mazzanti, C., Campione, E., Candi, E., Abeni, D., & Dellambra, E. (2019). Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. International journal of molecular sciences, 20(23), 5946. https://doi.org/10.3390/ijms20235946.
Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention
Nicotinamide (NAM), a precursor of NAD+, is crucial for cellular energy and DNA repair. NAD+ deficiency can lead to UV sensitivity, genomic instability, and increased cancer risk. NAM enhances cellular energy, improves DNA repair, and reduces skin cancer incidence and UV-induced immunosuppression, making it a promising strategy for skin cancer prevention and managing skin aging.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6929077/.
Kusumanchi, P., Zhang, Y., Jani, M. B., Jayaram, N. H., Khan, R. A., Tang, Y., Antony, A. C., & Jayaram, H. N. (2013). Nicotinamide mononucleotide adenylyltransferase2 overexpression enhances colorectal cancer cell-kill by Tiazofurin. Cancer gene therapy, 20(7), 403–412. https://doi.org/10.1038/cgt.2013.33.
Nicotinamide mononucleotide adenylyltransferase2 overexpression enhances colorectal cancer cell-kill by Tiazofurin
Colorectal cancer cells resistant to Tiazofurin, a drug that inhibits guanylate synthesis, can be made sensitive by overexpressing NMNAT2, which enhances the drug’s effectiveness. Additionally, Tiazofurin encapsulated in folate-tethered nanoparticles significantly improves its cytotoxicity against these cancer cells by targeting folate receptors, reducing the required dose and potentially minimizing toxicity.
You can read the abstract of the article at https://www.nature.com/articles/cgt201333.
Yoshino, M., Yoshino, J., Kayser, B. D., Patti, G. J., Franczyk, M. P., Mills, K. F., Sindelar, M., Pietka, T., Patterson, B. W., Imai, S. I., & Klein, S. (2021). Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science (New York, N.Y.), 372(6547), 1224–1229. https://doi.org/10.1126/science.abe9985.
Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women
A 10-week trial in postmenopausal women with prediabetes found that nicotinamide mononucleotide (NMN) supplementation improved insulin sensitivity and muscle insulin signaling, while also promoting muscle remodeling. These effects were significant compared to a placebo, highlighting NMN’s potential to enhance metabolic function in overweight or obese individuals.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8550608/.
Camacho-Pereira, J., Tarragó, M. G., Chini, C., Nin, V., Escande, C., Warner, G. M., Puranik, A. S., Schoon, R. A., Reid, J. M., Galina, A., & Chini, E. N. (2016). CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell metabolism, 23(6), 1127–1139. https://doi.org/10.1016/j.cmet.2016.05.006.
CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism
Aging increases the activity of NADase CD38, leading to reduced NAD+ levels and mitochondrial dysfunction, partly through SIRT3 regulation. CD38 also degrades the NAD+ precursor nicotinamide mononucleotide (NMN), highlighting its role in NAD+ depletion and its impact on NAD+ replacement therapies for aging and metabolic diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4911708/.
Escande, C., Nin, V., Price, N. L., Capellini, V., Gomes, A. P., Barbosa, M. T., O’Neil, L., White, T. A., Sinclair, D. A., & Chini, E. N. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes, 62(4), 1084–1093. https://doi.org/10.2337/db12-1139.
Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome
CD38, a key NAD+ase, regulates protein acetylation and sirtuin activity, impacting metabolic syndrome. Inhibiting CD38 with compounds like quercetin and apigenin increases NAD+ levels and reduces global protein acetylation, improving glucose and lipid homeostasis in obese mice. This suggests CD38 inhibition could be an effective strategy for treating metabolic diseases by enhancing NAD+-dependent pathways.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609577/.
Yoshino, J., Mills, K. F., Yoon, M. J., & Imai, S. (2011). Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell metabolism, 14(4), 528–536. https://doi.org/10.1016/j.cmet.2011.08.014.
Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice
High-fat diets and aging compromise NAD+ biosynthesis via NAMPT, leading to glucose intolerance and metabolic dysfunction. Nicotinamide mononucleotide (NMN), an NAD+ precursor, counteracts these effects by restoring NAD+ levels, enhancing insulin sensitivity, and improving glucose and lipid profiles in T2D mice, partly through SIRT1 activation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3204926/.
Choi, S. E., Fu, T., Seok, S., Kim, D. H., Yu, E., Lee, K. W., Kang, Y., Li, X., Kemper, B., & Kemper, J. K. (2013). Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging cell, 12(6), 1062–1072. https://doi.org/10.1111/acel.12135.
Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT
In obesity and aging, SIRT1 activity, which is crucial for preventing metabolic disorders, is reduced due to decreased NAD+ levels. Elevated hepatic microRNA-34a (miR-34a) targets and decreases NAMPT, the enzyme responsible for NAD+ biosynthesis, leading to reduced NAD+ and SIRT1 activity. This reduction in NAD+ and SIRT1 results in obesity-like symptoms and increased acetylation of SIRT1 target proteins. miR-34a antagonism can restore NAD+ levels and alleviate obesity-related issues, highlighting the miR-34a/NAMPT axis as a potential therapeutic target for treating obesity and aging-related diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3838500/.
Caton, P. W., Kieswich, J., Yaqoob, M. M., Holness, M. J., & Sugden, M. C. (2011). Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia, 54(12), 3083–3092. https://doi.org/10.1007/s00125-011-2288-0.
Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function
Chronic fructose feeding impairs islet function and lowers circulating extracellular NAMPT (eNAMPT) levels, leading to increased inflammation and reduced insulin secretion. Nicotinamide mononucleotide (NMN) administration improves insulin secretion and reduces inflammation in islets, counteracting the effects of pro-inflammatory cytokines. This improvement is partially mediated through sirtuin 1. Thus, NMN shows potential in addressing islet dysfunction linked to inflammation and reduced eNAMPT.
You can read the full article at https://link.springer.com/article/10.1007/s00125-011-2288-0.
Bordone, L., Motta, M. C., Picard, F., Robinson, A., Jhala, U. S., Apfeld, J., McDonagh, T., Lemieux, M., McBurney, M., Szilvasi, A., Easlon, E. J., Lin, S. J., & Guarente, L. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS biology, 4(2), e31. https://doi.org/10.1371/journal.pbio.0040031.
Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells
Sirt1 enhances insulin secretion in pancreatic beta cells by directly repressing the UCP2 gene. Reduced Sirt1 levels lead to elevated UCP2, impaired glucose-stimulated insulin secretion, and decreased ATP production. Knockdown of UCP2 restores insulin secretion in Sirt1-reduced cells, highlighting the critical role of Sirt1 in regulating insulin secretion through UCP2.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1318478/.
Ramsey, K. M., Mills, K. F., Satoh, A., & Imai, S. (2008). Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging cell, 7(1), 78–88. https://doi.org/10.1111/j.1474-9726.2007.00355.x.
Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice
In aged BESTO mice, the positive effects of Sirt1 on glucose-stimulated insulin secretion (GSIS) diminish, despite its initial benefits. This decline is linked to reduced plasma levels of nicotinamide mononucleotide (NMN), crucial for NAD biosynthesis and Sirt1 function. NMN supplementation restores GSIS and glucose tolerance, highlighting the potential of NAD-enhancing therapies to combat age-related metabolic disorders like type 2 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2238677/.
Nahle, A., Joseph, Y. D., Pereira, S., Mori, Y., Poon, F., Ghadieh, H. E., Ivovic, A., Desai, T., Ghanem, S. S., Asalla, S., Muturi, H. T., Jentz, E. M., Joseph, J. W., Najjar, S. M., & Giacca, A. (2021). Nicotinamide Mononucleotide Prevents Free Fatty Acid-Induced Reduction in Glucose Tolerance by Decreasing Insulin Clearance. International journal of molecular sciences, 22(24), 13224. https://doi.org/10.3390/ijms222413224.
Nicotinamide Mononucleotide Prevents Free Fatty Acid-Induced Reduction in Glucose Tolerance by Decreasing Insulin Clearance
NMN infusion improves glucose tolerance in mice with high free fatty acid (FFA) levels by protecting β cell function and reducing insulin clearance. However, NMN may impair glucose tolerance under normal FFA conditions due to decreased β cell function, indicating the need for caution in its therapeutic use.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8709165/.
Sheng, F., Ren, X., Dai, X., Xu, X., Dong, M., Pei, Q., Qu, J., Zhou, Z., Zhou, H., & Liu, Z. (2011). Effect of nicotinamide mononucleotide on insulin secretion and gene expressions of PDX-1 and FoxO1 in RIN-m5f cells. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences, 36(10), 958–963. https://doi.org/10.3969/j.issn.1672-7347.2011.10.005.
Effect of nicotinamide mononucleotide on insulin secretion and gene expressions of PDX-1 and FoxO1 in RIN-m5f cells
NMN enhances insulin secretion and increases the mRNA expression of the transcription factor PDX-1 in RIN-m5f cells, but does not significantly affect the expression of FoxO1 or the protein levels of PDX-1.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/22086006/.
Polo, V., Saibene, A., & Pontiroli, A. E. (1998). Nicotinamide improves insulin secretion and metabolic control in lean type 2 diabetic patients with secondary failure to sulphonylureas. Acta diabetologica, 35(1), 61–64. https://doi.org/10.1007/s005920050103.
Nicotinamide improves insulin secretion and metabolic control in lean type 2 diabetic patients with secondary failure to sulphonylureas
In a 6-month study of type 2 diabetes patients with secondary sulphonylurea failure, nicotinamide supplementation improved C-peptide release compared to placebo, though it did not significantly affect overall glycemic control compared to insulin alone or sulphonylureas.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s005920050103.
Liu, J., Zong, Z., Zhang, W., Chen, Y., Wang, X., Shen, J., Yang, C., Liu, X., & Deng, H. (2021). Nicotinamide Mononucleotide Alleviates LPS-Induced Inflammation and Oxidative Stress via Decreasing COX-2 Expression in Macrophages. Frontiers in molecular biosciences, 8, 702107. https://doi.org/10.3389/fmolb.2021.702107.
Nicotinamide Mononucleotide Alleviates LPS-Induced Inflammation and Oxidative Stress via Decreasing COX-2 Expression in Macrophages
Metabolomic analysis of LPS-activated macrophages revealed decreased NAD+ and increased NADPH levels, suggesting that NAD+ restoration can inhibit macrophage activation. Supplementation with nicotinamide mononucleotide (NMN) increased NAD+ levels, reduced cytokine production, and downregulated COX-2 and prostaglandin E2, effectively inactivating macrophages.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8290259/.
Ru, M., Wang, W., Zhai, Z., Wang, R., Li, Y., Liang, J., Kothari, D., Niu, K., & Wu, X. (2022). Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells. Food & function, 13(14), 7507–7519. https://doi.org/10.1039/d2fo00525e.
Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells
Nicotinamide mononucleotide (NMN) supplementation improves intestinal health during aging by increasing NAD+ content, enhancing jejunal structure, and up-regulating antioxidant and barrier function genes while reducing inflammation. NMN effectively restores damaged intestinal cells and maintains their function, suggesting its potential in mitigating age-related intestinal decline.
You can read the abstract of the article at https://pubs.rsc.org/en/content/articlelanding/2022/fo/d2fo00525e.
Cros, C., Margier, M., Cannelle, H., Charmetant, J., Hulo, N., Laganier, L., Grozio, A., & Canault, M. (2022). Nicotinamide Mononucleotide Administration Triggers Macrophages Reprogramming and Alleviates Inflammation During Sepsis Induced by Experimental Peritonitis. Frontiers in molecular biosciences, 9, 895028. https://doi.org/10.3389/fmolb.2022.895028.
Nicotinamide Mononucleotide Administration Triggers Macrophages Reprogramming and Alleviates Inflammation During Sepsis Induced by Experimental Peritonitis
Administration of β-nicotinamide mononucleotide (β-NMN) during sepsis improved survival and reduced inflammation by shifting macrophages from a pro-inflammatory to an anti-inflammatory phenotype. This was achieved through enhanced NAD+ metabolism, decreased bacterial load, and modulation of immune responses, highlighting β-NMN’s potential as a therapeutic agent in sepsis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9271973/.
Mateuszuk, Ł., Campagna, R., Kutryb-Zając, B., Kuś, K., Słominska, E. M., Smolenski, R. T., & Chlopicki, S. (2020). Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochemical pharmacology, 178, 114019. https://doi.org/10.1016/j.bcp.2020.114019.
Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside
Nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) both reduce endothelial inflammation and improve endothelial function. NMN’s effects are mediated through CD73-dependent pathways, including its extracellular conversion to NR, while NR acts via CD73-independent mechanisms. Both NMN and NR increase intracellular NAD levels and protect against endothelial dysfunction induced by inflammatory and oxidative stress.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S000629522030246X?via%3Dihub.
Miao, Y., Li, X., Shi, X., Gao, Q., Chen, J., Wang, R., Fan, Y., & Xiong, B. (2021). Nicotinamide Mononucleotide Restores the Meiotic Competency of Porcine Oocytes Exposed to Ethylene Glycol Butyl Ether. Frontiers in cell and developmental biology, 9, 628580. https://doi.org/10.3389/fcell.2021.628580.
Nicotinamide Mononucleotide Restores the Meiotic Competency of Porcine Oocytes Exposed to Ethylene Glycol Butyl Ether
Exposure to ethylene glycol butyl ether (EGBE) impairs porcine oocyte maturation by disrupting cytoskeleton dynamics, mitochondrial function, and increasing reactive oxygen species (ROS), leading to DNA damage and apoptosis. Nicotinamide mononucleotide (NMN) supplementation counteracts these effects by restoring NAD+ levels and mitochondrial function, thereby improving oocyte quality and suggesting potential benefits for fertility protection against environmental pollutants.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7884640/.
Bertoldo, M. J., Listijono, D. R., Ho, W. J., Riepsamen, A. H., Goss, D. M., Richani, D., Jin, X. L., Mahbub, S., Campbell, J. M., Habibalahi, A., Loh, W. N., Youngson, N. A., Maniam, J., Wong, A., Selesniemi, K., Bustamante, S., Li, C., Zhao, Y., Marinova, M. B., Kim, L. J., … Wu, L. E. (2020). NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell reports, 30(6), 1670–1681.e7. https://doi.org/10.1016/j.celrep.2020.01.058.
NAD+ Repletion Rescues Female Fertility during Reproductive Aging
Reproductive aging in female mammals reduces oocyte quality due to declining NAD+ levels. Nicotinamide mononucleotide (NMN) treatment rejuvenates oocyte quality and fertility in aged animals, and this effect is similar to the benefits observed with NAD+-dependent deacylase SIRT2 overexpression. NMN also improves developmental outcomes in embryos from older mothers, suggesting that restoring NAD+ levels can help reverse age-related declines in female reproductive function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7063679/.
Campbell, J. M., Mahbub, S. B., Bertoldo, M. J., Habibalahi, A., Goss, D. M., Ledger, W. L., Gilchrist, R. B., Wu, L. E., & Goldys, E. M. (2022). Multispectral autofluorescence characteristics of reproductive aging in old and young mouse oocytes. Biogerontology, 23(2), 237–249. https://doi.org/10.1007/s10522-022-09957-y.
Multispectral autofluorescence characteristics of reproductive aging in old and young mouse oocytes
Aging significantly impairs female fertility by deteriorating oocyte quality. Using label-free, multi-spectral imaging, researchers identified distinct autofluorescence profiles for oocytes from young versus aged animals. Treatment with nicotinamide mononucleotide (NMN) restored oocyte quality in aged animals, as evidenced by an autofluorescence profile similar to young oocytes and improved metabolic profiles. This approach suggests that spectral profiling of oocyte autofluorescence could be a non-invasive method to assess oocyte quality and the effects of interventions like NMN.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9023381/.
Song, M., Li, Y., Zhou, Y., Yan, J., Zhou, X., Gao, Q., Miao, Y., & Xiong, B. (2022). Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress. Journal of animal science and biotechnology, 13(1), 68. https://doi.org/10.1186/s40104-022-00716-0.
Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress
Heat stress negatively impacts porcine oocyte quality by disrupting meiotic organelles and increasing oxidative stress and DNA damage. Supplementation with nicotinamide mononucleotide (NMN) during heat stress effectively restores oocyte maturation, improving both nuclear and cytoplasmic functions. This suggests NMN is a viable strategy to mitigate heat stress-induced defects in oocytes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9202089/.
Miao, Y., Cui, Z., Zhu, X., Gao, Q., & Xiong, B. (2022). Supplementation of nicotinamide mononucleotide improves the quality of postovulatory aged porcine oocytes. Journal of molecular cell biology, 14(4), mjac025. https://doi.org/10.1093/jmcb/mjac025.
Supplementation of nicotinamide mononucleotide improves the quality of postovulatory aged porcine oocytes
Postovulatory aging impairs oocyte quality, potentially leading to early pregnancy failure and ART challenges. Supplementation with nicotinamide mononucleotide (NMN), a NAD+ precursor, effectively improves the quality of aged porcine oocytes by reducing fragmentation and maintaining morphological integrity, suggesting NMN as a promising approach to counteract postovulatory aging effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9345308/.
Yang, L., Lin, X., Tang, H., Fan, Y., Zeng, S., Jia, L., Li, Y., Shi, Y., He, S., Wang, H., Hu, Z., Gong, X., Liang, X., Yang, Y., & Liu, X. (2020). Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox. Aging cell, 19(9), e13206. https://doi.org/10.1111/acel.13206.
Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox
Aging leads to an accumulation of mitochondrial DNA (mtDNA) mutations, which adversely affect female fertility by impairing oocyte quality and reducing ovarian follicle numbers. Analysis of young and older women revealed that older oocytes had more mtDNA mutations and lower blastocyst formation rates. Using POLG mutator mice, it was found that mtDNA mutations decreased fertility, primarily through disruptions in the NADH/NAD+ redox state, a problem that nicotinamide mononucleotide (NMN) treatment could alleviate. This study identifies a causal link between mtDNA mutations and reduced fertility, highlighting a metabolic mechanism that could be targeted for therapeutic interventions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7511885/.
Ma, D., Hu, L., Wang, J., Luo, M., Liang, A., Lei, X., Liao, B., Li, M., Xie, M., Li, H., Gong, Y., Zi, D., Li, X., Chen, X., & Liao, X. (2022). Nicotinamide mononucleotide improves spermatogenic function in streptozotocin-induced diabetic mice via modulating the glycolysis pathway. Acta biochimica et biophysica Sinica, 10.3724/abbs.2022099. Advance online publication. https://doi.org/10.3724/abbs.2022099.
Nicotinamide mononucleotide improves spermatogenic function in streptozotocin-induced diabetic mice via modulating the glycolysis pathway
This study investigates the effects of nicotinamide mononucleotide (NMN) on spermatogenic dysfunction in streptozotocin-induced diabetic mice. NMN treatment improved body and testis weights, increased sperm count, and reduced sperm malformations. It also enhanced seminiferous tubule morphology and spermatogenic cell numbers. Mechanistically, NMN decreased apoptosis by upregulating anti-apoptotic factors and glycolysis-related enzymes. These findings suggest that NMN protects against diabetes-induced spermatogenic dysfunction, providing a potential therapeutic strategy for this condition.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9828322/.
Bai, S., & Sheline, C. T. (2013). NAD(+) maintenance attenuates light induced photoreceptor degeneration. Experimental eye research, 108, 76–83. https://doi.org/10.1016/j.exer.2012.12.007.
NAD(+) maintenance attenuates light induced photoreceptor degeneration
This study explores the role of NAD+ in protecting against light-induced retinal damage (LD). Increasing NAD+ levels through nicotinamide treatment, altering feeding schedules, or using transgenic mice that overexpress NAD+ synthesizing enzyme cytNMNAT1 reduced retinal damage, Zn2+ accumulation, and cell death after LD. These findings suggest that maintaining or restoring NAD+ levels can mitigate oxidative damage and cell loss in photoreceptors and retinal pigment epithelium cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578030/.
Lin, J. B., Kubota, S., Ban, N., Yoshida, M., Santeford, A., Sene, A., Nakamura, R., Zapata, N., Kubota, M., Tsubota, K., Yoshino, J., Imai, S. I., & Apte, R. S. (2016). NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell reports, 17(1), 69–85. https://doi.org/10.1016/j.celrep.2016.08.073.
NAD(+) Biosynthesis Is Essential for Vision In Mice
The retina, essential for vision, contains rods and cones that enable night vision and color perception, respectively. These photoreceptors, being non-proliferative, lead to blindness when they die. Researchers at Washington University School of Medicine found that replenishing the molecule nicotinamide adenine dinucleotide (NAD+) can prevent photoreceptor degeneration and restore vision in mice.
You can read the full article at https://www.nmn.com/news/nad-essential-for-vision-mice.
Mimura, T., Kaji, Y., Noma, H., Funatsu, H., & Okamoto, S. (2013). The role of SIRT1 in ocular aging. Experimental eye research, 116, 17–26. https://doi.org/10.1016/j.exer.2013.07.017.
The role of SIRT1 in ocular aging
Sirtuins, NAD+-dependent histone deacetylases, regulate lifespan and are linked to age-related diseases. Among the seven human sirtuins, SIRT1 is notable for its role in ocular aging, where its upregulation protects against diseases like cataract, retinal degeneration, and uveitis. SIRT1, activated by compounds like resveratrol, is present in various ocular structures and offers protection against oxidative stress-related damage.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014483513002157?via%3Dihub.
Zeng, Y., & Yang, K. (2015). Sirtuin 1 participates in the process of age-related retinal degeneration. Biochemical and biophysical research communications, 468(1-2), 167–172. https://doi.org/10.1016/j.bbrc.2015.10.139.
Sirtuin 1 participates in the process of age-related retinal degeneration
Sirtuins, NAD+-dependent histone deacetylases, regulate lifespan and are linked to age-related diseases. Among the seven human sirtuins, SIRT1 is notable for its role in ocular aging, where its upregulation protects against diseases like cataract, retinal degeneration, and uveitis. SIRT1, activated by compounds like resveratrol, is present in various ocular structures and offers protection against oxidative stress-related damage.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0006291X15308366?via%3Dihub.
Kowluru, R. A., Santos, J. M., & Zhong, Q. (2014). Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Investigative ophthalmology & visual science, 55(9), 5653–5660. https://doi.org/10.1167/iovs.14-14383.
Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy
In diabetic retinopathy, high glucose levels increase oxidative stress, which inhibits Sirtuin 1 (Sirt1) and leads to hyperacetylation of the NF-κB p65 subunit. This enhances the binding of p65 to the MMP-9 promoter, causing retinal mitochondrial damage and apoptosis. Resveratrol, a Sirt1 activator, mitigates these effects by preventing p65 acetylation and MMP-9 activation, offering a potential strategy to protect against diabetic retinopathy.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4160094/.
Zheng, Z., Chen, H., Li, J., Li, T., Zheng, B., Zheng, Y., Jin, H., He, Y., Gu, Q., & Xu, X. (2012). Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes, 61(1), 217–228. https://doi.org/10.2337/db11-0416.
Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin
Cellular metabolic memory in diabetic complications persists even after returning to normal glucose levels, involving elevated NF-κB and Bax expression. This study shows that SIRT1, which is downregulated by hyperglycemia-induced PARP activation, plays a crucial role in this memory effect. Metformin activates SIRT1 and the LKB1/AMPK pathway, reducing oxidative stress and inflammation, thus suppressing the memory of hyperglycemia. This suggests that targeting SIRT1 with metformin could be a promising strategy for managing diabetes-related cellular damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3237662/.
Kubota, S., Kurihara, T., Ebinuma, M., Kubota, M., Yuki, K., Sasaki, M., Noda, K., Ozawa, Y., Oike, Y., Ishida, S., & Tsubota, K. (2010). Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. The American journal of pathology, 177(4), 1725–1731. https://doi.org/10.2353/ajpath.2010.100098.
Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation
Resveratrol, a polyphenolic compound, was shown to protect against light-induced retinal degeneration in mice. Pretreatment with resveratrol reduced retinal damage, including cell apoptosis and outer nuclear layer thinning, and improved retinal function. This protection is linked to reduced activation of activator protein-1 (AP-1) and increased sirtuin 1 (SIRT1) activity, highlighting resveratrol’s potential as a therapeutic agent for retinal damage from light exposure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2947269/.
Chen, J., Michan, S., Juan, A. M., Hurst, C. G., Hatton, C. J., Pei, D. T., Joyal, J. S., Evans, L. P., Cui, Z., Stahl, A., Sapieha, P., Sinclair, D. A., & Smith, L. E. (2013). Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis, 16(4), 985–992. https://doi.org/10.1007/s10456-013-9374-5.
Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy
Sirtuin 1 (Sirt1) plays a crucial role in vascular regeneration in ischemic retinal tissue. In a mouse model of oxygen-induced ischemic retinopathy (OIR), Sirt1 was highly induced in ischemic retinas and its depletion led to reduced vascular regrowth and increased pathologic growth. Sirt1 regulates this process by modulating the stability of hypoxia-induced factors and angiogenic factor expression, suggesting it is vital for proper vascular response and regeneration in ischemic conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4006695/.
Meng, Y. F., Pu, Q., Dai, S. Y., Ma, Q., Li, X., & Zhu, W. (2021). Nicotinamide Mononucleotide Alleviates Hyperosmolarity-Induced IL-17a Secretion and Macrophage Activation in Corneal Epithelial Cells/Macrophage Co-Culture System. Journal of inflammation research, 14, 479–493. https://doi.org/10.2147/JIR.S292764.
Nicotinamide Mononucleotide Alleviates Hyperosmolarity-Induced IL-17a Secretion and Macrophage Activation in Corneal Epithelial Cells/Macrophage Co-Culture System
Nicotinamide mononucleotide (NMN) alleviates hyperosmosis stress (HS)-induced dry eye disease (DED) by enhancing NAD+ levels, improving cell viability, and reducing apoptosis in corneal epithelial cells (CEC). NMN treatment decreases HS-induced inflammation and mitochondrial damage, modulates SIRT1 activity, and lowers IL-17a expression. Additionally, NMN affects macrophage function and polarization through the Notch pathway, suggesting its potential for managing DED by improving cellular interactions and inflammatory responses.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7917392/.
Pu, Q., Guo, X. X., Hu, J. J., Li, A. L., Li, G. G., & Li, X. Y. (2022). Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 147, 112659. https://doi.org/10.1016/j.biopha.2022.112659.
Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway
Nicotinamide mononucleotide (NMN) improves cell viability and function in high-glucose-treated human corneal epithelial cells (HCECs) by reducing cell damage and apoptosis, enhancing cell migration, and restoring tight junctions. These protective effects are mediated through the SIRT1/Nrf2/HO-1 pathway.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0753332222000476?via%3Dihub.
Cimaglia, G., Votruba, M., Morgan, J. E., André, H., & Williams, P. A. (2020). Potential Therapeutic Benefit of NAD+ Supplementation for Glaucoma and Age-Related Macular Degeneration. Nutrients, 12(9), 2871. https://doi.org/10.3390/nu12092871.
Potential Therapeutic Benefit of NAD+ Supplementation for Glaucoma and Age-Related Macular Degeneration
Glaucoma and age-related macular degeneration, major causes of irreversible blindness, are driven by mitochondrial dysfunction, inflammation, and oxidative stress in the retina. Current treatments only manage symptoms, not the underlying pathology. This review highlights the role of decreased nicotinamide adenine dinucleotide (NAD) bioavailability in these diseases and suggests that supplementing NAD could become a potential therapeutic strategy.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7551676/.
Lee, D., Tomita, Y., Miwa, Y., Shinojima, A., Ban, N., Yamaguchi, S., Nishioka, K., Negishi, K., Yoshino, J., & Kurihara, T. (2022). Nicotinamide Mononucleotide Prevents Retinal Dysfunction in a Mouse Model of Retinal Ischemia/Reperfusion Injury. International journal of molecular sciences, 23(19), 11228. https://doi.org/10.3390/ijms231911228.
Nicotinamide Mononucleotide Prevents Retinal Dysfunction in a Mouse Model of Retinal Ischemia/Reperfusion Injury
Retinal ischemia/reperfusion (I/R) injury, which causes vision impairment through oxidative stress and inflammation, currently lacks effective therapies. This study demonstrates that nicotinamide mononucleotide (NMN) can significantly protect against retinal I/R injury by reducing retinal functional damage and inflammation. NMN also activates antioxidant pathways and shows protective effects against oxidative stress-induced cell death, suggesting its potential as a neuroprotective treatment for ischemic retinopathy.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9570481/.
Chen, X., Amorim, J. A., Moustafa, G. A., Lee, J. J., Yu, Z., Ishihara, K., Iesato, Y., Barbisan, P., Ueta, T., Togka, K. A., Lu, L., Sinclair, D. A., & Vavvas, D. G. (2020). Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging, 12(24), 24504–24521. https://doi.org/10.18632/aging.202453.
Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment
Photoreceptor degeneration lacks effective pharmacotherapy, but this study found that nicotinamide mononucleotide (NMN), a precursor to NAD+, significantly protects photoreceptors after retinal detachment. NMN administration reduces cell death, inflammation, oxidative stress, and preserves retinal structure by increasing NAD+ levels and activating the SIRT1/HO-1 signaling pathway. These findings suggest NMN as a potential neuroprotective treatment for photoreceptor degeneration.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7803565/.
Zhao, C., Li, W., Duan, H., Li, Z., Jia, Y., Zhang, S., Wang, X., Zhou, Q., & Shi, W. (2020). NAD+ precursors protect corneal endothelial cells from UVB-induced apoptosis. American journal of physiology. Cell physiology, 318(4), C796–C805. https://doi.org/10.1152/ajpcell.00445.2019.
NAD+ precursors protect corneal endothelial cells from UVB-induced apoptosis
Excessive UVB exposure causes corneal damage by inducing apoptosis in corneal endothelial cells. This study found that NAD+ precursors, including nicotinamide (NIC), nicotinamide mononucleotide (NMN), and NAD+, can protect against UVB-induced corneal endothelial cell apoptosis in both mouse and cell models. The protective effect is linked to the reactivation of AKT signaling, suggesting NAD+ precursors as potential treatments for UVB-induced corneal damage.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpcell.00445.2019?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Li, Y., Ma, X., Li, J., Yang, L., Zhao, X., Qi, X., Zhang, X., Zhou, Q., & Shi, W. (2019). Corneal Denervation Causes Epithelial Apoptosis Through Inhibiting NAD+ Biosynthesis. Investigative ophthalmology & visual science, 60(10), 3538–3546. https://doi.org/10.1167/iovs.19-26909.
Corneal Denervation Causes Epithelial Apoptosis Through Inhibiting NAD+ Biosynthesis
Trigeminal innervation of the corneal epithelium helps maintain its integrity and homeostasis by regulating NAD+ content. Corneal denervation in mice leads to reduced NAD+ levels, increased cell apoptosis, and epithelial defects, likely due to decreased NAMPT expression. Supplementing with NMN or NAD+ partially mitigates these effects, highlighting the importance of nerve-mediated NAD+ biosynthesis in corneal health.
You can read the full article at https://iovs.arvojournals.org/article.aspx?articleid=2748650.
Koetz, K., Bryl, E., Spickschen, K., O’Fallon, W. M., Goronzy, J. J., & Weyand, C. M. (2000). T cell homeostasis in patients with rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America, 97(16), 9203–9208. https://doi.org/10.1073/pnas.97.16.9203.
T cell homeostasis in patients with rheumatoid arthritis
Patients with rheumatoid arthritis (RA) experience a significant reduction in T cell receptor diversity and an accelerated loss of T cell receptor rearrangement excision circles (TREC), indicating impaired T cell production and homeostasis. This is coupled with excessive telomere erosion, suggesting increased self-replication of T cells. The loss of TREC-positive T cells may stem from either a primary defect in T cell homeostasis or impaired thymic function, leading to compensatory turnover of peripheral T cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC16846/.
Fyhrquist, F., Tiitu, A., Saijonmaa, O., Forsblom, C., Groop, P. H., & FinnDiane Study Group (2010). Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. Journal of internal medicine, 267(3), 278–286. https://doi.org/10.1111/j.1365-2796.2009.02139.x.
Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes
Short telomere length in blood leukocytes is an independent predictor of the progression of diabetic nephropathy in patients with type 1 diabetes, regardless of the degree of albuminuria, indicating its potential role in disease progression.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2796.2009.02139.x.
Testa, R., Olivieri, F., Sirolla, C., Spazzafumo, L., Rippo, M. R., Marra, M., Bonfigli, A. R., Ceriello, A., Antonicelli, R., Franceschi, C., Castellucci, C., Testa, I., & Procopio, A. D. (2011). Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association, 28(11), 1388–1394. https://doi.org/10.1111/j.1464-5491.2011.03370.x.
Leukocyte telomere length is associated with complications of type 2 diabetes mellitus
This study suggests that shorter leukocyte telomere length is associated with the presence and increasing number of complications in patients with type 2 diabetes, indicating that telomere attrition could be a marker for diabetic complications.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1464-5491.2011.03370.x.
Takeda, K., & Okumura, K. (2021). Nicotinamide mononucleotide augments the cytotoxic activity of natural killer cells in young and elderly mice. Biomedical research (Tokyo, Japan), 42(5), 173–179. https://doi.org/10.2220/biomedres.42.173.
Nicotinamide mononucleotide augments the cytotoxic activity of natural killer cells in young and elderly mice
Nicotinamide mononucleotide (NMN) enhances natural killer (NK) cell cytotoxic activity in both young and elderly mice, without increasing NK cell numbers, through a mechanism dependent on IFN-γ.
You can read the full article at https://www.jstage.jst.go.jp/article/biomedres/42/5/42_173/_article.
Maiese, K., Chong, Z. Z., Hou, J., & Shang, Y. C. (2009). The vitamin nicotinamide: translating nutrition into clinical care. Molecules (Basel, Switzerland), 14(9), 3446–3485. https://doi.org/10.3390/molecules14093446.
The vitamin nicotinamide: translating nutrition into clinical care
Nicotinamide, a form of vitamin B3, plays a crucial role in cellular energy metabolism and offers cytoprotective effects by modulating oxidative stress and various pathways involved in cell survival and death. It shows potential in treating immune dysfunction, diabetes, and aging-related diseases, but its therapeutic use depends on understanding the complex cellular pathways it influences.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2756609/.
Grahnert, A., Grahnert, A., Klein, C., Schilling, E., Wehrhahn, J., & Hauschildt, S. (2011). Review: NAD +: a modulator of immune functions. Innate immunity, 17(2), 212–233. https://doi.org/10.1177/1753425910361989.
Review: NAD +: a modulator of immune functions
Nicotinamide adenine dinucleotide (NAD+) is crucial for maintaining cell integrity, influencing energy metabolism, calcium homeostasis, gene transcription, DNA repair, and intercellular communication. This review highlights the life cycle of NAD+ in the cell, its metabolism by enzymes like CD38, PARPs, and sirtuins, and its essential role in immunological processes, emphasizing the importance of NAD+ in various cell functions and its potential impact on cell fate.
You can read the abstract of the article at https://journals.sagepub.com/doi/10.1177/1753425910361989?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Omran, H. M., & Almaliki, M. S. (2020). Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: A hypothesis. Journal of infection and public health, 13(9), 1196–1201. https://doi.org/10.1016/j.jiph.2020.06.004.
Influence of NAD+ as an ageing-related immunomodulator on COVID 19 infection: A hypothesis
Aging leads to a decline in biological functions, including heart, lung, and immune system impairments. This includes increased pro-inflammatory cytokines, reduced naive lymphocytes, and telomere shortening, which compromises immune function and increases susceptibility to infections and severe outcomes, especially in elderly COVID-19 patients. Enhancing NAD+ levels could counteract these aging effects by stabilizing telomeres, improving immune function, and reducing inflammation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7275989/.
Hiromatsu, Y., Yang, D., Miyake, I., Koga, M., Kameo, J., Sato, M., Inoue, Y., & Nonaka, K. (1998). Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism, 83(1), 121–124. https://doi.org/10.1210/jcem.83.1.4478.
Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy
Nicotinamide inhibits cytokine-induced activation of orbital fibroblasts in thyroid-associated ophthalmopathy by reducing the expression of HLA-DR and intercellular adhesion molecule 1, while not affecting HLA-A,B,C or CD44 expression. It also suppresses fibroblast proliferation and enhances Fas expression, potentially decreasing autoimmune injury in the orbit.
You can read the full article at https://academic.oup.com/jcem/article/83/1/121/2865087?login=false.
Hiromatsu, Y., Sato, M., Tanaka, K., Ishisaka, N., Kamachi, J., & Nonaka, K. (1993). Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology, 80(2), 330–332.
Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells
Nicotinamide and 3-aminobenzamide inhibit the expression of intercellular adhesion molecule-1 (ICAM-1) on thyroid cells from Graves’ disease patients in response to interferon-gamma and phytohaemagglutinin, independent of free radical scavenging. This suppression of ICAM-1 expression may help reduce autoimmune reactions in the thyroid gland.
You can read the abstract of the article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1422199/.
Silwal P., Shin K., Choi S., Namgung U., Lee C.Y., Heo J.-Y.-Y. Tryptophan negatively regulates IgE-mediated mast cell activation. Korean J Phys Anthropol. 2017;30:53. doi: 10.11637/kjpa.2017.30.2.53.
Tryptophan negatively regulates IgE-mediated mast cell activation
Tryptophan suppresses IgE-mediated allergic responses by inhibiting mast cell activation, including degranulation and the production of inflammatory mediators like LTB4, TNF-α, and IL-4, both in vivo and in vitro. This suggests tryptophan supplementation could be beneficial for managing IgE-mediated allergies.
You can read the full article at https://e-aba.org/DOIx.php?id=10.11637/kjpa.2017.30.2.53.
Imai, S., & Yoshino, J. (2013). The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes, obesity & metabolism, 15 Suppl 3(0 3), 26–33. https://doi.org/10.1111/dom.12171.
The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing
Aging leads to insulin resistance, declining β-cell function, and chronic inflammation, disrupting metabolic balance. The SIRT1-NAMPT pathway, part of the “NAD World,” is crucial for regulating insulin sensitivity and secretion, maintaining metabolic stability. This pathway offers potential targets for nutriceuticals to prevent and treat age-related metabolic issues like type 2 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3819727/.
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell metabolism, 27(3), 529–547. https://doi.org/10.1016/j.cmet.2018.02.011.
Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence
Nicotinamide adenine dinucleotide (NAD+) is crucial for energy metabolism and cell survival, acting as a signaling molecule that regulates various processes. NAD+ levels decline with age, leading to metabolic changes and increased disease risk. Restoring NAD+ levels in aging or diseased animals improves health and longevity, sparking interest in NAD-boosting compounds to enhance overall resilience and extend healthy lifespan.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6342515/.
Kim, M., Seol, J., Sato, T., Fukamizu, Y., Sakurai, T., & Okura, T. (2022). Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients, 14(4), 755. https://doi.org/10.3390/nu14040755.
Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study
This study found that taking nicotinamide mononucleotide (NMN) in the afternoon improved lower limb function and reduced drowsiness in older adults, suggesting NMN’s potential to enhance physical performance and reduce fatigue in this population.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8877443/.
Liao, B., Zhao, Y., Wang, D., Zhang, X., Hao, X., & Hu, M. (2021). Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. Journal of the International Society of Sports Nutrition, 18(1), 54. https://doi.org/10.1186/s12970-021-00442-4.
Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind studyNicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study
This study found that supplementing with nicotinamide mononucleotide (NMN) during exercise training improved the aerobic capacity of recreationally trained runners, likely by enhancing oxygen utilization in skeletal muscle, with greater benefits observed at higher NMN dosages.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8265078/.
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., Kim, L. J., Osborne, B., Joshi, S., Lu, Y., Treviño-Villarreal, J. H., Kang, M. J., Hung, T. T., Lee, B., Williams, E. O., Igarashi, M., Mitchell, J. R., Wu, L. E., Turner, N., Arany, Z., … Sinclair, D. A. (2018). Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell, 173(1), 74–89.e20. https://doi.org/10.1016/j.cell.2018.02.008.
Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging
Aging leads to a decline in capillary density and blood flow, contributing to mortality and morbidity. The study found that NAD+ boosters like nicotinamide mononucleotide (NMN) enhance blood flow and endurance in elderly mice by increasing capillary density through SIRT1 activation in endothelial cells, a process further boosted by exercise or hydrogen sulfide. These findings suggest potential strategies to improve blood flow, performance, and mobility in the elderly.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5884172/.
Crisol, B. M., Veiga, C. B., Braga, R. R., Lenhare, L., Baptista, I. L., Gaspar, R. C., Muñoz, V. R., Cordeiro, A. V., da Silva, A., Cintra, D. E., Moura, L. P., Pauli, J. R., & Ropelle, E. R. (2020). NAD+ precursor increases aerobic performance in mice. European journal of nutrition, 59(6), 2427–2437. https://doi.org/10.1007/s00394-019-02089-z.
NAD+ precursor increases aerobic performance in mice
Nicotinamide riboside (NR) supplementation alone does not improve aerobic performance in mice, but when combined with aerobic training, it enhances performance and increases mitochondrial proteins and type I muscle fibers. This suggests NR could be a promising strategy to boost mitochondrial metabolism and aerobic capacity, particularly in conjunction with exercise.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s00394-019-02089-z.
Dehhaghi, M., Panahi, H., Kavyani, B., Heng, B., Tan, V., Braidy, N., & Guillemin, G. J. (2022). The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging and disease, 13(3), 698–711. https://doi.org/10.14336/AD.2021.0824.
The Role of Kynurenine Pathway and NAD+ Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating illness characterized by neurological and gastrointestinal impairments and persistent malaise, with no current cure or biomarkers. Impaired tryptophan metabolism, particularly through the kynurenine pathway, may play a significant role in the disease by limiting NAD+ availability and exacerbating symptoms. Strategies to increase NAD+ levels, such as supplementation with nicotinamide mononucleotide or nicotinamide riboside, could potentially alleviate fatigue and improve quality of life in ME/CFS patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9116917/.
Kimura, S., Ichikawa, M., Sugawara, S., Katagiri, T., Hirasawa, Y., Ishikawa, T., Matsunaga, W., & Gotoh, A. (2022). Nicotinamide Mononucleotide Is Safely Metabolized and Significantly Reduces Blood Triglyceride Levels in Healthy Individuals. Cureus, 14(9), e28812. https://doi.org/10.7759/cureus.28812.
Nicotinamide Mononucleotide Is Safely Metabolized and Significantly Reduces Blood Triglyceride Levels in Healthy Individuals
Intravenous administration of nicotinamide mononucleotide (NMN) is safe and increases NAD+ levels in humans without affecting vital signs or metabolic markers. This method may also reduce blood triglyceride levels, suggesting potential benefits for preventing and treating diseases like fatty liver and diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9534732/.
Stromsdorfer, K. L., Yamaguchi, S., Yoon, M. J., Moseley, A. C., Franczyk, M. P., Kelly, S. C., Qi, N., Imai, S., & Yoshino, J. (2016). NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell reports, 16(7), 1851–1860. https://doi.org/10.1016/j.celrep.2016.07.027.
NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice
Adipocyte-specific deletion of nicotinamide phosphoribosyltransferase (NAMPT) in mice leads to severe insulin resistance across multiple organs and adipose tissue dysfunction. These effects include increased plasma free fatty acids, reduced adiponectin, and altered PPARγ signaling. The negative consequences were mitigated by administering rosiglitazone or nicotinamide mononucleotide (NMN), highlighting potential therapeutic strategies for obesity-related metabolic complications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5094180/.
Uddin, G. M., Youngson, N. A., Doyle, B. M., Sinclair, D. A., & Morris, M. J. (2017). Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Scientific reports, 7(1), 15063. https://doi.org/10.1038/s41598-017-14866-z.
Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise
Maternal overnutrition increases the risk of metabolic dysfunction in offspring, but interventions like exercise and nicotinamide mononucleotide (NMN) supplementation can mitigate these effects. In a study on mice, both treadmill exercise and NMN injections reduced adiposity and modestly improved glucose tolerance in offspring of obese mothers, with NMN showing stronger effects on liver fat metabolism. These findings suggest that NMN may be particularly beneficial in reversing metabolic dysfunction programmed by maternal obesity.
You can read the full article at https://www.nature.com/articles/s41598-017-14866-z.
Wei, C. C., Kong, Y. Y., Li, G. Q., Guan, Y. F., Wang, P., & Miao, C. Y. (2017). Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Scientific reports, 7(1), 717. https://doi.org/10.1038/s41598-017-00851-z.
Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway
Nicotinamide mononucleotide (NMN) was tested as a treatment for intracerebral hemorrhage (ICH) in a mouse model and found to significantly reduce brain edema, cell death, oxidative stress, and neuroinflammation, though it did not affect hematoma volume. NMN enhanced the expression and activation of cytoprotective proteins heme oxygenase 1 (HO-1) and nuclear factor-like 2 (Nrf2), and prolonged NMN treatment improved recovery of body weight and neurological function. These findings suggest that NMN offers neuroprotection in ICH through suppression of neuroinflammation and oxidative stress via the Nrf2/HO-1 signaling pathway.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429727/.
Sims, C. A., Guan, Y., Mukherjee, S., Singh, K., Botolin, P., Davila, A., Jr, & Baur, J. A. (2018). Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI insight, 3(17), e120182. https://doi.org/10.1172/jci.insight.120182.
Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock
In a rodent model of hemorrhagic shock, supplementation with nicotinamide mononucleotide (NMN) improved survival and metabolic resilience by reducing lactic acidosis, serum IL-6 levels, and mitochondrial dysfunction. NMN preserved NAD levels and mitochondrial function in the liver and kidneys, enhanced cellular energetics, and extended the time animals could endure severe shock before resuscitation. NMN’s benefits were observed both as a pretreatment and during resuscitation, highlighting its potential to mitigate inflammation and improve outcomes in hemorrhagic shock.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6171817/.
Wei, C. C., Kong, Y. Y., Hua, X., Li, G. Q., Zheng, S. L., Cheng, M. H., Wang, P., & Miao, C. Y. (2017). NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. British journal of pharmacology, 174(21), 3823–3836. https://doi.org/10.1111/bph.13979.
NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia
This study found that administering nicotinamide mononucleotide (NMN) in mice significantly reduced the harmful effects of tissue plasminogen activator (tPA) treatment following acute brain ischemia, including brain infarction, edema, and hemorrhagic transformation. NMN protected the blood-brain barrier (BBB) by preventing the down-regulation of tight junction proteins and reducing the activity of matrix metalloproteinases (MMPs), which are typically induced by delayed tPA treatment. These findings suggest that NMN may help mitigate the risks associated with tPA therapy in stroke patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5647191/.
Zhang, X. Q., Lu, J. T., Jiang, W. X., Lu, Y. B., Wu, M., Wei, E. Q., Zhang, W. P., & Tang, C. (2015). NAMPT inhibitor and metabolite protect mouse brain from cryoinjury through distinct mechanisms. Neuroscience, 291, 230–240. https://doi.org/10.1016/j.neuroscience.2015.02.007.
NAMPT inhibitor and metabolite protect mouse brain from cryoinjury through distinct mechanisms
This study investigated the effects of nicotinamide mononucleotide (NMN) and FK866, a nicotinamide phosphoribosyltransferase (NAMPT) inhibitor, on brain injury in a mouse model of traumatic brain injury (TBI). Both NMN and FK866 reduced neuronal loss and lesion volume, but through different mechanisms: NMN replenished NAD+ levels, while FK866 inhibited NAMPT activity in inflammatory cells, reducing inflammation and subsequent neuronal damage. These findings highlight the distinct therapeutic roles of NMN and FK866 in mitigating brain injury.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0306452215001499.
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., Machado De Oliveira, R., Leid, M., McBurney, M. W., & Guarente, L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature, 429(6993), 771–776. https://doi.org/10.1038/nature02583.
Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma
This study reveals that Sirtuin 1 (Sirt1), the mammalian equivalent of the yeast SIR2 gene, plays a crucial role in mediating the lifespan-extending effects of calorie restriction by promoting fat mobilization in white adipocytes. Sirt1 represses fat storage genes regulated by PPAR-gamma by interacting with cofactors NCoR and SMRT, leading to lipolysis and reduced fat storage. This repression is compromised in Sirt1+/- mice, indicating Sirt1’s essential role in fat metabolism and its potential link to extending lifespan through calorie restriction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820247/.
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., & Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature, 434(7029), 113–118. https://doi.org/10.1038/nature03354.
Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1
This study reveals that SIRT1, a protein linked to aging, plays a key role in glucose homeostasis by regulating the gluconeogenic and glycolytic pathways in the liver during fasting. SIRT1 interacts with and deacetylates the transcriptional coactivator PGC-1alpha, enhancing gluconeogenesis and hepatic glucose output, but does not affect PGC-1alpha’s role in mitochondrial gene regulation. This mechanism connects nutrient signaling to aging and has implications for energy homeostasis, diabetes, and lifespan.
You can read the abstract of the article at https://www.nature.com/articles/nature03354.
Yang, F., Vought, B. W., Satterlee, J. S., Walker, A. K., Jim Sun, Z. Y., Watts, J. L., DeBeaumont, R., Saito, R. M., Hyberts, S. G., Yang, S., Macol, C., Iyer, L., Tjian, R., van den Heuvel, S., Hart, A. C., Wagner, G., & Näär, A. M. (2006). An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature, 442(7103), 700–704. https://doi.org/10.1038/nature04942.
An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis.
This study identifies ARC105 (MED15) as a crucial mediator of SREBP-dependent gene regulation and lipid homeostasis. SREBPs, which regulate cholesterol and fatty acid balance, interact specifically with ARC105 to activate target genes, a mechanism distinct from other activators like CREB. In C. elegans, the SREBP homologue SBP-1 and ARC105 homologue MDT-15 are essential for fatty acid homeostasis, particularly in the desaturation of stearic acid to oleic acid. Supplementing oleic acid rescues defects caused by disrupting SBP-1 and MDT-15, highlighting their critical role in maintaining lipid balance.
You can read the abstract of the article at https://www.nature.com/articles/nature04942.
Assiri, M. A., Ali, H. R., Marentette, J. O., Yun, Y., Liu, J., Hirschey, M. D., Saba, L. M., Harris, P. S., & Fritz, K. S. (2019). Investigating RNA expression profiles altered by nicotinamide mononucleotide therapy in a chronic model of alcoholic liver disease. Human genomics, 13(1), 65. https://doi.org/10.1186/s40246-019-0251-1.
Investigating RNA expression profiles altered by nicotinamide mononucleotide therapy in a chronic model of alcoholic liver disease
This study explores the effects of nicotinamide mononucleotide (NMN) supplementation on early-stage alcoholic liver disease (ALD). Chronic alcohol consumption disrupts NAD+ levels, contributing to liver damage. In a murine model, NMN therapy increased hepatic NAD+ levels, prevented ethanol-induced liver damage, and altered the expression of key genes involved in pathways like MAPK signaling. Notably, NMN normalized Erk1/2 signaling and prevented Atf3 overexpression, suggesting potential protective mechanisms against ethanol hepatotoxicity. The findings highlight the need for further research on NAD+-based interventions for ALD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6902345/.
Guarino M, Dufour JF. Nicotinamide and NAFLD: Is There Nothing New Under the Sun?. Metabolites. 2019;9(9):180. Published 2019 Sep 10. doi:10.3390/metabo9090180.
Nicotinamide and NAFLD: Is There Nothing New Under the Sun?
This review highlights the critical role of nicotinamide adenine dinucleotide (NAD) in cellular metabolism and its potential therapeutic significance in nonalcoholic fatty liver disease (NAFLD). Reduced NAD levels contribute to metabolic imbalances that drive NAFLD pathogenesis. Supplementing or pharmacologically modulating NAD levels presents a promising strategy for NAFLD treatment, although further clinical studies are needed to validate these findings.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6780119/.
Wang S, Wan T, Ye M, et al. Nicotinamideriboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89-98. doi:10.1016/j.redox.2018.04.006.
Nicotinamideriboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway
This study demonstrates that nicotinamide riboside (NR), a precursor of NAD+, protects against ethanol-induced liver injuries by replenishing NAD+ levels, reducing oxidative stress, and enhancing mitochondrial function through the activation of the SirT1-PGC-1α pathway. The protective effects of NR against lipid accumulation and mitochondrial dysfunction induced by ethanol are dependent on SirT1, highlighting its key role in this process.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6007172/.
Zong, Z., Liu, J., Wang, N., Yang, C., Wang, Q., Zhang, W., Chen, Y., Liu, X., & Deng, H. (2021). Nicotinamide mononucleotide inhibits hepatic stellate cell activation to prevent liver fibrosis via promoting PGE2 degradation. Free radical biology & medicine, 162, 571–581. https://doi.org/10.1016/j.freeradbiomed.2020.11.014.
Nicotinamide mononucleotide inhibits hepatic stellate cell activation to prevent liver fibrosis via promoting PGE2 degradation
This study suggests that nicotinamide mononucleotide (NMN) may serve as a therapeutic approach for liver fibrosis by inactivating hepatic stellate cells (HSCs) and reducing the secretion of profibrotic proteins. NMN also decreased extracellular matrix accumulation in mouse models of liver fibrosis, potentially through inhibiting oxidation-mediated degradation of 15-PGDH and promoting prostaglandin E2 degradation, thereby preventing HSC activation.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0891584920316269?via%3Dihub.
Luo, C., Ding, W., Yang, C., Zhang, W., Liu, X., & Deng, H. (2022). Nicotinamide Mononucleotide Administration Restores Redox Homeostasis via the Sirt3-Nrf2 Axis and Protects Aged Mice from Oxidative Stress-Induced Liver Injury. Journal of proteome research, 21(7), 1759–1770. https://doi.org/10.1021/acs.jproteome.2c00167.
Nicotinamide Mononucleotide Administration Restores Redox Homeostasis via the Sirt3-Nrf2 Axis and Protects Aged Mice from Oxidative Stress-Induced Liver Injury
This study found that aging in mouse liver is associated with increased oxidative stress and activation of the Nrf2 pathway. Nicotinamide mononucleotide (NMN) administration reduced oxidative stress and Nrf2 activation, improved resistance to acetaminophen-induced liver injury, and restored adaptive homeostasis via the Sirt3-Nrf2 axis. These findings suggest that NMN supplementation could help protect the liver from age-related oxidative damage.
You can read the abstract of the article at https://pubs.acs.org/doi/10.1021/acs.jproteome.2c00167.
Guan, Y., Wang, S. R., Huang, X. Z., Xie, Q. H., Xu, Y. Y., Shang, D., & Hao, C. M. (2017). Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. Journal of the American Society of Nephrology : JASN, 28(8), 2337–2352. https://doi.org/10.1681/ASN.2016040385.
Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner
This study found that aging reduces the levels of the protective molecule SIRT1 and its cofactor NAD+ in kidneys, making them more susceptible to acute injury. Supplementation with nicotinamide mononucleotide (NMN) restored SIRT1 activity and NAD+ levels, protecting both young and aged mice from cisplatin-induced acute kidney injury (AKI). The protective effects of NMN were linked to the modulation of the JNK signaling pathway by SIRT1, suggesting that targeting SIRT1 with NMN could be a potential treatment for AKI in aged patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5533221/.
Jia, Y., Kang, X., Tan, L., Ren, Y., Qu, L., Tang, J., Liu, G., Wang, S., Xiong, Z., & Yang, L. (2021). Nicotinamide Mononucleotide Attenuates Renal Interstitial Fibrosis After AKI by Suppressing Tubular DNA Damage and Senescence. Frontiers in physiology, 12, 649547. https://doi.org/10.3389/fphys.2021.649547.
Nicotinamide Mononucleotide Attenuates Renal Interstitial Fibrosis After AKI by Suppressing Tubular DNA Damage and Senescence
Acute kidney injury (AKI) is a global health issue with no effective treatments to promote renal repair or prevent chronic fibrosis. This study shows that nicotinamide mononucleotide (NMN), an NAD+ precursor, can significantly reduce DNA damage, cellular senescence, and inflammation in kidney cells and mouse models of AKI. NMN also demonstrated antifibrosis effects when administered before or during the recovery phase of AKI, suggesting it may be a promising strategy for preventing or treating kidney fibrosis following AKI.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8021789/.
Hasegawa, K., Sakamaki, Y., Tamaki, M., & Wakino, S. (2022). Nicotinamide mononucleotide ameliorates adriamycin-induced renal damage by epigenetically suppressing the NMN/NAD consumers mediated by Twist2. Scientific reports, 12(1), 13712. https://doi.org/10.1038/s41598-022-18147-2.
Nicotinamide mononucleotide ameliorates adriamycin-induced renal damage by epigenetically suppressing the NMN/NAD consumers mediated by Twist2
This study shows that short-term nicotinamide mononucleotide (NMN) treatment provides renal protection in mice with adriamycin-induced focal glomerulosclerosis (FSGS). NMN alleviates urinary albumin excretion, mitigates glomerulosclerosis, and restores Sirt1 expression while reducing Claudin-1 expression. It also modulates histone methylation and suppresses enzymes that consume NAD+ and NMN, suggesting a potential new treatment strategy for FSGS through epigenetic regulation and Sirt1 activation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9374671/.
Yasuda, I., Hasegawa, K., Sakamaki, Y., Muraoka, H., Kawaguchi, T., Kusahana, E., Ono, T., Kanda, T., Tokuyama, H., Wakino, S., & Itoh, H. (2021). Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy. Journal of the American Society of Nephrology : JASN, 32(6), 1355–1370. https://doi.org/10.1681/ASN.2020081188.
Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy
Short-term nicotinamide mononucleotide (NMN) treatment in diabetic db/db mice significantly attenuated diabetic nephropathy (DN) symptoms, including urinary albumin excretion, mesangium expansion, and foot process effacement, without affecting blood glucose levels. The treatment also restored kidney NAD+ levels, Sirt1 expression, and histone modifications, while improving survival rates, suggesting NMN’s potential as a therapeutic strategy for DN.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8259649/.
Chen, Y., Liang, Y., Hu, T., Wei, R., Cai, C., Wang, P., Wang, L., Qiao, W., & Feng, L. (2017). Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-κB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Experimental and therapeutic medicine, 14(5), 4181–4193. https://doi.org/10.3892/etm.2017.5098.
Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-κB p65 and Sirt1 pathway; NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt
Endogenous Nicotinamide phosphoribosyltransferase (Nampt) upregulation in diabetic renal cells contributes to pro-inflammatory and pro-fibrotic signaling via the NF-κB p65 and Sirt1 pathways, leading to glomerular fibrosis in diabetic nephropathy (DN). The study suggests that inhibiting Nampt upregulation could be crucial in treating DN-related fibrosis, with nicotinamide mononucleotide (NMN) showing potential as a therapeutic agent.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5658765/.
Other Peptides
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

