GENEMEDICS APP
GENEMEDICS NUTRITION
Mitochondrial ORF Of The Twelve S C (MOTS-C)
Author: Dr. George Shanlikian, M.D. | Last Updated: November 21st, 2024
- Home
- >
- Health Library
- >
- Mitochondrial ORF of the twelve S c (MOTS-c)
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
- Key Takeaways
- What is MOTS-c?
- How MOTS-c Works
- Chemical Structure of MOTS-c
- Research on MOTS-c
- MOTS c Side Effects
- MOTS-c Peptide
- MOTS-c Injection
- MOTS c Cancer
- MOTS c 10mg
- MOTS c Supplement
- MOTS c Weight Loss
- MOTS c Dosage
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
- Key Takeaways
- What is MOTS-c?
- How MOTS-c Works
- Chemical Structure of MOTS-c
- Research on MOTS-c
- MOTS c Side Effects
- MOTS-c Peptide
- MOTS-c Injection
- MOTS c Cancer
- MOTS c 10mg
- MOTS c Supplement
- MOTS c Weight Loss
- MOTS c Dosage
- FAQ
- Reference
Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
The twelve S c (MOTS-c) benefits include improved metabolic function, enhanced mitochondrial biogenesis, increased insulin sensitivity, reduced inflammation, better endurance, and protection against age-related decline. Additionally, it supports cognitive function, promotes muscle regeneration, reduces oxidative stress, aids in weight management, enhances physical performance, and improves overall longevity.
- Treats obesity [1-12]
- Improves blood sugar levels and treats symptoms of diabetes [13-18]
- Improves heart health [19-24]
- Fights bone loss [25-27]
- Increases life expectancy [28-34]
- Improves exercise tolerance [35-51]
- Fights bacterial infection [52]
Key Takeaways
- Enhanced Metabolic Function: MOTS-c improves metabolic processes, aiding in efficient energy utilization and better overall metabolic health.
- Increased Insulin Sensitivity: It boosts insulin sensitivity, helping to regulate blood sugar levels and reduce the risk of diabetes.
- Reduced Inflammation: MOTS-c has anti-inflammatory properties that can help mitigate chronic inflammation and its associated health risks.
- Protection Against Age-Related Decline: This peptide supports mitochondrial biogenesis and function, which are crucial for maintaining vitality and slowing age-related decline.
- Improved Physical and Cognitive Performance: MOTS-c enhances endurance, muscle regeneration, cognitive function, and overall physical performance, contributing to better health and longevity.
What is MOTS-c?
The mitochondria are considered the “powerhouses of cells”. They are organelles that work like the digestive system which ingests nutrients, breaks them down, and produces fuel or energy for the cells’ biological functions. The mitochondria also pass down information via several signaling molecules in order to enhance communication between each cell. Mitochondrial ORF of the twelve S c (MOTS-c) is one of the mitochondrial-derived peptides (MDPs) that plays an integral role in a wide array of metabolic functions such as glucose metabolism, muscle synthesis, and maintenance of metabolic homeostasis (balance).
How MOTS-c Works
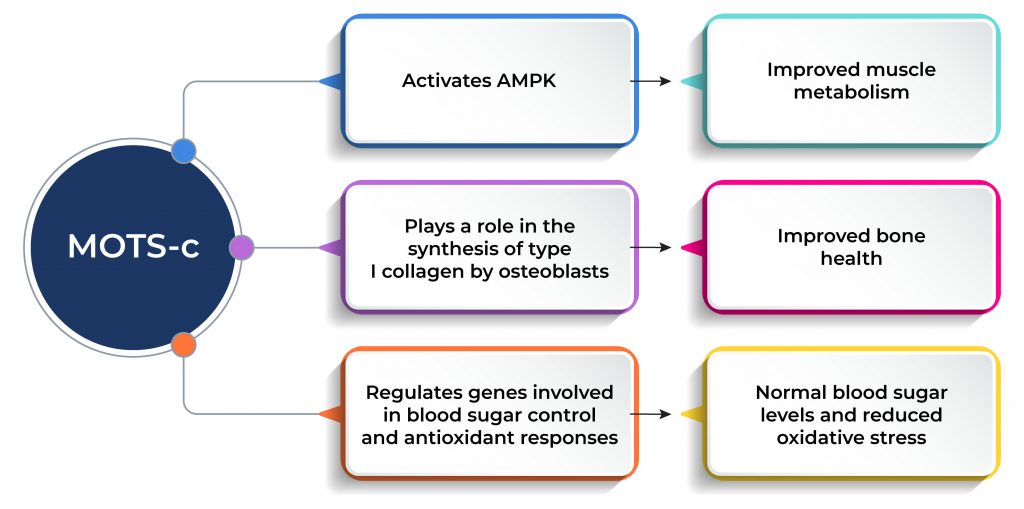
MOTS-c holds much potential as a therapeutic option for a multitude of medical conditions because of its unique ability to positively affect various bodily functions. It improves muscle metabolism and function via AMPK activation. Following metabolic stress, MOTS-c regulates certain genes involved in blood sugar restriction and antioxidant responses. In addition, it also plays a role in the synthesis of type I collagen by osteoblasts (bone-forming cells).
Chemical Structure of MOTS-c
Research on MOTS-c
A. Treats Obesity
Obesity is one of the world’s major health concerns. This debilitating medical condition can dramatically increase one’s risk for heart disease, cancer, stroke, blood vessel problems, and other chronic medical conditions. Numerous studies found that MOTS-c administration can help treat obesity by improving different health parameters and body processes:
- MOTS-c may help promote weight loss by reducing the levels of damaging reactive oxygen species during exercise, thereby stimulating physiological adaptation and increased tolerance to exercise. [1-8]
- A study found that plasma MOTS-c levels were higher in lean but not in obese individuals. [9]
- In rodents, MOTS-c treatment reduced abdominal fat and inhibited adipogenesis (fat cell formation) through the down-regulation of multiple genes involved in fat cell development. [10]
- In mice fed with a high-fat diet (60% by calories), MOTS-c treatment prevented diet-induced obesity as well as age-dependent and high-fat diet-induced insulin resistance. [11]
- In mice, MOTS-c treatment prevented ovariectomy-induced obesity by regulating the production of fat cells. [12]
B. Improves Blood Sugar Levels and Treats Symptoms of Diabetes
MOTS-c also exerts beneficial effects on blood sugar levels and diabetic symptoms. Studies show that this mitochondrial-derived peptide can help improve the body’s response to insulin hormone:
- MOTS-c regulates insulin sensitivity, thereby improving the effects of insulin on the body and reducing blood sugar levels. [13]
- MOTS-c balances blood sugar levels by stimulating cellular glucose uptake. [14]
- A study reported that reduced activity of MOTS-c was associated with an increased risk of diabetes mellitus. [15]
- In a postmenopausal animal model, MOTS-c treatment reduced weight gain and insulin resistance. [16]
- In non-obese diabetic mice, MOTS-c prevented pancreatic islet destruction (insulin-producing cells) by targeting T cells. [17]
- In diet-induced obese mice, MOTS-c protected against insulin resistance-induced skeletal muscle atrophy (muscle wasting). [18]
C. Improves Heart Health
Evidence also suggests that MOTS-c can help preserve heart function by combating the detrimental effects of inflammation on the body and that its levels correlate with cardiovascular health:
- In human subjects, lower circulating MOTS-c levels were associated with impaired coronary endothelial function. [19]
- In cold-exposed rats, MOTS-c prevented blood vessel dysfunction of the heart. [20]
- A study found that MOTS-c may help reduce endothelial dysfunction by suppressing inflammatory pathways such as MAPK/NF-κB. [21]
- In rats, MOTS-c improved myocardial mechanical efficiency and enhanced cardiac systolic function during exercise training. [22]
- In vitamin D3 plus nicotine-treated rats, injection with MOTS-c at a dose of 5 mg/kg once a day for 4 weeks prevented the deposition of minerals in the heart valves. [23]
- In a mouse model of gestational diabetes, daily administration of MOTS-c during pregnancy significantly alleviated hyperglycemia (high blood sugar levels), improved insulin sensitivity and glucose tolerance, and reduced the risk of low birth weight and death of offspring. [24]
D. Fights Bone Loss
The age-related decline in bone mineral density causes osteoporosis and fractures. Studies show that MOTS-c can help correct these problems by improving bone quality and preventing bone-damaging processes:
- In mice, MOTS-c injection at a dose of 5 mg/kg once a day for 12 weeks alleviated bone loss by inhibiting the process of bone breakdown (osteoclastogenesis). [25]
- In rats, MOTS-c treatment improved osteoporosis by boosting the production of bone mesenchymal stem cells (BMSCs), which are cells that can transform into bone-forming cells (osteoblasts). [26]
- A cell study found that MOTS-c treatment protected against osteoporosis by promoting the synthesis of type I collagen in osteoblasts. [27]
E. Increases Life Expectancy
Aside from correcting obesity and reducing the risk of various chronic debilitating medical conditions, MOTS-c can also increase life expectancy according to studies:
- A Japanese study found that the mitochondrial m.1382A>C polymorphism which occurs within the MOTS-c DNA sequence was associated with exceptional longevity. [28]
- A study reported that MOTS-c can help increase life expectancy by promoting cellular homeostasis and preventing metabolic stress. [29]
- MOTS-c increases the intracellular levels of NAD+ which is a key metabolic coenzyme involved in oxidation-reduction and prevention of age-related diseases. [30-32]
- In aged human placenta-derived mesenchymal stem cells, MOTS-c promoted homeostasis. [33]
- In healthy aging men, an increase in muscle MOTS-c with age is consistent with fast-to-slow type muscle fiber transition. [34]
F. Improves Exercise Tolerance
MOTS-c can also benefit athletes and physically active individuals. Studies show that this mitochondrial-derived peptide can improve exercise tolerance through various important mechanisms:
- MOTS-c stimulates physiological adaptation and increased tolerance to exercise by reducing the levels of damaging reactive oxygen species during exercise, thereby improving performance and results. [35-36]
- MOTS-c is actively engaged in transmitting exercise-induced signals to different body organs. [42-48]
- MOTS-c improves exercise performance by increasing cellular levels of AICAR and activating AMPK – both of these processes boost energy production. [49-51]
G. Fights Bacterial Infection
Sepsis, a condition characterized by the presence of harmful microorganisms in the blood and uncontrolled inflammatory responses, can be a life-threatening condition. Interestingly, a study found that MOTS-c can treat Methicillin-resistant Staphylococcus aureus (MRSA) infection, which is one of the most hard-to-treat bacterial infections and the major cause of sepsis. [52]
In this study, researchers observed that MOTS-c treatment significantly improved the survival rate and decreased bacteria loads in MRSA-challenged mice. In addition, researchers observed that the treatment significantly reduced the levels of inflammatory substances, suggesting that MOTS-c can be an effective therapeutic option for MRSA infections and other antibiotic-resistant infections.
MOTS c Side Effects
MOTS-c side effects are very uncommon. There have been some side effects associated with the use of this peptide wherein the patient had one of the issues listed below at some point while being on MOTS-c. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of MOTS-c. Despite this, it was listed as a side effect associated with MOTS-c even though these associated side effects are very uncommon.
Side effects associated with MOTS-c may include the following:
- Alteration in lipid profiles
- Development of antibodies
- Fluid retention
- High blood sugar
- Stomach upset
MOTS-c Peptide
MOTS-c is a mitochondrial-derived peptide that has garnered significant attention for its potential health benefits. Discovered relatively recently, MOTS-c plays a crucial role in regulating metabolic processes. By promoting efficient energy utilization, it helps enhance metabolic function, which is vital for maintaining overall health. This peptide’s ability to improve insulin sensitivity is particularly notable, as it aids in better blood sugar regulation and reduces the risk of metabolic disorders such as diabetes.
One of the remarkable aspects of MOTS-c is its anti-inflammatory properties. Chronic inflammation is a common underlying factor in various diseases, including cardiovascular diseases and certain types of cancer. By reducing inflammation, MOTS-c helps mitigate these risks, contributing to better long-term health. Additionally, its role in enhancing mitochondrial biogenesis ensures that cells maintain their energy production capabilities, which is especially important as we age. This function supports the maintenance of muscle mass and cognitive abilities, helping to protect against age-related decline.
Moreover, MOTS-c has been linked to improved physical and cognitive performance. Its ability to boost endurance and muscle regeneration makes it a valuable asset for athletes and individuals looking to enhance their physical fitness. Cognitive benefits are equally important, as MOTS-c supports brain health and function, potentially offering protection against neurodegenerative diseases. Overall, MOTS-c stands out as a peptide with multifaceted benefits, promoting both physical and cognitive well-being, and contributing to a healthier, more resilient body.
MOTS-c Injection
MOTS-c injection has emerged as a groundbreaking intervention in the field of metabolic health. This mitochondrial-derived peptide, known for its role in regulating metabolic processes, is administered directly into the bloodstream to ensure efficient absorption and action. By targeting the mitochondria, the energy powerhouses of cells, MOTS-c injections can enhance mitochondrial function, promoting better energy utilization and metabolic efficiency. This has significant implications for individuals suffering from metabolic disorders such as obesity, diabetes, and metabolic syndrome.
The mechanisms behind MOTS-c injections involve its ability to activate key metabolic pathways that enhance insulin sensitivity and glucose uptake, making it a powerful tool in managing blood sugar levels. Additionally, MOTS-c reduces inflammation and oxidative stress, both of which are critical factors in the progression of chronic diseases. Regular administration of MOTS-c injections can lead to improved endurance, muscle regeneration, and overall physical performance. This makes it not only beneficial for those with metabolic disorders but also for athletes and individuals looking to optimize their physical fitness and recovery.
Beyond its metabolic benefits, MOTS-c injections hold promise in combating age-related decline. As we age, mitochondrial function tends to deteriorate, leading to reduced energy levels and increased susceptibility to various diseases. MOTS-c helps counteract this decline by promoting mitochondrial biogenesis and maintaining mitochondrial health. This contributes to enhanced vitality and potentially greater longevity. Research is ongoing, but early findings suggest that MOTS-c injections could be a valuable tool in age management, providing a means to maintain physical and cognitive function well into older age.
MOTS c Cancer
MOTS-c, a mitochondrial-derived peptide, has garnered attention for its potential role in cancer prevention and treatment. This peptide regulates metabolic homeostasis and has shown promise in counteracting the metabolic reprogramming observed in cancer cells. By enhancing mitochondrial function and biogenesis, MOTS-c can disrupt the altered energy production pathways that cancer cells rely on, thereby inhibiting their growth and proliferation.
Research has indicated that MOTS-c may also play a role in modulating the immune response against cancer. It can reduce chronic inflammation, a condition that often contributes to cancer progression. By dampening inflammatory pathways, MOTS-c helps create an environment less conducive to cancer cell survival and spread. Furthermore, its ability to improve insulin sensitivity and regulate glucose levels can indirectly affect cancer risk, as hyperinsulinemia and high glucose levels are linked to certain cancers.
In addition to these metabolic and immunomodulatory effects, MOTS-c has been observed to influence the cellular stress response. It helps protect cells from oxidative damage, which is a common feature in the tumor microenvironment. This protective effect can prevent the DNA damage that often leads to cancerous mutations. While research is still ongoing, the multifaceted actions of MOTS-c suggest it could be a valuable component in comprehensive cancer prevention strategies and therapeutic interventions.
MOTS c 10mg
MOTS-c 10mg is a synthetic peptide derived from mitochondrial DNA, known for its significant role in metabolic regulation and health. By mimicking the effects of exercise, this peptide activates the AMPK pathway, which is crucial for maintaining energy balance and enhancing cellular metabolism. Research suggests that a 10mg dose of MOTS-c can help improve insulin sensitivity, reduce inflammation, and support overall metabolic health, making it a promising therapeutic option for conditions like obesity, diabetes, and age-related metabolic decline.
The benefits of MOTS-c extend beyond metabolic health, as it also plays a vital role in promoting mitochondrial biogenesis and function. This is particularly important for aging populations, as mitochondrial dysfunction is a common hallmark of aging and various age-related diseases. A 10mg dose of MOTS-c can help boost mitochondrial health, enhance endurance, and protect against oxidative stress, thus contributing to improved physical performance and longevity.
Additionally, MOTS-c 10mg has shown potential in supporting cognitive health. By reducing oxidative stress and inflammation, and enhancing mitochondrial function, this peptide may help protect against neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Its ability to promote muscle regeneration and overall vitality further underscores its comprehensive benefits, making MOTS-c a valuable tool in the pursuit of improved health and well-being.
MOTS c Supplement
MOTS-c, a peptide encoded by the mitochondrial genome, plays a significant role in regulating metabolic processes and promoting overall health. As a supplement, MOTS-c is gaining attention for its potential benefits, including enhanced metabolic function, increased insulin sensitivity, and reduced inflammation. This peptide influences cellular metabolism by activating pathways that improve energy production and utilization, making it a promising candidate for addressing metabolic disorders and supporting healthy aging.
Supplementing with MOTS-c offers a range of benefits that extend beyond metabolic health. Research suggests that MOTS-c can enhance mitochondrial biogenesis, leading to better energy production and cellular health. Additionally, it has been shown to improve insulin sensitivity, which is crucial for maintaining stable blood sugar levels and preventing diabetes. The anti-inflammatory properties of MOTS-c further contribute to its potential in reducing chronic inflammation, a common underlying factor in various diseases. These combined effects make MOTS-c a valuable supplement for those looking to support their metabolic health and overall well-being.
While the benefits of MOTS-c supplementation are promising, ongoing research is essential to fully understand its mechanisms and long-term effects. Potential applications of MOTS-c extend to athletic performance, where it may improve endurance and muscle regeneration, and to neurodegenerative diseases, where its role in mitochondrial health could offer protective effects. As scientific interest in MOTS-c continues to grow, future studies will likely explore its therapeutic potential in greater detail, paving the way for more targeted and effective uses of this powerful peptide supplement.
MOTS c Weight Loss
MOTS-c, a mitochondrial-derived peptide, has garnered attention for its potential role in weight loss and metabolic health. One of its primary mechanisms involves enhancing insulin sensitivity, which aids in the effective regulation of blood glucose levels. By improving how the body processes and utilizes glucose, MOTS-c helps prevent excess glucose from being stored as fat, thereby reducing overall fat accumulation. This improvement in metabolic efficiency can lead to a more balanced energy expenditure and support weight loss efforts.
In addition to its effects on insulin sensitivity, MOTS-c has been shown to stimulate mitochondrial biogenesis and function. Mitochondria are the powerhouses of cells, playing a crucial role in energy production. By promoting the creation of new, healthy mitochondria, MOTS-c enhances the body’s ability to burn calories efficiently. This increased mitochondrial activity not only supports greater energy levels and endurance but also contributes to a higher basal metabolic rate, making it easier to shed excess weight and maintain a healthier body composition.
Moreover, MOTS-c’s anti-inflammatory properties play a significant role in weight management. Chronic inflammation is often linked to obesity and metabolic disorders, as it can disrupt normal metabolic functions and promote fat storage. By reducing inflammation, MOTS-c helps restore metabolic balance and improve overall health. This reduction in inflammation, combined with enhanced metabolic function and improved insulin sensitivity, makes MOTS-c a promising tool in the fight against obesity and for supporting long-term weight management.
MOTS c Dosage
Optimal Dosage for Health Benefits: The optimal dosage of MOTS-c for achieving health benefits can vary depending on the individual’s health status, age, and specific health goals. Research suggests that a common dosage range for MOTS-c peptide is between 5 mg to 10 mg administered two to three times per week. This dosage is often sufficient to enhance metabolic function, increase insulin sensitivity, and promote mitochondrial health. It’s crucial to consult with a healthcare provider to determine the most appropriate dosage tailored to individual needs.
Administration Methods: MOTS-c is typically administered via subcutaneous injections, allowing for direct entry into the bloodstream and ensuring efficient absorption. This method helps in bypassing the digestive system, where peptides can be broken down and rendered less effective. For those new to peptide therapy, healthcare professionals can provide guidance on the proper technique for self-administration to maximize benefits and minimize potential side effects.
Monitoring and Adjustments: Regular monitoring of health markers, such as blood glucose levels, inflammation markers, and mitochondrial function, can help in assessing the effectiveness of the MOTS-c dosage. Adjustments may be necessary based on these health markers and any side effects experienced. Personalized dosage adjustments, under the supervision of a healthcare professional, ensure that the therapy remains safe and effective over the long term. Continuous communication with a healthcare provider is essential for optimizing the benefits of MOTS-c while mitigating any risks.
FAQ
What does the MOTS-c peptide do?
MOTS-c peptide improves metabolic function, enhances mitochondrial biogenesis, increases insulin sensitivity, reduces inflammation, supports muscle homeostasis, and protects against age-related decline. By maintaining muscle homeostasis, MOTS-c helps ensure muscle health and function, which is essential for overall well-being. Additionally, its role in muscle homeostasis contributes to better endurance and physical performance.
Does MOTS-c reduce appetite?
There is no strong evidence suggesting that MOTS-c specifically reduces appetite. Its primary benefits are related to metabolic and mitochondrial health, particularly in addressing age-dependent physical decline. By improving mitochondrial function, MOTS-c can help mitigate age-dependent physical decline, supporting overall vitality. Additionally, enhancing metabolic processes can also contribute to reducing the effects of age-dependent physical decline.
Does MOTS-c have side effects?
MOTS-c is generally well-tolerated, but potential side effects may include mild injection site reactions and temporary fatigue. The peptide’s role in adaptive nuclear gene expression can sometimes influence how side effects manifest. As with any peptide therapy, side effects can vary among individuals, and the process of adaptive nuclear gene expression may contribute to this variability. Understanding the interplay between MOTS-c and adaptive nuclear gene expression can help manage and anticipate these potential side effects more effectively.
What does the MOTS-c peptide do?
MOTS-c peptide regulates metabolic functions, enhances insulin sensitivity, reduces inflammation, and supports mitochondrial health, contributing to overall improved health and longevity. Additionally, MOTS-c plays a crucial role in skeletal muscles, where it helps improve metabolic functions. By enhancing insulin sensitivity and supporting mitochondrial health in skeletal muscles, it significantly contributes to overall muscle health and longevity.
What is C-peptide used for?
C-peptide is used to evaluate insulin production and pancreatic function. It helps diagnose diabetes and monitor treatment efficacy. Monitoring mots c levels can provide additional insights into metabolic health. Understanding both C-peptide and mots c levels can enhance the accuracy of diabetes diagnosis and treatment monitoring. Keeping track of mots c levels alongside C-peptide levels can offer a comprehensive view of an individual’s metabolic and pancreatic function.
Does MOTS-c increase appetite?
There is no evidence that MOTS-c increases appetite. Its primary role is in improving metabolic processes and mitochondrial function. As one of the mitochondria derived peptides, MOTS-c enhances energy production and overall cellular health. Research on mitochondria derived peptides, including MOTS-c, highlights their potential in addressing metabolic disorders.
What are the benefits of MOTS-c?
The benefits of MOTS-c include enhanced metabolic function, increased insulin sensitivity, reduced inflammation, improved endurance, cognitive function, muscle regeneration, and overall longevity. Incorporating an exercise intervention can further amplify these benefits, as physical activity complements the metabolic and mitochondrial enhancements provided by MOTS-c. Additionally, combining MOTS-c with an exercise intervention may lead to even greater improvements in muscle regeneration and overall longevity.
What is the MOTS-c treatment?
MOTS-c treatment typically involves subcutaneous injections of the peptide to improve metabolic health, enhance mitochondrial function, and provide anti-aging benefits. This peptide is encoded in mammalian mitochondrial DNA, which plays a crucial role in regulating cellular energy production. By targeting mammalian mitochondrial DNA, MOTS-c enhances mitochondrial function, leading to improved metabolic health and anti-aging benefits. The unique properties of mammalian mitochondrial DNA make MOTS-c an effective treatment for promoting overall vitality and longevity.
Does MOTS-c increase energy?
Yes, MOTS-c can increase energy by enhancing mitochondrial function, which is crucial for efficient glucose utilization and energy production in cells. Improved glucose utilization through MOTS-c contributes to better overall cellular efficiency and vitality. By supporting mitochondrial health, MOTS-c helps optimize glucose utilization, thus boosting energy levels.
Is MOTS-c safe?
While preliminary studies suggest MOTS-c is generally safe and well-tolerated, it is important to use it under medical supervision. This is crucial because, although MOTS-c shows promise in improving metabolic functions, there is limited information on its effects in cases involving pancreatic islet destruction. Ongoing research will help clarify whether MOTS-c can mitigate or exacerbate conditions associated with pancreatic islet destruction.
What is MOTS-c used for?
MOTS-c is used to improve metabolic function, increase insulin sensitivity, reduce inflammation, enhance mitochondrial health, and support overall longevity and vitality.
What is MOTS-c in mice?
In mice, MOTS-c has been shown to improve metabolic health, enhance lipid metabolism, and physical performance, reduce obesity, and provide protection against age-related decline. Additionally, it supports lipid metabolism, contributing to better overall metabolic function. MOTS-c’s effects on lipid metabolism highlight its potential for addressing metabolic disorders and promoting healthy aging.
How does MOTS-c affect metabolism?
MOTS-c influences metabolism by promoting the uptake of glucose and the utilization of fatty acids. It enhances insulin sensitivity and helps regulate blood sugar levels, which can be beneficial for metabolic health, particularly in obese male children. Improved glucose uptake and fatty acid utilization can support better metabolic outcomes, which is crucial for managing conditions in obese male children. By enhancing insulin sensitivity, MOTS-c may contribute to better overall metabolic health in obese male children.
Can MOTS-c help with weight loss?
MOTS-c may assist with weight loss by improving metabolic efficiency and increasing energy expenditure, which can be particularly beneficial for individuals experiencing cardiac dysfunction. Its effects on enhancing insulin sensitivity and promoting fat oxidation contribute to better weight management, potentially alleviating some factors associated with cardiac dysfunction. By improving metabolic health, MOTS-c may help reduce the burden of cardiac dysfunction and support overall cardiovascular well-being.
How is MOTS-c administered?
MOTS-c is typically administered via subcutaneous injection. This method allows the peptide to be absorbed into the bloodstream and exert its effects on the body’s metabolism, potentially influencing factors related to cardiovascular disease. By improving metabolic function and reducing inflammation, MOTS-c may contribute to a lower risk of cardiovascular disease. However, further research is needed to fully understand its impact on cardiovascular disease and overall heart health.
Is MOTS-c naturally occurring?
Yes, MOTS-c is a naturally occurring peptide encoded by the mitochondrial DNA. It is produced within the cells and plays a critical role in regulating mitochondrial metabolism. By influencing mitochondrial metabolism, MOTS-c helps optimize cellular energy production and overall metabolic function.
What is the role of MOTS-c in exercise performance?
MOTS-c has been shown to enhance exercise performance in diabetes subjects by improving muscle function and increasing energy availability. In addition, it may help reduce fatigue and improve endurance during physical activities for diabetes subjects. This peptide’s benefits are particularly valuable for diabetes subjects seeking to boost their physical activity and overall health.
Can MOTS-c be used to treat diabetes?
Research suggests that MOTS-c may have potential in treating type 2 diabetes by improving insulin sensitivity and regulating blood glucose levels.
What are the potential anti-aging benefits of MOTS-c?
MOTS-c may have anti-aging benefits due to its role in promoting cellular health and metabolic efficiency. It helps protect cells from oxidative stress and supports mitochondrial function, which are crucial factors in reducing neuronal cell death. By mitigating factors that contribute to neuronal cell death, MOTS-c supports overall cellular health and longevity. Additionally, its role in enhancing mitochondrial function helps to further protect against neuronal cell death and the negative impacts of aging.
Does MOTS-c improve cognitive function?
Studies indicate that MOTS-c may support cognitive function by enhancing energy metabolism in the brain and protecting neurons from damage. Its effects on reducing inflammation and oxidative stress also contribute to brain health by improving overall energy metabolism. This holistic approach to energy metabolism helps maintain cognitive function and neuron health over time.
How does MOTS-c interact with other hormones?
MOTS-c interacts with various hormones, including insulin and leptin, to regulate metabolic functions. It helps improve insulin signaling and can influence appetite-regulating hormones, contributing to better metabolic control and balanced levels of MOTS-c. By affecting these hormones, MOTS-c plays a role in maintaining optimal levels of MOTS-c and overall metabolic health. Monitoring levels of MOTS-c can provide insights into its effectiveness in managing metabolic processes.
Can MOTS-c be used for muscle recovery?
MOTS-c may aid in muscle recovery by enhancing energy production and inhibiting inflammation in muscle tissues. It supports the repair and regeneration of muscle cells after exercise or injury by inhibiting inflammation and improving overall cellular function. Additionally, by inhibiting inflammation, MOTS-c contributes to a quicker recovery and better muscle repair.
What are the effects of MOTS-c on cardiovascular health?
MOTS-c has been shown to improve cardiovascular health by promoting better blood flow and reducing oxidative stress in blood vessels, which is particularly beneficial for individuals with chronic kidney disease. It may also help regulate blood pressure and support overall heart function, potentially offering advantages for those suffering from chronic kidney disease. Additionally, MOTS-c’s role in reducing oxidative stress and improving vascular health can be crucial in managing complications associated with chronic kidney disease.
Is MOTS-c effective for all age groups?
While MOTS-c has shown promising results in various studies, its effectiveness in addressing age-related diseases may vary among different age groups. Research is ongoing to determine its specific benefits and potential applications across various age demographics, particularly in the context of age-related diseases. The aim is to better understand how MOTS-c might influence the progression and management of these age-related diseases.
How long does it take to see the effects of MOTS-c?
The timeframe for observing the effects of MOTS-c can vary depending on the individual and the specific health condition being addressed. Some users may notice improvements within a few weeks, while others may require longer periods of consistent use. Monitoring circulating MOTS-c levels can provide insights into the effectiveness of the treatment and help adjust dosages as needed. Additionally, tracking changes in circulating MOTS-c levels over time can be useful in assessing overall progress and ensuring optimal benefits.
Are there any known drug interactions with MOTS-c?
There are currently no well-documented drug interactions with MOTS-c, a type of mitochondrial derived peptide, but it is always important to consult with a healthcare provider before combining it with other medications or treatments. As with other mitochondrial derived peptides, individual responses can vary, so professional guidance is crucial. Ensuring safe use of mitochondrial derived peptides like MOTS-c helps in achieving the desired health benefits while minimizing potential risks.
Can MOTS-c be used to enhance athletic performance?
MOTS-c has the potential to enhance athletic performance by improving energy production, reducing fatigue, and supporting muscle function. Its benefits for metabolic health and endurance make it a promising option for athletes.
What are the main sources of MOTS-c in the body?
MOTS-c is produced within the mitochondria of cells throughout the body. Its production is influenced by various factors, including physical activity, diet, and overall metabolic health.
How does MOTS-c affect mitochondrial function?
MOTS-c supports mitochondrial function by promoting the efficient production of ATP, the energy currency of the cell. It helps maintain mitochondrial health and protect against oxidative damage.
Can MOTS-c be used to support immune function?
Research suggests that MOTS-c may support immune function by reducing inflammation and enhancing the body’s metabolic responses. Its role in cellular health also contributes to a more robust immune system.
What are the long-term benefits of using MOTS-c?
The long-term benefits of MOTS-c include improved metabolic health, better weight management, enhanced muscle function, and potential anti-aging effects. Continued research is needed to fully understand its long-term impact on various health outcomes.
Reference
Lee C, Kim KH, Cohen P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free RadicBiol Med. 2016;100:182–187. doi:10.1016/j.freeradbiomed.2016.05.015.
MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism
The study titled “MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism” was published in “Free Radical Biology & Medicine” in 2016 by Lee C, Kim KH, and Cohen P. The study investigates a mitochondrial-derived peptide called MOTS-c and its role in regulating metabolism, specifically in muscle and fat tissues.
Mitochondria, the energy-producing organelles within cells, are known to release various peptides that can influence cellular functions. MOTS-c is one such peptide that has been identified as having potential metabolic effects. The study aims to characterize MOTS-c and elucidate its mechanisms of action in muscle and fat metabolism.
The findings of this research suggest that MOTS-c plays a role in regulating glucose metabolism, insulin sensitivity, and energy expenditure in muscle and fat tissues. It may act as a signaling molecule that communicates between mitochondria and the nucleus to coordinate metabolic responses.
For more details https://www.sciencedirect.com/science/article/pii/S0891584916302507
Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766.
Mitohormesis
The article titled “Mitohormesis” was published in “Cell Metabolism” in 2014 by authors Yun J and Finkel T. The concept of mitohormesis explores the phenomenon where exposure to low levels of mitochondrial stress leads to beneficial effects on cellular health and longevity.
Mitochondria are essential organelles responsible for energy production in cells, but they also play a role in regulating cellular signaling pathways related to stress response, metabolism, and aging. Mitohormesis suggests that mild mitochondrial stress, such as that induced by moderate levels of reactive oxygen species (ROS) or by caloric restriction, can activate adaptive cellular pathways that enhance stress resistance and promote longevity.
The article discusses the mechanisms underlying mitohormesis, including the involvement of signaling pathways such as the AMP-activated protein kinase (AMPK) pathway, the sirtuin pathway, and the mitochondrial unfolded protein response (UPRmt). These pathways help cells adapt to stress by promoting mitochondrial biogenesis, improving mitochondrial function, and enhancing antioxidant defenses.
For more details https://www.cell.com/cell-metabolism/pdf/S1550-4131(14)00017-5.pdf
Thevis M, Schanzer W. Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis – Potential implications for sports drug testing programs. Rapid Commun Mass Spectrom. 2016;30(5):635–651.
Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis–Potential implications for sports drug testing programs
The article titled “Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis – Potential implications for sports drug testing programs” was published in “Rapid Communications in Mass Spectrometry” in 2016 by authors Thevis M and Schanzer W.
This article explores the potential impact of emerging drugs on skeletal muscle function and mitochondrial biogenesis, particularly in the context of sports drug testing programs.
Skeletal muscle function and mitochondrial biogenesis are crucial aspects of athletic performance, and athletes may seek to enhance these processes through various means, including the use of performance-enhancing drugs (PEDs). The article discusses the potential mechanisms of action and effects of emerging drugs that target skeletal muscle function and mitochondrial biogenesis, such as selective androgen receptor modulators (SARMs), peroxisome proliferator-activated receptor delta (PPARδ) agonists, and mitochondrial uncouplers.
For more details https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/rcm.7470
Merry TL, Ristow M. Mitohormesis in exercise training. Free RadicBiol Med. 2015
Mitohormesis in exercise training
The article titled “Mitohormesis in exercise training” was published in “Free Radical Biology & Medicine” in 2015 by authors Merry TL and Ristow M.
Mitohormesis refers to the concept that exposure to mild stressors, such as exercise-induced mitochondrial stress, can lead to beneficial adaptations in cells and tissues. This phenomenon has been observed in various contexts, including aging, calorie restriction, and exercise training.
In this article, the authors focus specifically on the role of mitohormesis in exercise training. They discuss how moderate levels of exercise-induced mitochondrial stress can activate adaptive cellular pathways that enhance mitochondrial biogenesis, improve mitochondrial function, and increase antioxidant defenses. These adaptations contribute to improved metabolic health, enhanced exercise performance, and protection against age-related diseases.
For more details https://www.sciencedirect.com/science/article/pii/S0891584915011417
Handschin C. Caloric restriction and exercise “mimetics”: ready for prime time? Pharmacol Res. 2015;103:158–166.
Caloric restriction and exercise “mimetics”: ready for prime time?
The article titled “Caloric restriction and exercise ‘mimetics’: ready for prime time?” was authored by Handschin C and published in “Pharmacological Research” in 2015.
This article explores the concept of caloric restriction and exercise mimetics, which are compounds that mimic the effects of caloric restriction and exercise on metabolism and healthspan. Caloric restriction and regular exercise are well-established interventions that have been shown to extend lifespan and improve metabolic health in various organisms, including humans.
The article discusses the potential benefits of caloric restriction and exercise mimetics in promoting health and longevity, particularly in the context of aging-related diseases such as obesity, type 2 diabetes, and cardiovascular disease. These compounds activate signaling pathways that regulate energy metabolism, mitochondrial function, and cellular stress response, leading to improvements in metabolic health and resilience to age-related stressors.
For more details https://www.sciencedirect.com/science/article/pii/S104366181530178X
Hunter P. Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome. EMBO Rep. 2016.
Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome
The article titled “Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome” was authored by Hunter P and published in “EMBO Reports” in 2016.
This article discusses the concept of elucidating the mechanisms by which exercise confers health benefits, with the goal of identifying new therapeutic options for various diseases, including cancer and metabolic syndrome. Exercise is well-known to have numerous health benefits, including improving cardiovascular health, enhancing metabolic function, and reducing the risk of chronic diseases. However, the precise mechanisms underlying these benefits are not fully understood.
The article highlights recent advances in understanding the molecular pathways and physiological processes involved in exercise-induced health benefits. These include improvements in mitochondrial function, regulation of inflammation and oxidative stress, and activation of signaling pathways such as AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α).
For more details https://www.embopress.org/doi/abs/10.15252/embr.201541835
Li S, Laher I. Exercise pills: at the starting line. Trends PharmacolSci. 2015.
Exercise pills: at the starting line
The article titled “Exercise pills: at the starting line” was authored by Li S and Laher I, and it was published in “Trends in Pharmacological Sciences” in 2015.
This article discusses the concept of “exercise pills,” which are pharmacological agents designed to mimic the beneficial effects of exercise on health and fitness. Exercise is known to have numerous health benefits, including improving cardiovascular health, enhancing metabolic function, and reducing the risk of chronic diseases. However, adherence to exercise regimens can be challenging for many individuals due to factors such as time constraints, physical limitations, or lack of motivation.
The article explores recent advances in understanding the molecular pathways and physiological processes involved in exercise-induced health benefits. These include improvements in mitochondrial function, regulation of inflammation and oxidative stress, and activation of signaling pathways such as AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α).
For more details https://www.sciencedirect.com/science/article/pii/S0891584916302507
Lee DE, et al. Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice. Acta Physiol. 2016.
Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice
The study titled “Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice” was conducted by Lee DE and colleagues. It was published in “Acta Physiologica” in 2016.
This study investigates the effects of physical activity on the translational machinery of mitochondrial mRNA in Western diet-induced obese mice. Western diet-induced obesity is associated with mitochondrial dysfunction, which contributes to metabolic disturbances and insulin resistance. Physical activity is known to have beneficial effects on mitochondrial function and metabolic health, but the underlying molecular mechanisms are not fully understood.
The researchers conducted experiments using Western diet-induced obese mice that were subjected to physical activity interventions. They assessed the expression and activity of the translational machinery involved in mitochondrial mRNA translation, including ribosomal proteins and initiation factors.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1111/apha.12687
Cataldo LR, Fernández-verdejo R, Santos JL, Galgani JE. Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J Investig Med. 2018;66(6):1019-1022.
Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals
The study titled “Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals” was conducted by Cataldo LR, Fernández-verdejo R, Santos JL, and Galgani JE. It was published in the “Journal of Investigative Medicine” in 2018.
This study aimed to investigate the association between plasma MOTS-c levels and insulin sensitivity in both lean and obese individuals. MOTS-c is a mitochondrial-derived peptide that has been implicated in the regulation of metabolism and insulin sensitivity.
The researchers measured plasma MOTS-c levels in a cohort of lean and obese individuals and assessed insulin sensitivity using standardized techniques. They then analyzed the relationship between MOTS-c levels and insulin sensitivity in both groups.
For more details https://journals.sagepub.com/doi/abs/10.1136/jim-2017-000681
Available from https://www.qscience.com/content/papers/10.5339/qfarc.2016.HBPP1855#abstract_content.
Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals
The article titled “Novel Mitochondrial-Derived Peptide MOTS-c Inhibits Adipogenesis through Down Regulation of Master Gene PPAR in Murine Adipocytes” investigates the effects of MOTS-c on adipocyte development and metabolism in cultured mouse adipocytes. It found that MOTS-c inhibits adipogenesis through down-regulation of multiple genes involved in the development of adipocytes.
Lee C, Zeng J, Drew BG, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–454. doi:10.1016/j.cmet.2015.02.009
The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance
The study titled “The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance” was conducted by Lee C, Zeng J, Drew BG, and colleagues. It was published in “Cell Metabolism” in 2015.
This study investigates the effects of MOTS-c, a mitochondrial-derived peptide, on metabolic homeostasis, obesity, and insulin resistance. The researchers conducted experiments using cell culture models and animal models to assess the metabolic effects of MOTS-c.
The findings of the study suggest that MOTS-c promotes metabolic homeostasis by enhancing glucose uptake, improving insulin sensitivity, and reducing adiposity. These effects contribute to a reduction in obesity and insulin resistance in animal models of metabolic dysfunction.
For more details https://www.cell.com/article/S1550-4131%252815%252900061-3/abstract
Lu H, Wei M, Zhai Y, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med. 2019;97(4):473-485.
MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction
The study titled “MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction” was conducted by Lu H, Wei M, Zhai Y, and colleagues. It was published in the “Journal of Molecular Medicine” in 2019.
This study investigates the role of MOTS-c peptide in regulating adipose tissue homeostasis and preventing metabolic dysfunction induced by ovariectomy, a surgical procedure that mimics menopause in female rodents. Menopause is associated with metabolic disturbances, including increased adiposity and insulin resistance.
The researchers conducted experiments using ovariectomized female rodents treated with MOTS-c peptide to assess its effects on adipose tissue function and metabolic parameters. They measured adipose tissue morphology, lipid metabolism, and insulin sensitivity in response to MOTS-c treatment.
For more details https://link.springer.com/article/10.1007/s00109-018-01738-w
Lee C, Zeng J, Drew BG, Sallam T, Martin‐Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial‐derived peptide MOTS‐c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454.
The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance
The study titled “The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance” was conducted by Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, and Cohen P. It was published in Cell Metabolism in 2015.
This study investigates the effects of MOTS-c, a mitochondrial-derived peptide, on metabolic homeostasis, obesity, and insulin resistance. Using cell culture models and animal models, the researchers found that MOTS-c enhances glucose uptake, improves insulin sensitivity, and reduces adiposity, ultimately leading to a reduction in obesity and insulin resistance in animal models of metabolic dysfunction.
The findings of this study suggest that MOTS-c has therapeutic potential for promoting metabolic health and mitigating obesity and insulin resistance. Further research is needed to elucidate the underlying mechanisms of action and explore the clinical applications of MOTS-c for the treatment of metabolic disorders.
For more details https://www.cell.com/article/S1550-4131%252815%252900061-3/abstract
Lee C, Kim KH, Cohen P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free RadicBiol Med. 2016;100:182–187. doi:10.1016/j.freeradbiomed.2016.05.015.
MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism
The study titled “MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism” was published in “Free Radical Biology & Medicine” in 2016 by Lee C, Kim KH, and Cohen P. The study investigates a mitochondrial-derived peptide called MOTS-c and its role in regulating metabolism, specifically in muscle and fat tissues.
Mitochondria, the energy-producing organelles within cells, are known to release various peptides that can influence cellular functions. MOTS-c is one such peptide that has been identified as having potential metabolic effects. The study aims to characterize MOTS-c and elucidate its mechanisms of action in muscle and fat metabolism.
The findings of this research suggest that MOTS-c plays a role in regulating glucose metabolism, insulin sensitivity, and energy expenditure in muscle and fat tissues. It may act as a signaling molecule that communicates between mitochondria and the nucleus to coordinate metabolic responses.
For more details https://www.sciencedirect.com/science/article/pii/S0891584916302507
Zempo, H., Kim, S. J., Fuku, N., Nishida, Y., Higaki, Y., Wan, J., Yen, K., Miller, B., Vicinanza, R., Miyamoto-Mikami, E., Kumagai, H., Naito, H., Xiao, J., Mehta, H. H., Lee, C., Hara, M., Patel, Y. M., Setiawan, V. W., Moore, T. M., Hevener, A. L., … Cohen, P. (2021). A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c. Aging, 13(2), 1692–1717. https://doi.org/10.18632/aging.202529.
A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c
The study titled “A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c” was conducted by Zempo H, Kim SJ, Fuku N, Nishida Y, Higaki Y, Wan J, Yen K, Miller B, Vicinanza R, Miyamoto-Mikami E, Kumagai H, Naito H, Xiao J, Mehta HH, Lee C, Hara M, Patel YM, Setiawan VW, Moore TM, Hevener AL, and Cohen P. It was published in Aging in 2021.
This study investigates a pro-diabetogenic mitochondrial DNA (mtDNA) polymorphism in the mitochondrial-derived peptide MOTS-c. The researchers found that this polymorphism is associated with an increased risk of diabetes and metabolic dysfunction. The findings highlight the importance of understanding genetic variations in MOTS-c and their implications for metabolic health and disease risk.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7880332/
Kim SJ, Miller B, Kumagai H, Yen K, Cohen P. MOTS-c: an equal opportunity insulin sensitizer. J Mol Med. 2019;97(4):487-490.
MOTS-c: an equal opportunity insulin sensitizer
The article titled “MOTS-c: an equal opportunity insulin sensitizer” was authored by Kim SJ, Miller B, Kumagai H, Yen K, and Cohen P. It was published in the “Journal of Molecular Medicine” in 2019.
This article discusses MOTS-c, a mitochondrial-derived peptide, as an insulin sensitizer with equal efficacy across different populations. MOTS-c has been shown to improve insulin sensitivity and glucose metabolism in various experimental models, regardless of age, sex, or genetic background.
The authors highlight the potential of MOTS-c as a therapeutic target for insulin resistance and metabolic disorders, emphasizing its broad applicability and effectiveness in diverse populations. They discuss the underlying mechanisms of action of MOTS-c and its implications for metabolic health.
For more details https://link.springer.com/article/10.1007/s00109-019-01758-0
Kong, B. S., Min, S. H., Lee, C., & Cho, Y. M. (2021). Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell reports, 36(4), 109447. https://doi.org/10.1016/j.celrep.2021.109447.
Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes
The study titled “Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes” was conducted by Kong BS, Min SH, Lee C, and Cho YM. It was published in Cell Reports in 2021.
This study investigates the role of mitochondrial-encoded MOTS-c in preventing pancreatic islet destruction in autoimmune diabetes. The researchers found that MOTS-c, which is encoded by mitochondrial DNA, protects pancreatic islets from destruction by autoimmune processes in a mouse model of autoimmune diabetes.
The findings suggest that MOTS-c has potential therapeutic applications for preventing or treating autoimmune diabetes by preserving pancreatic islet function and integrity. Further research is needed to elucidate the mechanisms underlying the protective effects of MOTS-c and to explore its clinical potential for the treatment of autoimmune diabetes.
For more details https://www.cell.com/cell-reports/pdf/S2211-1247(21)00864-0.pdf
Kumagai, H., Coelho, A. R., Wan, J., Mehta, H. H., Yen, K., Huang, A., Zempo, H., Fuku, N., Maeda, S., Oliveira, P. J., Cohen, P., & Kim, S. J. (2021). MOTS-c reduces myostatin and muscle atrophy signaling. American journal of physiology. Endocrinology and metabolism, 320(4), E680–E690. https://doi.org/10.1152/ajpendo.00275.2020.
MOTS-c reduces myostatin and muscle atrophy signaling. American journal of physiology
The study titled “MOTS-c reduces myostatin and muscle atrophy signaling” was conducted by Kumagai H, Coelho AR, Wan J, Mehta HH, Yen K, Huang A, Zempo H, Fuku N, Maeda S, Oliveira PJ, Cohen P, and Kim SJ. It was published in the “American Journal of Physiology. Endocrinology and Metabolism” in 2021.
This study investigates the effects of MOTS-c on myostatin signaling and muscle atrophy. Myostatin is a negative regulator of muscle growth, and its overexpression contributes to muscle wasting and atrophy. The researchers found that MOTS-c reduces myostatin expression and signaling, leading to attenuation of muscle atrophy in experimental models.
The findings suggest that MOTS-c has potential therapeutic applications for preventing or treating muscle wasting conditions by inhibiting myostatin signaling and preserving muscle mass. Further research is needed to elucidate the underlying mechanisms of MOTS-c action in muscle metabolism and to explore its clinical potential for the treatment of muscle disorders.
For more details https://journals.physiology.org/doi/abs/10.1152/ajpendo.00275.2020
Qin Q, Delrio S, Wan J, et al. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. International journal of cardiology. 2018; 254:23-27.
Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction
The study titled “Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction” was conducted by Qin Q, Delrio S, Wan J, and colleagues. It was published in the “International Journal of Cardiology” in 2018.
This study investigates the association between MOTS-c levels and coronary endothelial dysfunction in patients. Coronary endothelial dysfunction is characterized by impaired vasodilation of coronary arteries and is considered an early marker of cardiovascular disease.
The researchers found that circulating MOTS-c levels were significantly downregulated in patients with coronary endothelial dysfunction compared to healthy controls. This suggests that MOTS-c may play a role in the pathogenesis of coronary endothelial dysfunction and could serve as a potential biomarker for cardiovascular disease.
For more details https://www.sciencedirect.com/science/article/pii/S0167527317347915
Available from https://www.fasebj.org/doi/abs/10.1096/fasebj.31.1_supplement.1015.2.
Li H, Ren K, Jiang T, Zhao GJ. MOTS-c attenuates endothelial dysfunction via suppressing the MAPK/NF-κB pathway. Int J Cardiol. 2018;268:40.
MOTS-c attenuates endothelial dysfunction via suppressing the MAPK/NF-κB pathway
The research article titled “MOTS-c attenuates endothelial dysfunction via suppressing the MAPK/NF-κB pathway” investigates the role of the mitochondrial-derived peptide MOTS-c in reducing endothelial dysfunction. This study demonstrates that MOTS-c exerts a protective effect on endothelial cells primarily through the inhibition of the MAPK/NF-κB signaling pathway. By suppressing this pathway, MOTS-c potentially lowers inflammation and oxidative stress within the vascular system, offering insights into new therapeutic strategies for cardiovascular diseases associated with endothelial dysfunction. The findings contribute to a deeper understanding of the molecular mechanisms behind MOTS-c’s beneficial effects on vascular health.
For more details https://pubmed.ncbi.nlm.nih.gov/30041797/
Yuan, J., Wang, M., Pan, Y., Liang, M., Fu, Y., Duan, Y., Tang, M., Laher, I., & Li, S. (2021). The mitochondrial signaling peptide MOTS-c improves myocardial performance during exercise training in rats. Scientific reports, 11(1), 20077. https://doi.org/10.1038/s41598-021-99568-3.
The mitochondrial signaling peptide MOTS-c improves myocardial performance during exercise training in rats.
The study by Yuan et al. (2021) explored the effects of the mitochondrial signaling peptide MOTS-c on myocardial performance in rats undergoing exercise training. The research found that MOTS-c administration improved cardiac function and enhanced exercise performance, suggesting that this peptide plays a significant role in promoting cardiovascular health through its mitochondrial mechanisms. This highlights MOTS-c’s potential as a therapeutic agent for improving myocardial performance during physical activity.
For more details https://doi.org/10.1038/s41598-021-99568-3
Wei, M., Gan, L., Liu, Z., Liu, L., Chang, J. R., Yin, D. C., Cao, H. L., Su, X. L., & Smith, W. W. (2020). Mitochondrial-Derived Peptide MOTS-c Attenuates Vascular Calcification and Secondary Myocardial Remodeling via Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway. Cardiorenal medicine, 10(1), 42–50. https://doi.org/10.1159/000503224.
Mitochondrial-Derived Peptide MOTS-c Attenuates Vascular Calcification and Secondary Myocardial Remodeling via Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway
The study by Wei et al. (2020) investigates how the mitochondrial-derived peptide MOTS-c impacts vascular calcification and subsequent myocardial remodeling. It was found that MOTS-c, through the AMP-activated protein kinase signaling pathway, can significantly reduce vascular calcification, thereby preventing secondary changes in myocardial structure and function. This suggests MOTS-c’s potential therapeutic value for cardiovascular diseases linked to vascular calcification
For more details https://doi.org/10.1159/000503224
Yin, Y., Pan, Y., He, J., Zhong, H., Wu, Y., Ji, C., Liu, L., & Cui, X. (2022). The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacological research, 175, 105987. https://doi.org/10.1016/j.phrs.2021.105987.
The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus
The study conducted by Yin et al. (2022) delves into the therapeutic potential of the mitochondrial-derived peptide MOTS-c in addressing gestational diabetes mellitus (GDM), a condition characterized by hyperglycemia and insulin resistance during pregnancy. Their findings reveal that MOTS-c administration significantly mitigates these symptoms, offering a novel approach to manage GDM. By focusing on the mitochondrial pathways, this research underscores the importance of cellular metabolism in pregnancy-related diabetes and opens up new avenues for treatment strategies aimed at enhancing maternal and fetal health outcomes.
For more details https://doi.org/10.1016/j.phrs.2021.105987
Ming W, Lu G, Xin S, et al. Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. BiochemBiophys Res Commun. 2016;476(4):412-419.
Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation
The study by Ming W et al. explores the effects of the mitochondrial-derived peptide MOTS-c on bone health, specifically its ability to counteract bone loss induced by ovariectomy in a model system. They discovered that MOTS-c promotes bone preservation through the activation of the AMPK signaling pathway. This mechanism suggests that MOTS-c could be a potential therapeutic agent for treating osteoporosis, especially in postmenopausal women who are at increased risk due to hormonal changes. The findings are significant for understanding the molecular underpinnings of bone metabolism and developing new treatments for bone loss.
For more details https://www.sciencedirect.com/science/article/pii/S0006291X16308579
Hu BT, Chen WZ. MOTS-c improves osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via TGF-β/Smad pathway. Eur Rev Med Pharmacol Sci. 2018;22(21):7156-7163.
MOTS-c improves osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via TGF-β/Smad pathway
The study by Hu BT and Chen WZ investigates the role of MOTS-c in improving osteoporosis. It focuses on how MOTS-c enhances the osteogenic differentiation of bone marrow mesenchymal stem cells through the TGF-β/Smad signaling pathway. This pathway is crucial for bone formation and regeneration, indicating that MOTS-c could be a promising therapeutic agent for osteoporosis by promoting bone health at a cellular level. The research, published in the European Review for Medical and Pharmacological Sciences in 2018, provides valuable insights into osteoporosis treatment strategies.
For more details https://www.europeanreview.org/wp/wp-content/uploads/7156-7163.pdf
Che, N., Qiu, W., Wang, J. K., Sun, X. X., Xu, L. X., Liu, R., & Gu, L. (2019). MOTS-c improves osteoporosis by promoting the synthesis of type I collagen in osteoblasts via TGF-β/SMAD signaling pathway. European review for medical and pharmacological sciences, 23(8), 3183–3189. https://doi.org/10.26355/eurrev_201904_17676.
MOTS-c improves osteoporosis by promoting the synthesis of type I collagen in osteoblasts via TGF-β/SMAD signaling pathway
The study by Che et al. (2019) demonstrates that MOTS-c can effectively improve osteoporosis by enhancing the synthesis of type I collagen in osteoblasts. This process is facilitated through the activation of the TGF-β/SMAD signaling pathway, a critical route for bone formation and repair. This research presents MOTS-c as a potential therapeutic agent for osteoporosis by promoting bone density and health at the molecular level.
For more details https://www.europeanreview.org/wp/wp-content/uploads/3183-3189.pdf
Alexe G, et al. Enrichment of longevity phenotype in mtDNAhaplogroups D4b2b, D4a, and D5 in the Japanese population. Hum Genet. 2007;121(3–4):347–356.
Enrichment of longevity phenotype in mtDNA haplogroups D4b2b, D4a, and D5 in the Japanese population
The study by Alexe G. et al. (2007) highlights a significant correlation between specific mitochondrial DNA (mtDNA) haplogroups (D4b2b, D4a, and D5) and longevity in the Japanese population. This research suggests that individuals belonging to these haplogroups may have a genetic advantage contributing to longer life spans. The findings provide valuable insights into the genetic factors associated with aging and longevity.
For more details https://link.springer.com/article/10.1007/s00439-007-0330-6
Kim, K. H., Son, J. M., Benayoun, B. A., & Lee, C. (2018). The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell metabolism, 28(3), 516–524.e7. https://doi.org/10.1016/j.cmet.2018.06.008.
The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress
The study by Kim, K.H., et al. (2018) reveals that the mitochondrial-encoded peptide MOTS-c can move to the nucleus to influence nuclear gene expression in response to metabolic stress. This finding sheds light on the intricate mechanisms through which mitochondria communicate with the nucleus to regulate cellular responses under stress conditions, highlighting the versatility and significance of MOTS-c in cellular metabolism and stress response.
For more details https://doi.org/10.1016/j.cmet.2018.06.008
Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471.
NAD+ and sirtuins in aging and disease
Imai and Guarente (2014) discuss the crucial roles of NAD+ and sirtuins in the aging process and disease. They highlight how NAD+ serves as a vital cofactor for sirtuins, enzymes involved in cellular regulation. The review explores the impact of these molecules on metabolism, stress resistance, and longevity, suggesting their potential as therapeutic targets for age-related diseases.
For more details https://www.cell.com/trends/cell-biology/fulltext/S0962-8924(14)00063-4?elsca1=etoc&elsca2=email&elsca3=0962-8924_201408_24_8_&elsca4=Cell+Press
Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–1213.
NAD+ in aging, metabolism, and neurodegeneration
In the article by Verdin (2015), the importance of NAD+ in aging, metabolism, and neurodegeneration is discussed. Verdin outlines how NAD+ plays a pivotal role in regulating cellular and metabolic processes, impacting health span and susceptibility to various neurodegenerative diseases. This work emphasizes the potential therapeutic benefits of targeting NAD+ pathways to combat age-related decline and improve overall health outcomes.
For more details https://www.science.org/doi/abs/10.1126/science.aac4854
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217.
The hallmarks of aging
Lopez-Otin et al. (2013) present a comprehensive overview of the key biological mechanisms that contribute to aging. These include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Understanding these processes provides insights into the complex nature of aging and highlights potential therapeutic targets for age-related diseases.
For more details https://www.cell.com/fulltext/S0092-8674(13)00645-4?source=post_page—————————
Yu, W. D., Kim, Y. J., Cho, M. J., Seok, J., Kim, G. J., Lee, C. H., Ko, J. J., Kim, Y. S., & Lee, J. H. (2021). The mitochondrial-derived peptide MOTS-c promotes homeostasis in aged human placenta-derived mesenchymal stem cells in vitro. Mitochondrion, 58, 135–146. https://doi.org/10.1016/j.mito.2021.02.010.
The mitochondrial-derived peptide MOTS-c promotes homeostasis in aged human placenta-derived mesenchymal stem cells in vitro
The study by Yu et al. (2021) explores the effect of the mitochondrial-derived peptide MOTS-c on aged human placenta-derived mesenchymal stem cells (hPDMSCs) in vitro. They found that MOTS-c enhances cellular homeostasis in these cells, implying potential therapeutic benefits for aging-related degeneration and diseases. This research underscores the significance of mitochondrial peptides in maintaining stem cell function and offers insights into developing regenerative medicine strategies.
For more details https://doi.org/10.1016/j.mito.2021.02.010
Lee C, Kim KH, Cohen P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free RadicBiol Med. 2016;100:182–187. doi:10.1016/j.freeradbiomed.2016.05.015.
MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism.
The study by Lee, Kim, and Cohen (2016) delves into the discovery and functional roles of MOTS-c, a mitochondrial-derived peptide, in the regulation of muscle and fat metabolism. This research uncovers MOTS-c’s significant effects on metabolic pathways, suggesting its potential as a therapeutic target for addressing metabolic diseases. The findings contribute to our understanding of mitochondrial peptides in metabolic regulation, opening new avenues for treatment strategies in metabolic disorders.
For more details https://www.sciencedirect.com/science/article/pii/S0891584916302507
Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766.
Mitohormesis
Yun and Finkel’s article on “Mitohormesis” (2014) explores the concept that mild mitochondrial stress can induce a beneficial adaptive response, enhancing cellular and organismal performance and longevity. This process involves the activation of stress response pathways that improve cellular function and resistance to further stress, suggesting potential strategies for promoting health and longevity through controlled mitochondrial stress induction.
For more details https://www.cell.com/cell-metabolism/pdf/S1550-4131(14)00017-5.pdf
Thevis M, Schanzer W. Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis – Potential implications for sports drug testing programs. Rapid Commun Mass Spectrom. 2016;30(5):635–651.
Emerging drugs affecting skeletal muscle function and mitochondrial biogenesis – Potential implications for sports drug testing programs
Thevis and Schänzer discuss emerging drugs that influence skeletal muscle function and stimulate mitochondrial biogenesis, highlighting their potential impact on sports drug testing programs. These substances, which can enhance athletic performance by improving muscle strength and energy production, pose challenges for anti-doping efforts. The article emphasizes the need for updated detection methods to address these advancements.
For more details https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/abs/10.1002/rcm.7470
Merry TL, Ristow M. Mitohormesis in exercise training. Free RadicBiol Med. 2015.
Mitohormesis in exercise training
Merry and Ristow (2015) delve into the concept of mitohormesis in the context of exercise training, explaining how physical activity induces mild stress on mitochondria, leading to beneficial adaptations in muscle cells. This adaptive response enhances cellular defense mechanisms and metabolic efficiency, contributing to improved health and longevity. The paper highlights the significance of this process in designing effective exercise programs for health promotion.
For more details https://www.sciencedirect.com/science/article/pii/S0891584915011417
Handschin C. Caloric restriction and exercise “mimetics”: ready for prime time? Pharmacol Res. 2015;103:158–166.
Caloric restriction and exercise “mimetics”: Ready for prime time?
Handschin (2015) evaluates the potential of caloric restriction and exercise mimetics, substances that mimic the effects of dieting and physical activity on health, for mainstream use. He discusses their mechanisms, impacts on metabolism and muscle function, and the scientific evidence supporting their benefits and limitations. This review considers whether these mimetics can realistically replicate the health benefits of exercise and caloric restriction without the associated lifestyle changes.
For more details https://www.sciencedirect.com/science/article/pii/S104366181530178X
Hunter P. Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome. EMBO Rep. 2016.
Exercise in a bottle: elucidating how exercise conveys health benefits might lead to new therapeutic options for a range of diseases from cancer to metabolic syndrome
Hunter (2016) discusses the pursuit of understanding how physical exercise delivers its health benefits, which could pave the way for new treatments across various diseases, including cancer and metabolic syndrome. The article explores the potential for “exercise mimetics,” drugs that could simulate the effects of exercise, offering therapeutic options for those unable to engage in physical activity. This research could revolutionize treatment strategies for chronic diseases by encapsulating the benefits of exercise into pharmacological forms.
For more details https://www.embopress.org/doi/abs/10.15252/embr.201541835
Li S, Laher I. Exercise pills: at the starting line. Trends PharmacolSci. 2015.
Exercise pills: at the starting line.
Li and Laher (2015) explore the development of “exercise pills,” a concept for pharmacological agents designed to mimic the health benefits of physical exercise. These pills aim to offer an alternative for improving health in individuals unable to perform regular physical activity due to various constraints. This innovative approach could potentially revolutionize the management of diseases associated with sedentary lifestyles by providing the metabolic and physiological advantages of exercise through medication.
For more details https://www.cell.com/fulltext/S0165-6147(15)00187-X
Lee DE, et al. Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice. Acta Physiol. 2016.
Translational machinery of mitochondrial mRNA is promoted by physical activity in Western diet-induced obese mice
Lee DE and colleagues (2016) examine the impact of physical activity on the translational machinery of mitochondrial mRNA in mice fed a Western diet, leading to obesity. The study suggests that exercise enhances the efficiency of mitochondrial protein synthesis, potentially counteracting the negative metabolic effects of a high-fat diet. This research underscores the importance of physical activity in maintaining mitochondrial function and overall metabolic health in the context of obesity.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1111/apha.12687
Reynolds, J. C., Lai, R. W., Woodhead, J., Joly, J. H., Mitchell, C. J., Cameron-Smith, D., Lu, R., Cohen, P., Graham, N. A., Benayoun, B. A., Merry, T. L., & Lee, C. (2021). MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nature communications, 12(1), 470. https://doi.org/10.1038/s41467-020-20790-0.
MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis
The research by Reynolds et al. (2021) highlights that the mitochondrial-encoded peptide MOTS-c is not only upregulated by exercise but also plays a pivotal role in combating age-associated physical decline and preserving muscle health. Their findings point towards the significance of MOTS-c in the broader context of exercise physiology and aging, indicating its potential as a therapeutic target for enhancing muscle function and delaying the onset of age-related physical limitations.
For more details https://doi.org/10.1038/s41467-020-20790-0
Zarse K, Ristow M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015;21(3):355–356.
A mitochondrially encoded hormone ameliorates obesity and insulin resistance
In their commentary, Zarse and Ristow (2015) highlight the identification of a hormone encoded within mitochondrial DNA, emphasizing its significant impact on mitigating obesity and insulin resistance. This groundbreaking finding illuminates a novel pathway for developing treatments aimed at metabolic disorders, suggesting a potential shift in how these conditions might be managed in the future. The research underscores the intricate relationship between mitochondrial function and systemic metabolic health. For a thorough review, the article can be found in Cell Metabolism.
For more details https://www.cell.com/cell-metabolism/pdf/S1550-4131(15)00065-0.pdf
Li S, Laher I. Exercise pills: at the starting line. Trends Pharmacol Sci. 2015;36(12):906–917.
Exercise pills: at the starting line
Li and Laher (2015) explore the concept of “exercise pills,” which are pharmacological agents designed to mimic the health benefits of physical activity. These pills represent an innovative approach to improving health and managing diseases associated with sedentary lifestyles, potentially offering the metabolic, cardiovascular, and muscular benefits of exercise to those unable to engage in traditional physical activities. The article discusses the potential mechanisms, challenges, and future directions in the development of these mimetics.
For more details https://www.cell.com/fulltext/S0165-6147(15)00187-X
Fuku N, et al. The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell. 2015;14(6):921–923.
The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell
Fuku et al. (2015) discuss the mitochondrial-derived peptide MOTS-c and its potential role in promoting exceptional longevity. They explore how MOTS-c might influence aging processes and contribute to the extended lifespan observed in some individuals. This peptide’s function in metabolic regulation and its implications for health span extension present intriguing possibilities for understanding the mechanisms behind aging and longevity.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1111/acel.12389
Merry TL, Ristow M. Mitohormesis in exercise training. Free RadicBiol Med. 2015.
Mitohormesis in exercise training
Merry and Ristow (2015) delve into the concept of mitohormesis, highlighting its role in exercise training. They propose that low doses of mitochondrial stress, induced by exercise, can activate signaling pathways that ultimately improve cellular and systemic health. This adaptive response to mild stress enhances metabolic efficiency and resistance to diseases, pointing to potential therapeutic strategies that mimic the health benefits of physical activity.
For more details https://www.sciencedirect.com/science/article/pii/S0891584915011417
Handschin C. Caloric restriction and exercise “mimetics”: ready for prime time? Pharmacol Res. 2015;103:158–166.
Caloric restriction and exercise “mimetics”: Ready for prime time?
Handschin (2015) evaluates the potential of caloric restriction and exercise mimetics, exploring whether these substances, designed to mimic the health benefits of diet and physical activity, are ready for widespread use. The discussion includes their mechanisms, benefits, and challenges, pointing toward their future in health and disease management.
For more details https://www.sciencedirect.com/science/article/pii/S104366181530178X
Yang, B., Yu, Q., Chang, B., Guo, Q., Xu, S., Yi, X., & Cao, S. (2021). MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochimica et biophysica acta. Molecular basis of disease, 1867(6), 166126. https://doi.org/10.1016/j.bbadis.2021.166126.
MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway
Yang, Yu, Chang, Guo, Xu, Yi, and Cao (2021) explore how the mitochondrial-derived peptide MOTS-c, combined with exercise, influences glucose metabolism and insulin resistance in mice. Their findings suggest that MOTS-c and exercise synergistically increase PGC-1α expression via the AMPK signaling pathway, improving insulin sensitivity and glucose management. This study underscores the potential of combining MOTS-c with physical activity for treating metabolic disorders. For more details, see the article in Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease.
For more details https://www.sciencedirect.com/science/article/pii/S0925443921000594
Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273(6 Pt 1):E1107–E1112.
AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle
Merrill, Kurth, Hardie, and Winder (1997) conducted a study that demonstrated the effects of AICA riboside on increasing AMP-activated protein kinase (AMPK) activity, fatty acid oxidation, and glucose uptake in rat muscle. This research contributes to understanding how AICA riboside can influence metabolic pathways, potentially offering insights into treatments for metabolic disorders.
For more details https://journals.physiology.org/doi/abs/10.1152/ajpendo.1997.273.6.e1107
Narkar VA, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–415.
AMPK and PPARdelta agonists are exercise mimetics
Narkar et al. (2008) explored how activating AMPK and PPARδ through specific agonists could replicate the physiological benefits of exercise, such as improved endurance and metabolic health, in a study that underscores the potential of these compounds as therapeutic options for individuals who are unable to engage in physical activity due to health constraints. This groundbreaking work opens avenues for medical treatments aimed at mimicking exercise’s beneficial effects, offering hope for enhancing physical fitness and combating metabolic diseases through pharmacological means.
For more details https://www.cell.com/fulltext/S0092-8674(08)00838-6
Fujii N, et al. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. BiochemBiophys Res Commun. 2000;273(3):1150–1155.
Exercise induces isoform-specific increase in 5′ AMP-activated protein kinase activity in human skeletal muscle
The study by Fujii et al. (2000) focuses on how physical activity impacts AMP-activated protein kinase (AMPK) in human skeletal muscle, highlighting that exercise triggers a selective increase in specific AMPK isoforms. This distinction is crucial for understanding the muscle’s metabolic response to exercise, illustrating how different AMPK isoforms contribute to the body’s adaptation to physical activity, potentially influencing fitness and metabolic health. Their research provides a deeper insight into the molecular pathways activated by exercise.
For more details https://www.sciencedirect.com/science/article/pii/S0006291X00930730
Zhai D, Ye Z, Jiang Y, et al. MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. MolImmunol. 2017;92:151-160.
MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA
In the study conducted by Zhai et al. (2017), the researchers investigated the potential of the mitochondrial-derived peptide MOTS-c as a therapeutic agent against Methicillin-resistant Staphylococcus aureus (MRSA) infection in mice. Their findings revealed that treatment with MOTS-c led to a significant improvement in survival rates among infected mice. Furthermore, MOTS-c administration resulted in a notable reduction in bacterial load in the infected animals.
These promising results suggest that MOTS-c may possess antimicrobial properties and could be a valuable addition to the arsenal of treatments for MRSA infections, which are notoriously difficult to manage due to their resistance to many antibiotics. This study highlights the potential of MOTS-c as a novel therapeutic agent for combating bacterial infections and underscores the importance of further research to explore its mechanisms of action and potential clinical applications in infectious diseases.
For more details https://www.sciencedirect.com/science/article/pii/S0161589017305461
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

