Health Library
Methylene Blue
Author: Dr. George Shanlikian, M.D. | Last Updated: November 30th, 2024
- Home
- >
- Health Library
- >
- Methylene Blue
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Methylene Blue
- Key Takeaways of Methylene Blue
- What is Methylene Blue?
- How does Methylene Blue Work?
- Chemical Structure of Methylene Blue
- Research on Methylene Blue Benefits
- Methylene Blue Uses
- Methylene Blue Contraindications
- Intravenous Methylene Blue vs Oral Methylene Blue Treatment
- Methylene Blue Side Effects
- Methylene Blue Dosage
- FAQs
- Blogs
- Reference
Table of Contents
- Overall Health Benefits of Methylene Blue
- Key Takeaways of Methylene Blue
- What is Methylene Blue?
- How does Methylene Blue Work?
- Chemical Structure of Methylene Blue
- Research on Methylene Blue Benefits
- Methylene Blue Uses
- Methylene Blue Contraindications
- Intravenous Methylene Blue vs Oral Methylene Blue Treatment
- Methylene Blue Side Effects
- Methylene Blue Dosage
- FAQs
- Blogs
- Reference
Overall Health Benefits of Methylene Blue
Methylene blue offers a wide range of benefits, including producing anti-aging effects, improving cognitive function, and reducing mortality and morbidity. It also plays a role in enhancing mood, aiding in the treatment of conditions like methemoglobinemia and malaria, improving skin health, and contributing to the fight against infectious diseases and cancer, making it a versatile and valuable compound in various medical applications.
- Produces anti-aging effects [1-11]
- Improves cognitive function [12-40]
- Reduces mortality and morbidity [41-50]
- Improves mood [51-58]
- Improves skin health [59-61]
- Fights various infectious diseases [62-73]
- Fights cancer and aids in cancer treatment [74-77]
- Helps treat malaria [78-82]
- Helps treat methemoglobinemia [83-85]
Key Takeaways of Methylene Blue
- Methylene blue is a synthetic chemical compound that has been used for centuries as a dye and stain.
- It has been studied for its potential health benefits, including antioxidant, anti-inflammatory, anticancer, neuroprotective, and metabolic effects.
- Methylene blue is generally safe when used as directed, but it can cause some side effects depending on the route of administration (by mouth or injection).
- The dosage of methylene blue depends on the condition being treated. It is important to follow the directions on the label or the instructions of your doctor.
What is Methylene Blue?

Methylene blue, also known as methylthioninium chloride, is a synthetic dye that has been widely used for various purposes, including as a stain in histology and microbiology. Beyond its traditional applications, methylene blue has found its way into the realm of health and medicine due to its unique properties.
Methylene blue was first prepared in 1876 by Heinrich Caro, a German chemist. This medication has a variety of medical and industrial applications. It is on the List of Essential Medicines by the World Health Organization (WHO), which means that it is considered to be an essential medication for a basic health system.
4. How does Methylene Blue Work?
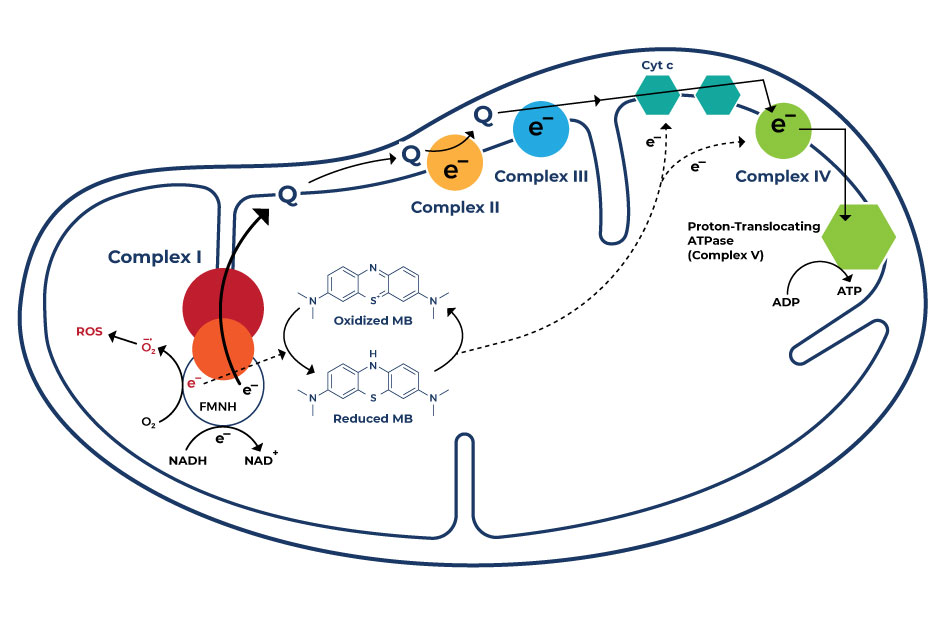
Methylene blue exerts its effects through several mechanisms due to its unique chemical properties. It interacts with various cellular components, leading to a range of potential benefits:
- Mitochondrial Function: Methylene blue acts as an alternative electron acceptor by facilitating electron transfer in the mitochondrial respiratory chain. It bypasses certain complex deficiencies, enhancing electron flow and preventing electron leakage, which reduces oxidative stress. This mechanism ultimately improves mitochondrial function, promoting cellular energy production and potentially offering therapeutic benefits for neurodegenerative conditions.
- Antioxidant Activity: Methylene blue acts as an antioxidant, meaning it can neutralize harmful molecules called free radicals that can damage cells and contribute to aging and disease. By scavenging free radicals, it helps protect cells from oxidative stress and potential damage.
- Nitric Oxide Regulation: Methylene blue can influence the regulation of nitric oxide, a molecule involved in various physiological processes, including blood vessel dilation. By modulating nitric oxide levels, it might have implications for cardiovascular health and blood flow regulation.
- Neuroprotection: Some studies suggest that methylene blue may offer neuroprotective effects. It can help preserve nerve cells’ viability and function, potentially reducing the risk of neurodegenerative disorders.
- Cognitive Enhancement: Research indicates that methylene blue might enhance cognitive function by increasing blood flow to the brain and potentially improving memory and attention.
- Antimicrobial Action: Methylene blue’s ability to interact with cellular components might contribute to its antimicrobial properties. This medication could disrupt bacterial or microbial functions, making it a potential adjunct to traditional antimicrobial therapies.
Chemical Structure of Methylene Blue
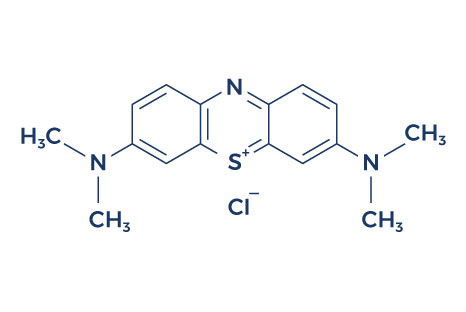
Research on Methylene Blue Benefits
A. Produces Anti-Aging Effects

Numerous studies provide empirical evidence supporting the potential anti-aging effects attributed to methylene blue:
- Methylene blue (MB) has gained attention for its potential to counteract brain aging, as oxidative metabolism plays a pivotal role in sustaining brain activity. Mitochondrial dysfunction, implicated in neuronal loss and brain diseases like Alzheimer’s and Parkinson’s, underscores the significance of MB’s role. Its lipophilic nature facilitates efficient blood-brain barrier penetration, with higher brain concentration observed after oral or intravenous administration in rats, suggesting its promising anti aging effects. [1]
- The process of aging is closely linked to the occurrence of mitochondrial dysfunction, concomitant with heightened levels of free radical production. Methylene blue (MB) exhibits a notable attraction to mitochondria. In contrast to alternative antioxidants like MitoQ and MitoVitE, MB has the capacity to diminish the generation of free radicals not through radical scavenging, but by circumventing the activity of Complex I/III. Notably, in mice and rats, MB can partially reinstate the membrane potential in mitochondria where Complex III function is hindered. [2]
- Mitochondria, the cellular powerhouses, play a crucial role in maintaining brain health by producing dopamine, a chemical essential for proper brain function. Dysfunction in these mitochondria due to harmful free radicals can lead to the demise of dopamine-dependent brain cells. This is especially concerning in Parkinson’s disease, an age-related neurodegenerative condition. Interestingly, methylene blue, recognized for enhancing mitochondrial function, shows promise as a potential therapy for Parkinson’s disease, as observed in rat models exposed to rotenone, a substance simulating the disease’s effects. [3-4] These models demonstrated reduced loss of dopamine-producing brain cells and improved motor abilities after the treatment, suggesting a potential avenue for addressing Parkinson’s disease.
- In the context of human IMR90 fibroblasts, MB and related diaminophenothiazines extend cellular lifespan by over 20 population doublings, or PDLs. [5] Operating at nanomolar levels, MB effectively postpones cellular senescence by enhancing mitochondrial function, leading to a 30% increase in mitochondrial complex IV and a substantial rise in cellular oxygen consumption by 37-70%. Moreover, MB amplifies heme synthesis, counteracts premature senescence caused by factors like H2O2 or cadmium, and prompts the activation of phase-2 antioxidant enzymes in hepG2 cells. The process of MB transformation between its oxidized and reduced states, facilitated by flavin-dependent enzymes and cytochrome c, contributes to its protective role. This dynamic cycling potentially inhibits mitochondrial oxidant production, mitigating mitochondrial dysfunction and oxidative stress — crucial factors implicated in cellular senescence and aging.
- A study investigated the effects of methylene blue (MB) on mitochondrial function in diabetic rat hearts. [6] Researchers observed that MB enhanced mitochondrial respiration and reduced oxidative stress, with benefits dependent on the substrate type. These results indicate MB’s potential as a therapeutic agent to improve cardiac mitochondrial function in diabetes.
- Research explored methylene blue (MB) as an anti-aging agent, focusing on its ability to enhance mitochondrial function, reduce oxidative stress, and improve cellular health. [7] The findings highlighted MB’s promise in mitigating age-related cellular decline, supporting its potential applications in anti-aging therapies.
- A study examined the neuroprotective effects of methylene blue (MB) in a Parkinson’s disease model. [8] The research found that MB activated the Nrf2 pathway, reducing oxidative stress and improving mitochondrial function in neurons exposed to MPP+ toxicity. These outcomes suggest MB as a potential therapeutic for neurodegenerative diseases.
- Research explored the impact of methylene blue (MB) on mitochondrial dysfunction in rat kidneys during acute pancreatitis. [9] The study demonstrated that MB treatment mitigated mitochondrial damage, indicating a protective role for MB in managing renal injury associated with acute pancreatitis.
- A review examined the protective potential of methylene blue (MB) in Alzheimer’s disease, highlighting its role in supporting mitochondrial function and enhancing cytochrome c oxidase activity. [10] The analysis underscored MB’s therapeutic promise in addressing mitochondrial dysfunction in neurodegenerative conditions.
- Researchers investigated the effects of methylene blue (MB) and mitoquinone on skeletal aging by targeting mitochondrial dysfunction. [11] The study revealed that both compounds improved mitochondrial health and reduced markers of aging in skeletal tissues, suggesting their potential as therapies for skeletal aging-related issues.
B. Improves Cognitive Function
According to studies, methylene blue has demonstrated the potential to enhance cognitive function via several important mechanisms:
- A study highlighted the promising benefits of methylene blue on cognitive function. [12] Methylene blue works in a unique way, not relying on traditional drug-receptor interactions. At low doses, it acts as an electron cycler in the mitochondria, the energy powerhouses of cells, boosting antioxidant properties and cell respiration. This enhances nervous system function and memory consolidation, offering potential memory improvement.
- A study demonstrated that methylene blue (MB) holds promise in improving cognitive function, especially in conditions linked to reduced blood flow to the brain. [13] Rats with decreased blood flow to the brain showed poorer performance in memory-related tasks, but those treated with daily doses of MB exhibited better memory and learning abilities.
- In recent studies, researchers have found that a substance called MB holds promise for protecting the brain, especially by helping the energy factories in our cells, known as mitochondria, work better. [14] This is important for keeping our brain cells healthy. The review also talks about how MB might help the brain make new cells and boost thinking abilities, especially as we get older and our thinking skills decline.
- Scientists found that methylene blue can act like an antidepressant, something that helps reduce anxiety, and a protector for the brain. [15] They’ve seen these effects in both animals and humans. One way it helps is by keeping the energy factories in our cells stable and by controlling harmful molecules that can damage our cells.
- To comprehend how methylene blue affects Alzheimer’s disease’s progression and uncover its working mechanism, researchers used mice with a condition resembling Alzheimer’s disease. [16] Their study showed that a consistent diet of methylene blue decreased harmful substances associated with Alzheimer’s disease and enhanced the mice’s ability to learn and remember. The way methylene blue seemed to work against these harmful substances involved improving the brain’s waste disposal system.
- In a study using rats with symptoms of hepatic encephalopathy (a loss of brain function due to impaired removal of toxins from the blood due to liver damage), researchers tried two treatments: one involving a blue dye called methylene blue (MB) and another using a specific type of light on the head (photobiomodulation or PBM). [17] The results showed that rats with HE-like symptoms treated with methylene blue injection exhibited improved memory, as evidenced by improved performance in water maze tests, compared with rats treated with PBM.
- A review of studies reported that methylene blue (MB) is a chemical that can affect different parts of our nervous system by interacting with various molecules involved in how our nerves work. [18] This happens because of its special properties, like how it behaves when it comes to sharing or receiving electrons, its electric charge, and the kind of light it interacts with.
- A group of researchers did a study with healthy adults to see if a small amount of methylene blue (MB) taken by mouth could change how different parts of the brain communicate. [19] They used brain scans while the participants were doing tasks and when they were just resting, both before and after they took MB or a fake pill (placebo). The researchers found that oral methylene blue made some parts of the brain work less during a task, and it made other parts of the brain associated with visuomotor tasks, perception, and memory functions communicate better when not doing anything.
- A study focused on a potential way to protect and enhance the brain using a specific part of our cells called mitochondria, which are like tiny power plants for the brain. [20] The researchers suggested that improving the way these power plants function could be effectively used to treat diseases like Alzheimer’s, which affect memory and brain function. They have three ideas for how to do this: using certain drugs, using special types of light, and changing our diet to include specific substances.
- A substance called methylene blue (MB) was tested in a new study to see if it could help with memory problems in Alzheimer’s patients. [21] The study showed that MB can make brain cells healthier by reducing the harmful Aβ and by making sure amyloid-binding alcohol dehydrogenase (ABAD) doesn’t cause damage to the brain cells. It was tested on mice with brain inflammation and the results were promising. MB not only helped the brain cells survive better, but it also made sure that ABAD didn’t cause issues and improved the levels of another substance called estradiol that’s important for the brain.
- A study provided a recent overview of how low amounts of methylene blue and near-infrared light can protect the brain. [22] The study also explained that even though these two methods are different, they both work in the same way by boosting the energy production in cell parts called mitochondria, which helps the brain stay healthy.
- A group of researchers investigated how methylene blue, when ingested orally, impacts the brain’s capacity to maintain focus over extended periods and enhance memory in healthy individuals. [23] Employing specialized brain scans, the researchers observed the participants’ brain activity while they undertook tasks assessing focus and memory before and after taking the substance or placebo. Upon analyzing the findings, the researchers discovered that individuals who consumed methylene blue exhibited increased brain activity when concentrating and attempting to recall information, as opposed to those who received the placebo. Furthermore, the participants’ ability to remember information also demonstrated slight improvement.
- A study reviewed how methylene blue (MB) impacts different parts of cells and the brain, and how this could be useful for Alzheimer’s disease (AD). [24] It was found that MB can help reduce the buildup of harmful proteins in the brain, improve cell energy production, and influence important brain chemicals. These effects combined might be why MB could be helpful for AD. Scientists are now trying to create new treatments for AD based on these findings.
- Previous studies have shown that macroautophagy (a fundamental cellular process that involves the degradation and recycling of cellular components) helps cells stay healthy. In a current study, the researchers showed that methylene blue can safeguard brain cells from dying when they lack essential nutrients, and this protection seems to happen alongside macroautophagy. [25] Interestingly, the researchers found that if they stop macroautophagy, the protective effect of methylene blue goes away.
- The use of methylene blue for treating ifosfamide-induced encephalopathy (a side effect of the chemotherapy drug ifosfamide that causes brain dysfunction) was evaluated through a search of MEDLINE and International Pharmaceutical Abstracts. [26] The compound seemed to rapidly alleviate cognitive symptoms of ifosfamide-induced encephalopathy but demonstrated modest efficacy overall.
- Researchers reviewed the therapeutic potential of methylene blue in treating traumatic brain injury, brain ischemia, and Alzheimer’s disease. [27] The study highlighted methylene blue’s neuroprotective properties, including its ability to enhance mitochondrial function and reduce oxidative stress. These findings suggest that methylene blue could be a promising candidate for managing various neurodegenerative conditions.
- A study investigated the molecular pathways through which methylene blue exerts its neuroprotective effects. [28] The researchers found that methylene blue modulates mitochondrial activity, reduces reactive oxygen species, and influences neurotransmitter systems. These mechanisms collectively contribute to its potential in preventing neuronal damage.
- In a randomized controlled trial, scientists examined the impact of methylene blue on brain functional connectivity using fMRI. [29] The results demonstrated that methylene blue administration led to increased connectivity in brain regions associated with memory and attention. This suggests its potential role in enhancing cognitive functions.
- Researchers explored the effects of methylene blue on Alzheimer’s disease pathology in transgenic mice. [30] The study revealed that methylene blue inhibited β-secretase activity, reduced amyloid plaque formation, and improved cognitive performance in the mice. These findings indicate its potential as a therapeutic agent for Alzheimer’s disease.
- A study investigated methylene blue’s protective effects on the blood-brain barrier during ischemia/reperfusion injury. [31] The findings showed that methylene blue preserved the integrity of the blood-brain barrier and reduced neuronal damage. This suggests its potential in mitigating brain injuries caused by ischemic events.
- Researchers explored how methylene blue enhances memory and provides neuroprotection by influencing neurometabolic pathways. [32] The study demonstrated that methylene blue improves mitochondrial function, leading to increased energy production in neurons. These findings suggest potential therapeutic applications for methylene blue in neurodegenerative diseases.
- A study investigated the effects of methylene blue on postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery. [33] The results indicated that methylene blue administration significantly reduced the incidence of early postoperative cognitive dysfunction. This suggests methylene blue could be beneficial in preserving cognitive function post-surgery in elderly patients.
- In a study, researchers examined the impact of administering methylene blue after exposure therapy sessions on fear extinction and contextual memory in adults with claustrophobia. [34] The findings revealed that methylene blue enhanced the retention of fear extinction and improved contextual memory. This suggests its potential as an adjunctive treatment in exposure-based therapies for anxiety disorders.
- A commentary discussed the methodological considerations of using intravenous methylene blue to mitigate postoperative cognitive disorders in elderly patients. [35] The author emphasized the importance of dosage, timing, and patient selection to maximize the therapeutic benefits of methylene blue. This highlights the need for careful planning in clinical applications to ensure efficacy and safety.
- Researchers found that methylene blue administration protected aged mice from cognitive dysfunction induced by sevoflurane anesthesia. [36] The protective effect was attributed to the suppression of Drp1 deSUMOylation, a process involved in mitochondrial dynamics. This study suggests methylene blue’s potential in preventing anesthesia-related cognitive impairments in the elderly.
- Researchers investigated the effects of preventive methylene blue treatment on cognitive function in mice expressing human Tau protein associated with neurodegenerative diseases. [37] The results showed that methylene blue preserved cognitive abilities and reduced Tau pathology in these mice. This indicates its potential as a preventive treatment for Tau-related neurodegenerative conditions.
- Researchers examined the effects of methylene blue on rats subjected to chronic cerebral hypoperfusion, a condition that mimics vascular dementia. [38] The study found that methylene blue preserved cytochrome oxidase activity, prevented neurodegeneration, and maintained memory function in these rats. These findings suggest its potential in treating vascular-related cognitive impairments.
- A study looked into the combined use of focused ultrasound and methylene blue to enhance drug delivery across the blood-brain barrier in a model of Alzheimer’s disease. [39] The treatment led to reduced neural damage and decreased amyloid-beta plaques, associated with upregulation of AQP-4. This approach shows promise for improving therapeutic outcomes in neurodegenerative diseases.
- In a study, researchers evaluated both therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in transgenic mice. [40] The findings demonstrated that methylene blue treatment reduced amyloid plaques and tau tangles, leading to improved cognitive function. This suggests its potential as a treatment strategy for Alzheimer’s disease.
C. Reduces Mortality and Morbidity

Research indicates that methylene blue has been found to lower both mortality and morbidity rates in individuals affected by a range of medical conditions:
- A study looked at patients who experienced low blood pressure after heart surgery, a condition known as cardiac postoperative vasoplegia or vasoplegic syndrome. [41] They found that around 8.8% of these patients had this problem. Unfortunately, it led to higher rates of death (10.7% of those with the issue compared to 3.6% of others). However, when they gave some patients methylene blue, the death rate was much lower (0% vs. 21.4%). Plus, the recovery time was faster with the drug – less than 6 hours compared to more than 48 hours for those who didn’t get it.
- Shock that doesn’t respond to fluid and catecholamine therapy is a serious issue with notable harm and fatalities in children. To address this, a study aimed to gather and summarize existing literature while also understanding how doctors employ methylene blue for treating shock in children. [42] They systematically searched various databases for studies involving methylene blue in catecholamine-resistant shock up to 2019. After assessing numerous studies, it was observed that methylene blue generally appeared safe and helped raise blood pressure in refractory shock scenarios caused by various factors.
- A systematic review and meta-analysis aimed to assess the effectiveness and safety of methylene blue (MB) in patients with vasodilatory shock. [43] After analyzing 15 studies encompassing 832 patients, it was found that administering MB alongside vasopressors significantly decreased mortality rates and reduced the need for vasopressors. Additionally, MB improved hemodynamics by increasing mean arterial pressure, heart rate, and peripheral vascular resistance. Organ function was positively impacted as well, with a lower incidence of renal failure, and oxygen metabolism was improved through reduced lactate levels. Notably, no serious side effects that require medical attention were observed. These findings suggest that combining MB with vasopressors can enhance survival, hemodynamics, and organ function in vasodilatory shock patients.
- Several studies found that methylene blue has been linked to increased mean arterial pressure, decreased need for other medications, reduced length of hospital stay, and improved heart function and oxygenation in patients with septic shock. [44-48]
- A study evaluated the effectiveness of methylene blue (MB) in treating vasoplegia (a condition of low blood pressure despite medication) following cardiopulmonary bypass (CPB) during cardiac surgery. [49] The study compared patients who received MB between 2010 and 2015 with matched historical controls from 2004 to 2009. The results showed that the MB group had a significantly shorter time to improvement of vasoplegia, a faster discontinuation of vasopressors, lower 30-day mortality, reduced cardiac surgical Intensive Care Unit (CSICU) morbidity, shorter hospital stays, and a lower incidence of renal failure compared to the control group. The use of MB was associated with improved hemodynamics, decreased need for vasopressors, and better outcomes post-surgery in patients who were at high risk for vasoplegic syndrome.
- Researchers investigated whether low-dose methylene blue (MB) could slow down the progression of at-risk brain tissue towards becoming damaged during a stroke. [50] They conducted experiments on rats with a blocked artery and used MRI scans to assess the effects of MB. The study found that MB significantly delayed the transition of at-risk tissue to infarct (damage), moderately improved blood flow to the affected area, and maintained a stable energy supply.
D. Improves Mood

Research studies have demonstrated that methylene blue has shown positive effects on mood:
- Studies show that methylene blue can improve mood by inhibiting multiple amine oxidase activities, particularly MAO-A, which increases serotonin. [51-53]
- Studies report that methylene blue can treat psychotic and mood disorders, enhance memory in fear-extinction training, and show promise in the short- and long-term management of bipolar disorder. [54-55] Notably, it offers antidepressant and anxiolytic effects in bipolar disorder treatment without inducing manic episodes. Long-term usage has demonstrated improved stabilization and reduced residual symptoms.
- The dysfunction of the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway is closely linked to mood, anxiety, and psychosis in neurobiology. [56] Inhibiting NOS and/or guanylate cyclase has shown antidepressant effects, a mechanism that methylene blue (MB) may leverage for its psychotropic activity.
- In one study, five recently developed methylene blue (MB) analogues with low monoamine oxidase-A (MAO-A) activity were examined for their antidepressant-like effects in rats using the forced swim test (FST). [57] The research found that all five analogues demonstrated antidepressant-like properties in the FST, comparable to imipramine and MB, without impacting locomotor activity.
- In a 3-week trial, the efficacy of 15 mg/day of methylene blue was assessed against a placebo for severe depressive illness treatment. [58] The study design aimed to prevent both placebo response bias and observer bias. Results indicated that patients receiving methylene blue exhibited significantly greater improvement compared to those on placebo, suggesting its potential as a potent antidepressant at this dosage.
E. Improves Skin Health

Methylene blue has been recognized for its potential anti-aging effects on the skin. Emerging research suggests that the compound’s antioxidant properties and ability to enhance cellular energy production may contribute to improved skin health and appearance:
- Methylene blue (MB), a traditional antioxidant targeting mitochondria, effectively scavenged ROS in skin fibroblasts from healthy donors and patients with progeria, a genetic aging disorder. [59] Compared to other general and mitochondrial antioxidants, MB showed enhanced skin fibroblast proliferation, delayed cellular senescence, and passed a skin irritation test on a 3D human skin model. Application of MB improved skin viability, wound healing, hydration, dermis thickness, and altered gene expression of extracellular matrix proteins, suggesting its potential for skin care.
- Methylene blue (MB), a historical medicine and antioxidant, has potential as a UV radiation protection ingredient due to its molecular structure and light absorption properties. In one study, the researchers observed that MB treatment reduced DNA damage and cell death caused by UVB rays in human keratinocytes (skin cells). [60] Compared to Oxybenzone, a commonly used sunscreen ingredient harmful to coral reefs, MB showed superior UVB absorption and protection against DNA damage and cellular ROS. Our findings suggest that MB could be a coral reef-friendly sunscreen ingredient offering broad-spectrum UVA and UVB protection.
- A study aimed to evaluate the effectiveness and side effects of injecting methylene blue into the perianal skin of patients with chronic refractory idiopathic pruritus ani (IPA), a condition characterized by persistent itching and discomfort in the anal area. [61] Patients who didn’t respond to standard treatments were given intradermal injections of 1% methylene blue solution up to the dentate line, which is the boundary between the upper anal canal and the lower rectum. Results showed that symptoms resolved within 4 weeks, with no serious side effects, and a 20% success rate was observed over a 60-month period.
F. Fights various Infectious Diseases
According to research findings, methylene blue has exhibited the potential to combat a diverse range of infections:
- SARS-CoV-2 infection typically initiates within the respiratory tract, leading to bilateral pneumonia. The disease’s progression can result in acute respiratory distress syndrome and multi-organ failure, propelled by viral dissemination through the bloodstream and an exacerbated inflammatory response encompassing a cytokine storm.Methylene blue, a cost-effective dye recognized for its antiseptic properties and employed effectively in treating conditions such as malaria, urinary tract infections, shock, and methemoglobinemia, stands as the singular known substance capable of restraining the excessive generation of reactive species and cytokines, suggesting its potential to treat COVID-19 and SARS-CoV 2 infections. [62-65]
- A research aimed to evaluate the safety and efficacy of two distinct urinary antiseptic combinations for addressing recurrent cystitis symptoms. [66] One combination consisted of methenamine 120mg + methylene blue 20mg (Group A), while the other included acriflavine 15mg + methenamine 250mg + methylene blue 20mg + Atropa belladonna L. 15mg (Group B).The study involved participants receiving a 3-day course of oral treatment with the assigned combination, followed by 3 days of antibiotic therapy based on urine culture, alongside continued treatment with the study drug. The Urinary Tract Infection Symptoms Assessment Questionnaire (UTISA) was employed to gauge treatment efficacy, with the primary focus on improvement in the “Urination Regularity” domain.Ultimately, outcomes revealed the effectiveness of both treatments in alleviating UTI symptoms. Notably, the combination of methenamine + methylene blue demonstrated a more favorable profile in terms of treatment-related adverse effects compared to the alternative combination.
- A present study systematically investigated methylene blue’s virucidal potential against influenza virus H1N1 and SARS-CoV-2, exploring various factors such as incubation times and the influence of light activation. [67] The research delves into both preventive and therapeutic aspects, probing the impact on infected cells and even in conjunction with immune serum. The data underscores methylene blue’s ability to exert virucidal effects against these viruses at low micromolar concentrations without the need for UV activation.Moreover, the study sheds light on the intricate mechanisms through which methylene blue operates, especially in terms of genomic RNA degradation and the influence of light exposure. In conclusion, this work strongly advocates for clinical investigations to confirm the potential preventive and therapeutic value of methylene blue against both influenza virus H1N1 and SARS-CoV-2 infections.
- In addressing the limitations of existing methods for identifying infected tissue in periprosthetic joint infection (PJI), a study aimed to evaluate the efficacy of methylene blue-guided surgical debridement as an innovative approach. [68] This prospective investigation encompassed sixteen patients who met the criteria outlined by the Musculoskeletal Infection Society for PJI, all undergoing the initial stage of a two-stage exchange arthroplasty procedure.Employing a diluted solution of methylene blue (0.1%), the surgical area was infused before debridement, with subsequent analysis of stained and unstained tissue samples collected from various anatomical sites. The application of methylene blue was associated with heightened detection of bacterial presence and increased neutrophil count in stained tissue. These findings underscore the potential utility of methylene blue in visually guiding and enhancing surgical debridement as a treatment strategy for PJI.
- Candida albicans is a microorganism known to cause a spectrum of infections, varying from surface-level to systemic fungal infections in individuals with weakened immune systems. An investigation looked into the mechanism by which methylene blue (MB) exerts its antifungal effects against C. albicans. [69] The study demonstrates MB’s efficacy not only against C. albicans but also against two clinical isolates and two non-albicans species.Importantly, MB’s antifungal impact appears to function independently of major drug efflux transporter activity. The research suggests that MB’s influence on Candida cells is linked to mitochondrial inhibition and disturbances in redox balance, leading to disruptions in membrane integrity and lipid composition. Furthermore, the study highlights MB’s ability to hinder the transformation of Candida cells into a more invasive hyphal form, a significant virulence trait. The findings underscore the potential of MB as a promising agent in the fight against fungal infections caused by Candida.
- The contamination of blood products with hepatitis C virus (HCV) poses a significant risk, leading to infections that can result in acute and chronic liver diseases. To address this concern, innovative pathogen reduction techniques, specifically employing photodynamic treatment utilizing methylene blue (MB) combined with visible light, as well as shortwave ultraviolet (UVC) irradiation, have been developed. [70] These methods aim to neutralize viruses and other pathogens present in plasma and platelet concentrates (PCs).Cell culture-derived HCV and bovine viral diarrhea virus (BVDV), used as a model for HCV, were subjected to inactivation procedures. Plasma units and PCs were intentionally exposed to high viral titers cultivated in cell cultures. Following treatment with MB and light using the Theraflex MB-Plasma system, or UVC through the Theraflex UV-Platelets system, the residual viral infectivity was meticulously evaluated through sensitive cell culture assays.Results demonstrated that HCV was effectively susceptible to inactivation by both pathogen reduction techniques. Plasma-associated HCV was efficiently neutralized by MB plus light at a mere fraction of the full light dose, effectively rendering it undetectable. In conclusion, the application of technologies such as MB plus light treatment and UVC irradiation exhibits the potential to substantially mitigate the risk of transfusion-transmitted HCV infections, representing a significant advancement in enhancing blood product safety.
- In one study, the remarkable efficacy of methylene blue, an FDA-approved drug, as a potent and broad-spectrum antiviral against both Zika and Dengue viruses, has been illuminated both in laboratory settings and animal models. [71] Methylene blue’s capacity to substantially impede the interactions between the viral protease NS3 and its NS2B co-factor, inhibit viral protease activity, curb viral replication, safeguard 3D mini-brain organoids from ZIKV infection, and diminish viremia in a mouse model has been observed.Detailed investigations into the mechanism of action indicate that methylene blue operates during both the viral entry and post-entry phases, suppresses virus production in replicon cells, and hampers the generation of processed NS3 protein. Collectively, these findings underscore methylene blue’s potent antiviral potential for managing flavivirus infections, particularly for Zika virus.
- Photodynamic therapy involves the use of a light-activated drug that generates highly reactive radicals upon exposure to specific wavelengths of light. This process, termed “photodynamic antimicrobial chemotherapy” (PACT), holds promise for treating microbial infections. However, conventional application of this therapy for chronic wounds faces challenges due to the presence of thick layers of necrotic tissue, limiting its effectiveness.To address this, a novel approach involving microneedles (MNs) has been investigated, allowing painless and efficient delivery of photosensitizing agents such as methylene blue to the skin. [72] This study aimed to explore the characteristics and antimicrobial activity of dissolving MNs loaded with methylene blue.The results demonstrated significant reduction in microbial viability, exceeding 96% for Staphylococcus aureus and over 99% for Escherichia coli and Candida albicans, when exposed to PACT using methylene blue concentrations ranging from 0.1 to 2.5 mg/mL. This approach exhibited potential for effectively combating various infections through targeted delivery of methylene blue via microneedles.
- Bartonella henselae, a Gram-negative bacterium, is transmitted to humans through scratches from cats in the presence of ectoparasites. Infections with this bacterium can lead to various clinical conditions, ranging from local lymph node swelling to more severe systemic diseases like persistent bacteremia (presence of bacteria in the bloodstream) and endocarditis (inflammation of the heart’s lining).Treating persistent B. henselae infections remains challenging, as current treatments are not very effective. In an effort to find better solutions, a study assessed a range of drugs and drug combinations, including those used in current treatments and promising candidates from recent drug screenings. [73] The results showed that ciprofloxacin, gentamicin, and nitrofurantoin were highly effective against stationary phase B. henselae, while clofazimine and miconazole showed poor activity.Notably, combinations of drugs like azithromycin/ciprofloxacin, azithromycin/methylene blue, rifampin/ciprofloxacin, and rifampin/methylene blue demonstrated rapid eradication of stationary phase and biofilm B. henselae. This suggests that combining methylene blue with certain antibiotics can help treat persistent Bartonella infections.
G. Fights Cancer and Aids in Cancer Treatment
According to research studies, methylene blue has shown potential in combating cancer and contributing to cancer treatment strategies.
- A study proposed employing poly(N-isopropylacrylamide) (PNIPAM) microgels to encapsulate methylene blue, an anticancer agent, for breast cancer treatment in the MCF-7 cell line. [74] The researchers created biocompatible microgels using nonfunctionalized PNIPAM and its anionically functionalized PNIPAM and polyacrylic acid (PNIPAM-co-PAA) counterparts.Methylene blue was chosen as the photosensitizer due to its ability to produce harmful reactive oxygen species when exposed to light at 664 nm. Notably, core PNIPAM microgels retained methylene blue longer and demonstrated enhanced photodynamic efficacy against MCF-7 cells (human breast cancer cell line), surpassing the performance of free methylene blue.In conclusion, the study highlights the potential of core PNIPAM and core/shell PNIPAM-co-PAA microgels for improving methylene blue encapsulation. Core PNIPAM microgels exhibited controlled drug release and effectively inhibited the growth of MCF-7 cells, showcasing their promise as a platform for enhancing the efficacy of photodynamic therapy.
- A study investigated the potential anti-cancer effects of methylene blue (MB), a known inhibitor of Heat shock protein 70 (Hsp70), in comparison to novobiocin (NB), an established Hsp90 inhibitor, and their combination. [75] Lung cancer cells rely on chaperones like Hsp70 and Hsp90 for survival and growth. Through in vitro assays using A549 non-small cell lung cancer cells, MB exhibited lower cell viability compared to NB, and their combination further intensified this effect.The combination demonstrated promising results for inducing early and late apoptosis (programmed cell death). In vivo experiments on benzo[a]pyrene-induced lung carcinogenesis in mice revealed that MB not only significantly inhibited Hsp70 but also improved tumor biomarkers and lung histopathology, suggesting its potent anticancer potential.
- A research delved into the effectiveness of methylene blue (MB) in photodynamic therapy (PDT) against breast epithelial cells, representing both non-malignant conditions and diverse molecular subtypes of breast tumors. [76] Cells were subjected to PDT with MB and 640 nm irradiation at 4.5 J/cm², revealing significant tumor cell death while non-malignant cells exhibited greater resistance.Unveiling unique mechanisms beyond conventional apoptosis, the findings pointed towards MB-PDT inducing distinct pathways, including autophagy (a cellular process that involves the degradation and recycling of damaged or unnecessary cellular components), to impact cell viability. Even when inhibiting these pathways, MB-PDT continued to drive cell fatality, demonstrating its potential as an impactful adjunct therapy for breast and potentially other tumor types, with implications for reducing disease recurrence.
- Oral mucositis, a complication of cancer therapy, causes severe pain impacting oral function, nutrition, and quality of life, potentially leading to treatment nonadherence and dose-limiting toxicity. Methylene blue (MB) oral rinse has been suggested as a potential solution for this oral pain.In one study, a group of researchers examined 281 patients who had persistent pain despite conventional treatments to assess the efficacy and safety of MB oral rinse for mucositis-related oral pain in cancer patients. [77] The results demonstrated significant pain reduction after MB oral rinse, with most patients achieving relief within the initial three doses. The treatment’s effectiveness remained consistent across various patient factors and cancer types, indicating that MB oral rinse holds promise as an effective and safe approach for managing refractory oral pain caused by mucositis in cancer therapy.
H. Helps Treat Malaria
Malaria is a mosquito-borne infectious disease caused by parasites of the Plasmodium genus and characterized by symptoms like fever, chills, and anemia. Numerous studies have indicated that methylene blue serves as an effective antimalarial agent, demonstrating its potential in the field of malaria treatment:
- A review of existing studies confirmed the effectiveness and safety of methylene blue (MB) in treating malaria. [78] While more clinical trials are needed for its effects on P. vivax malaria, strong evidence supports its efficacy against P. falciparum parasites (causative agent of malaria), particularly gametocytes. MB could be an alternative to primaquine (PQ) in falciparum malaria combination therapy, especially for malaria elimination programs, and its addition to artemisinin-based combination therapy (ACT) regimens could decrease transmission intensity, improve treatment outcomes, and reduce the risk of artemisinin and ACT resistance development and spread.
- A 2004 study published in the Malaria Journal on the combination of methylene blue and chloroquine (MB-CQ) in 435 children with uncomplicated falciparum malaria in Burkina Faso showed that while CQ monotherapy had a high clinical failure rate in the area in 2003, the combination did not yield favorable results. [79] However, MB alone had some efficacy against malaria. Therefore, it was concluded that MB alone can help treat malaria compared with the MB-CQ combination.
- Methylene blue (MB) was the first synthetic antimalarial discovered and used in the late 19th and early 20th centuries against all types of malaria. Studies have shown MB’s effectiveness in inhibiting Plasmodium falciparum, with potent ex vivo activity against drug-resistant isolates. [80] This suggests MB could be a valuable partner drug for artemisinin-based combination therapy (ACT) to prevent resistance and reduce transmission.
- A study reported that methylene blue exhibits intrinsic antimalarial activity and can enhance the effectiveness of chloroquine, an antimalarial agent. [81] It’s also important for preventing methemoglobinemia, a complication of malarial anemia. Acting as an antiparasitic agent, methylene blue interferes with hemoglobin and heme metabolism, inhibits Plasmodium falciparum glutathione reductase, and depletes glutathione to sensitize the parasite for chloroquine.
- Untreated malaria can rapidly progress to severe forms in less than 24 hours, and drug resistance poses a global threat to malaria prevention efforts. To address this, a group of researchers conducted experiments using methylene blue (MB) in combination with common antimalarial drugs (mefloquine and amodiaquine) to treat malaria and cerebral malaria. [82] The study, using a C57BL6/J mouse model infected with Plasmodium berghei ANKA, showed that MB-based combination therapies were effective even when treatment was initiated at a late stage, with significant survival rate differences observed in comparison to untreated and individual drug-treated groups. Combining MB with AQ proved to be a promising option for preventing cerebral malaria.
I. Helps Treat Methemoglobinemia
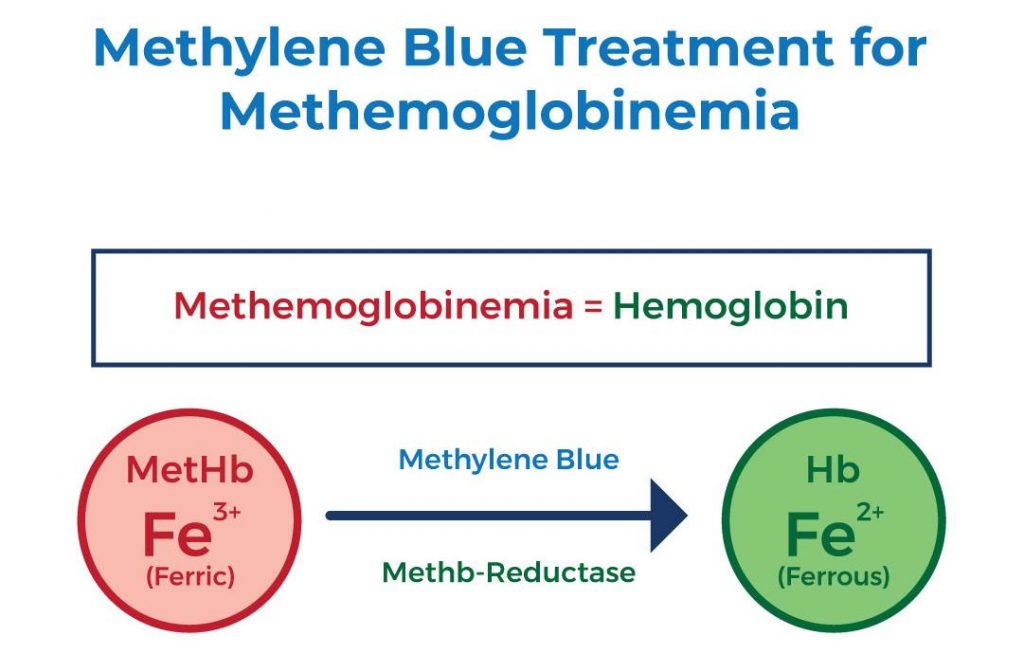
Methemoglobinemia is a medical condition characterized by elevated levels of methemoglobin, a modified form of hemoglobin containing ferric iron, in the blood. Hemoglobin, responsible for transporting oxygen in red blood cells, is normally in a ferrous iron state, allowing efficient oxygen binding and release to body tissues. However, in methemoglobin, the iron is oxidized to its ferric state, preventing effective oxygen exchange. At lower levels, one might experience difficulty breathing, nausea, and increased pulse rate, while higher levels can lead to symptoms progressing to lethargy, stupor, declining consciousness, cardiac arrhythmias (abnormal heart rhythms), and even death.
Treatment of methemoglobinemia frequently involves the use of reducing agents, including methylene blue, which is converted to leukomethylene blue (LB). LB acts as a potent reducing agent, facilitating the reduction of ferric iron back to ferrous iron within the methemoglobin molecule. This conversion effectively restores hemoglobin’s ability to transport oxygen, thus remedying the symptoms associated with methemoglobinemia and improving oxygen delivery to body tissues.
Research findings indicate that methylene blue demonstrates therapeutic efficacy in the treatment of methemoglobinemia:
- A review explored methylene blue use in treating acquired methemoglobinemia and ifosfamide neurotoxicity, as well as its role in critically ill patients with refractory vasoplegic shock. [83] It has been found effective for treating acquired methemoglobinemia, ifosfamide neurotoxicity, and refractory vasoplegic shock in both pediatric and adult critical care settings, expanding its applications.
- A study recommends that the usual starting dose of methylene blue for the treatment of methemoglobinemia is 1–2 mg/kg (0.2 mL/kg of a 1% solution) infused intravenously over 3 to 5 minutes, with the option of a repeated 1 mg/kg dose if needed within 30–60 minutes. [84] However, caution is needed as repeated doses can worsen methemoglobinemia, with toxic levels reached at a total dose > 7 mg/kg, and significant reduction in MetHb levels is expected in less than an hour.
- The over-the-counter availability of topical anesthetics and their frequent use in medical procedures like intubation and endoscopy has led to a notable increase in cases of methemoglobinemia in the last decade. Suspicion for methemoglobin should arise in hypoxic and cyanotic patients who show no improvement with 100% FiO2 oxygen therapy. A study recommends methylene blue infusion when methemoglobin levels are 30% for asymptomatic patients and 20% for symptomatic patients. [85]
Methylene Blue Uses
Methylene blue is used for the treatment of the following conditions:
- Methemoglobinemia: Methylene blue is commonly used to treat methemoglobinemia, a condition where hemoglobin in red blood cells can’t effectively carry oxygen. It helps convert methemoglobin back to normal hemoglobin, enhancing oxygen delivery and improving the capacity to carry oxygen.
- Cyanide Poisoning: Large doses of methylene blue are utilized in the treatment of cyanide poisoning, helping to counteract the toxic effects by supporting cellular respiration.
- Septic Shock: Methylene blue has been investigated as an adjunct treatment for septic shock, as it might improve blood pressure regulation and assist in managing shock-related complications.
- Neurodegenerative Disorders: Methylene blue’s antioxidant and energy-enhancing properties have sparked interest in its potential to mitigate oxidative stress and support mitochondrial function in neurodegenerative conditions like Alzheimer’s and Parkinson’s disease.
- Cognitive Decline: Some research suggests methylene blue could have cognitive-enhancing effects, making it a candidate for addressing cognitive decline and memory impairment.
- Ischemic Stroke: Studies have explored methylene blue’s ability to delay the progression of at-risk brain tissue to infarct in certain types of stroke, potentially offering neuroprotective effects.
- Antimicrobial Therapy: Methylene blue’s antimicrobial properties could be beneficial as an adjunct therapy in combating infections, particularly those caused by antibiotic-resistant bacteria.
- Mitochondrial Disorders: Due to its potential to support mitochondrial function, methylene blue might be considered for certain mitochondrial disorders where cellular energy production is impaired.
- Hypotension and Shock: Methylene blue’s ability to stabilize blood pressure, enhance blood flow, and manage refractory hypotension (a severe and persistent drop in blood pressure that does not respond adequately to standard medical interventions) could be valuable in managing various forms of shock.
- Ifosfamide neuropsychiatric toxicity: Methylene Blue also finds application in addressing ifosfamide neurotoxicity, a lesser-recognized application. The mitochondrial respiratory chain disruption caused by chloroacetaldehyde, a toxic metabolite of ifosfamide, leads to the accumulation of nicotinamide adenine dinucleotide hydrogen (NADH). Methylene blue plays a crucial role in mitigating this neurotoxicity by effectively reversing the NADH inhibition of hepatic gluconeogenesis (a process by which the liver synthesizes glucose from non-carbohydrate sources).
Methylene Blue Contraindications
There are certain situations in which the use of methylene blue is contraindicated, meaning it should be avoided due to potential risks or interactions. Contraindications for methylene blue include:
- Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency: Individuals with G6PD deficiency are at risk of developing hemolytic anemia (a potentially fatal condition characterized by shortage of healthy red blood cells) when exposed to methylene blue, as the deficiency impairs the ability of red blood cells to handle oxidative stress.
- Pulmonary Hypertension: Methylene blue should be used with caution or avoided in individuals with pulmonary hypertension, as it can potentially worsen this condition.
- Preexisting Heart Conditions: Individuals with certain heart conditions, such as certain types of heart failure, should be cautious when using methylene blue, as it can influence cardiac function.
- Hypertensive Crisis: Methylene blue can cause an increase in blood pressure, so its use should be avoided in individuals with uncontrolled high blood pressure.
- Allergic Reactions: Individuals with a known hypersensitivity or allergy to methylene blue should avoid its use. Therefore, a health care professional should assess for a history of an allergic reaction to this medication before prescribing it.
- Pregnancy and Breastfeeding: Methylene blue administration to pregnant women in the second trimester has been linked to fetal death, while unintended exposure in the first trimester might lead to comparatively less fetal damage. The medication is also not recommended for breastfeeding women because it passes into the breast milk.
- Drug Interactions: When used together with certain medicines for depression such as selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOI), it can increase the levels of a neurotransmitter (brain chemical) known as serotonin, leading to serious serotonin toxicity or also known as serotonin syndrome. It’s important to discuss all current medications, including dietary supplements, non-prescription drugs, illegal drugs, and other medicines, with a healthcare provider before using methylene blue to prevent serotonin toxicity.
- Children and Infants: Methylene blue use in children and infants should be approached with caution and under the guidance of a healthcare professional, especially due to the risk of methemoglobinemia.
- Renal Impairment: Individuals with severe kidney impairment should be cautious when using methylene blue, as it can affect kidney function.
Always consult a healthcare provider before using methylene blue, especially if you have any preexisting medical conditions, are taking dietary supplements, non-prescription drugs and other medicines, or are unsure about its suitability for your specific situation.
Intravenous Methylene Blue vs Oral Methylene Blue Treatment
When it comes to using methylene blue as a treatment, there are two main ways it can be given: intravenous and oral. Intravenous (IV) methylene blue means the substance is injected directly into the bloodstream using a needle, while oral methylene blue is taken by swallowing a pill or liquid.
Intravenous methylene blue is often used in more critical situations or medical settings. It can quickly reach the bloodstream, making it a faster-acting option. This method is commonly used to treat conditions like methemoglobinemia (a blood disorder) or to support certain medical procedures. Because methylene blue injection goes directly into the bloodstream, it can have more immediate effects. However, methylene blue must be injected intravenously very slowly over a period of several minutes to avoid having too much of the compound in one place, which could cause more methemoglobin to form.
On the other hand, oral methylene blue involves taking the substance through the digestive system, like other medications. It is usually used for less urgent situations or chronic conditions. The effects might take longer to appear compared to the IV form because the body needs time to process and absorb the substance through the digestive tract.
Both methods have their own advantages and considerations, and the choice between intravenous and oral methylene blue depends on the specific condition being treated, the desired speed of action, and the overall health of the patient. Always follow the guidance of a health care professional on which form of treatment is best for your situation.
Methylene Blue Side Effects
Methylene blue side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on methylene blue. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of methylene blue.
The side effects of methylene blue can vary depending on whether it’s injected or taken orally. Methylene blue injection might cause local discomfort at the injection site, temporary urine discoloration (blue staining of the urine), an allergic reaction, and skin sensitivity. When taken orally, it could lead to gastrointestinal discomfort, nausea, and possibly discolored stool. Seek medical attention immediately if you experience any untoward signs and symptoms or other side effects.
Methylene Blue Dosage
The dosage of methylene blue can vary depending on the condition being treated. For conditions like methemoglobinemia, a single dose of 1-2 milligrams per kilogram of body weight is often used. However, it’s important to follow the guidance of a medical professional for the correct dosage.
FAQ
What is methylene blue used for?
This medication is used for various purposes, including microbiology staining, potential cognitive enhancement, support for mitochondrial function, antioxidant properties, and as an adjunct in antimicrobial therapies.
Is methylene blue harmful to humans?
Like other medicines, this medication can be safe when used appropriately and under medical guidance of a health care professional. However, improper use or excessive consumption can lead to side effects and potential harm. For instance, methylene blue is known to have the potential to induce hemolytic anemia in individuals with a deficiency of the enzyme glucose-6-phosphate dehydrogenase (G6PD). It can also cause drug interactions when used together with certain medicines for depression or anxiety.
What are the benefits of methylene blue for humans?
Methylene blue’s potential benefits include enhancing cognitive function, supporting mitochondrial health, providing antioxidant effects, aiding in microbial control, and assisting in studying microscopic organisms.
Is methylene blue dye toxic?
This medication can be toxic if consumed at high doses. It’s important to use any form of methylene blue, including the dye, with proper caution and guidance of a health care professional.
Is methylene blue safe for humans?
When used appropriately and under medical supervision, methylene blue is generally considered safe for humans. However, individual reactions and conditions can vary.
Does methylene blue increase oxygen levels?
Yes, this medication has the potential to increase oxygen levels. The way methylene blue works is by changing the oxidized form of hemoglobin (Fe3+) back to its normal form (Fe2+). This helps hemoglobin pick up more oxygen and carry it to the body’s tissues, improving oxygen delivery.
How long does methylene blue last in the body?
The duration of methylene blue’s effects in the body can vary based on factors such as dosage, individual metabolism, and the purpose for which it’s used. It’s best to follow medical guidance.
What is methylene blue used for?
This medication is used for staining in microbiology, potential cognitive enhancement, supporting mitochondrial function, providing antioxidants, and aiding antimicrobial therapies.
Is methylene blue safe for humans?
When used properly and under medical supervision, methylene blue is generally considered safe for humans. However, individual responses may differ.
What are the benefits of methylene blue for humans?
Methylene blue may offer benefits such as cognitive enhancement, support for mitochondria, antioxidant effects, microbial control, and the ability to stain microscopic samples.
What is the common name for methylene blue?
The common name for methylene blue is methylthioninium chloride.
What does methylene blue do for your body?
Methylene blue’s effects on the body include potential cognitive enhancement, support for cellular energy production, antioxidant activity, and assistance in microbial control.
Is methylene blue safe for daily use?
Using methylene blue daily should be done under medical guidance, as excessive or prolonged use might lead to adverse effects. It’s important to follow recommended dosages and usage patterns.
Does methylene blue reduce inflammation?
Methylene blue’s anti-inflammatory effects have been explored in research, but its precise impact on inflammation can vary and further studies are needed.
Is methylene blue good for your liver?
Some studies have suggested that methylene blue might have a protective effect on the liver, but its overall impact depends on various factors and requires more research.
What can methylene blue be used for?
Methylene blue can be used for staining in microbiology, potential cognitive enhancement, supporting mitochondrial function, providing antioxidant effects, and aiding antimicrobial therapies.
What are the benefits of methylene blue for humans?
Potential benefits of methylene blue include cognitive enhancement, support for mitochondria, antioxidant effects, microbial control, and its use as a stain in research.
What does methylene blue detect?
Methylene blue can detect various biological substances and cellular structures when used as a stain in microbiological and histological research.
What does methylene blue do to bacteria?
Methylene blue can stain bacterial cells, making them more visible under a microscope. It’s also been explored for its potential antimicrobial properties.
Who should not take methylene blue?
Individuals with certain medical conditions, such as certain types of glucose-6-phosphate dehydrogenase deficiency, and those on specific medications should avoid methylene blue. Consultation with a healthcare professional is advised to prevent potential adverse effects of the following medications. During the consultation period, it is recommended to tell your doctor if you smoke, drink alcohol, take over the counter medications, or use illegal drugs to avoid any potential interactions.
Avoid taking this medicine with the following medications:
- Bupropion
- Certain medicines for depression or anxiety
- Clomipramine
- Doxepin
- Duloxetine
- Fluoxetine
- MAOIs like Marplan, Nardil, and Parnate
- Milnacipran
- Mirtazapine
- Rasagiline
- Selegiline
- St. John’s wort
- Trazodone
- Tryptophan
Is methylene blue safe for daily use?
Using methylene blue daily should be approached cautiously and under medical guidance to prevent potential adverse effects. It’s important to follow recommended usage instructions.
How does methylene blue make you feel?
The effects of methylene blue can vary depending on its purpose and dosage. It’s not meant for recreational use, and any effects should be discussed with a healthcare provider.
How is methylene blue used in medicine?
Methylene blue is used in medicine for various purposes, including treating methemoglobinemia, acting as a diagnostic stain, and potentially offering neuroprotective effects.
What are the applications of methylene blue in staining?
Methylene blue is commonly employed as a stain in histology and microbiology to enhance contrast and visibility of cellular structures.
How does methylene blue treat methemoglobinemia?
Methylene blue helps to turn the abnormal “rusty” form of blood (methemoglobin) back into the normal red form that can carry oxygen effectively.
What are the potential neuroprotective effects of methylene blue?
Methylene blue has shown promise in potentially mitigating neurodegenerative conditions by influencing mitochondrial function and reducing oxidative stress.
How does methylene blue enhance memory function?
Methylene blue has been investigated for its potential to enhance memory and cognitive function, possibly through its effects on mitochondrial function and neurotransmitter systems.
Can methylene blue be used for mood regulation?
Methylene blue has been explored as a potential mood regulator due to its effects on mitochondrial function and interactions with neurotransmitter systems.
What are the industrial applications of methylene blue?
Methylene blue is used in various industries, including textiles, as a dye and colorant, and in analytical chemistry as an indicator.
How is methylene blue used as an indicator in titrations?
In analytical chemistry, methylene blue serves as a redox indicator, undergoing color changes that signal the endpoint of titrations.
What is the role of methylene blue in photodynamic therapy?
Methylene blue is used in photodynamic therapy as a photosensitizer that, when activated by light, produces reactive oxygen species to target and destroy cancer cells.
How does methylene blue interact with other medications?
Methylene blue can interact with certain medications, particularly those affecting serotonin levels, potentially leading to a condition known as serotonin syndrome.
Can methylene blue be used in nanomedicine and drug delivery?
Methylene blue’s properties make it a candidate for applications in nanomedicine and drug delivery, potentially aiding in targeted therapy.
Does methylene blue have antimicrobial properties?
Methylene blue has demonstrated antimicrobial effects against various bacteria, fungi, and parasites, suggesting its potential as an antimicrobial agent.
How does methylene blue affect mitochondrial function?
Methylene blue has been found to influence mitochondrial function by promoting electron transport and enhancing cellular energy production.
What is ifosfamide-induced encephalopathy?
Ifosfamide-induced encephalopathy is a neurological condition associated with the use of ifosfamide chemotherapy.
Is methylene blue used in histological staining?
Yes, methylene blue is commonly used in histological staining to enhance the visualization of cellular structures under a microscope.
What is the relationship between methylene blue and serotonin?
Methylene blue can inhibit the reuptake of serotonin, potentially leading to increased serotonin levels and affecting mood and other physiological processes.
How is methylene blue administered?
Methylene blue can be administered through various routes, including oral, intravenous, and topical application, depending on the intended medical use.
What is the mechanism of action of methylene blue?
Methylene blue’s mechanisms of action include its roles as a redox agent, mitochondrial enhancer, and potential modulator of neurotransmitter systems.
Can methylene blue be used as an antidepressant?
Methylene blue’s effects on neurotransmitters and mitochondrial function have led to investigations into its potential as an adjunctive treatment for depression.
What are the potential future applications of methylene blue?
Methylene blue’s diverse properties open up possibilities for future applications in areas such as neuroprotection, cancer therapy, and drug delivery.
Does methylene blue have antioxidant properties?
Methylene blue exhibits antioxidant properties by reducing oxidative stress and potentially contributing to cellular protection.
How does methylene blue impact cognitive function?
Methylene blue’s influence on mitochondrial function and neurotransmitter systems may contribute to its impact on cognitive function and memory enhancement.
Is methylene blue used in analytical chemistry?
Yes, methylene blue is used as a redox indicator in analytical chemistry to signal the endpoint of titrations.
What is the connection between methylene blue and aquaculture?
Methylene blue is used in aquaculture to treat fungal and bacterial infections in fish, providing a safe environment for aquatic organisms.
How is methylene blue relevant in redox titration?
Methylene blue’s color change in response to redox reactions makes it useful as an indicator in redox titrations.
Can methylene blue be used for cancer treatment?
Methylene blue’s photodynamic properties have led to its investigation as a potential agent for cancer treatment through targeted cell destruction.
What are the safety considerations when using methylene blue?
Methylene blue should be used with caution, considering potential interactions with medications and its adverse effects.
Does methylene blue have potential in drug delivery?
Methylene blue’s properties suggest it could play a role in drug delivery systems, potentially enhancing targeted therapies.
How does methylene blue affect mood disorders?
Methylene blue’s impact on neurotransmitter systems and mitochondrial function has led to its exploration as a potential treatment for mood disorders.
What are the potential drawbacks of methylene blue in therapy?
Drawbacks of methylene blue therapy include potential interactions with medications and adverse effects such as serotonin syndrome.
Can methylene blue be used in combination therapies?
Methylene blue’s diverse mechanisms of action make it a candidate for combination therapies in various medical applications.
How does methylene blue compare to other dyes in staining?
Methylene blue offers distinct staining capabilities compared to other dyes, making it valuable in specific histological and microbiological contexts.
What are the effects of methylene blue on mitochondrial function?
Methylene blue has been shown to enhance mitochondrial function by promoting electron transport and cellular energy production.
Is methylene blue used in veterinary medicine?
Yes, methylene blue is used in veterinary medicine to treat various conditions, including fish parasites and cyanide poisoning.
What role does methylene blue play in oxidative stress?
Methylene blue’s antioxidant properties contribute to its role in mitigating oxidative stress and protecting cells from damage.
How does methylene blue interact with neurotransmitter systems?
Methylene blue interacts with neurotransmitter systems by affecting serotonin reuptake and potentially influencing mood and cognitive function.
What are the potential effects of methylene blue on mood?
Methylene blue’s impact on neurotransmitters and mitochondrial function may lead to mood-enhancing effects.
Can methylene blue be used in targeting cancer cells?
Methylene blue’s photodynamic properties make it a candidate for targeting and destroying cancer cells through light activation.
How is methylene blue relevant to neurodegenerative diseases?
Methylene blue’s potential neuroprotective effects have sparked interest in its application for neurodegenerative disease treatment.
What are the challenges in using methylene blue in therapy?
Challenges in methylene blue therapy include optimizing dosages, understanding potential interactions, and managing adverse effects.
How does methylene blue impact mitochondrial electron transport?
Methylene blue enhances mitochondrial electron transport, potentially leading to increased cellular energy production.
What are the future prospects of methylene blue research?
Future research on methylene blue may uncover new applications and further elucidate its mechanisms of action.
Is methylene blue a versatile compound across different fields?
Yes, methylene blue’s diverse properties make it versatile and applicable in various fields, from medicine to industry.
Reference
Peter C, Hongwan D, Küpfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000 Jun;56(3):247-50. doi: 10.1007/s002280000124. PMID: 10952480.
Pharmacokinetics and organ distribution of intravenous and oral methylene blue
This study aimed to understand the pharmacokinetics and organ distribution of intravenous (i.v.) and oral methylene blue, used to prevent ifosfamide-induced encephalopathy in cancer patients. The researchers measured methylene blue concentration in blood after i.v. and oral doses in volunteers, and in various tissues in rats. I.v. administration showed a multiphasic time course with a 5.25-hour half-life. Oral administration resulted in much lower blood levels. Co-administration with mesna didn’t affect distribution. Urinary excretion was moderately higher for i.v. administration. In rats, intraduodenal administration led to higher intestinal and liver concentrations but lower blood and brain levels than i.v. administration. The differences in organ distribution account for distinct pharmacokinetics, suggesting i.v. methylene blue might be more effective if targeting the central nervous system, while oral and i.v. forms could be equally effective in the liver.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10952480/.
Sváb G, Kokas M, Sipos I, Ambrus A, Tretter L. Methylene Blue Bridges the Inhibition and Produces Unusual Respiratory Changes in Complex III-Inhibited Mitochondria. Studies on Rats, Mice and Guinea Pigs. Antioxidants (Basel). 2021 Feb 16;10(2):305. doi: 10.3390/antiox10020305. PMID: 33669457; PMCID: PMC7920423.
Methylene Blue Bridges the Inhibition and Produces Unusual Respiratory Changes in Complex III-Inhibited Mitochondria. Studies on Rats, Mice and Guinea Pigs
Methylene blue (MB) holds therapeutic potential for various human conditions, especially in neurodegenerative diseases. Its beneficial effects are linked to mitochondrial function, where it can donate electrons to cytochrome c independently of complex I and III through an “alternative electron transport” process. This counters the harmful effects of inhibitors on these complexes. Recent debate concerned MB’s effects on complex III-inhibited mitochondria, with new evidence suggesting MB can enhance bioenergetic parameters like respiration and membrane potential by reducing cytochrome c in mitochondria treated with complex III inhibitors. Unusual respiratory responses and consistent outcomes across rodent species underscore MB’s impact, reflecting its distribution across mitochondrial compartments and indicating its role in influencing mitochondrial metabolism.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7920423/.
Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F. Striatal neuroprotection with methylene blue. Neuroscience. 2009 Oct 20;163(3):877-89. doi: 10.1016/j.neuroscience.2009.07.012. Epub 2009 Jul 24. PMID: 19596056; PMCID: PMC2765788.
Striatal neuroprotection with methylene blue
Recent research suggests that low-dose methylene blue (MB), known for its antioxidant and metabolic-enhancing properties, could potentially counteract neural damage caused by neurotoxins. In a study involving rats infused with the neurotoxin rotenone, MB was examined for its effects. Results showed that MB reduced the size of rotenone-induced anatomical lesions and prevented decreases in cytochrome oxidase activity and oxidative stress in the striatum and related motor regions. It also maintained functional connectivity in motor circuits and partially prevented behavioral asymmetries caused by rotenone. This study highlights MB’s potential as a protective intervention against neural damage associated with oxidative stress and energy deficits in the brain.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2765788/.
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011 May 6;286(18):16504-15. doi: 10.1074/jbc.M110.208447. Epub 2011 Mar 18. PMID: 21454572; PMCID: PMC3091255.
Alternative mitochondrial electron transfer as a novel strategy for neuroprotection
In the past two decades, numerous neuroprotective approaches, including agents targeting oxidative stress, ion channels, and inflammation, have been investigated for treating neurological disorders, but none have shown efficacy in clinical trials. This study highlights methylene blue (MB) as an alternative electron carrier that bypasses complex I/III blockage in mitochondria by accepting electrons from NADH and transferring them to cytochrome c. A derivative of MB lacking its redox center showed no impact on mitochondrial complexes. MB increased oxygen consumption and decreased anaerobic glycolysis in neuronal cells, displaying protective effects at low concentrations against various insults in vitro. Animal models demonstrated MB’s remarkable capacity to mitigate impairments in Parkinson’s disease and cerebral ischemia-reperfusion injury, suggesting a novel strategy for neuroprotection in conditions linked to mitochondrial dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3091255/.
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008 Mar;22(3):703-12. doi: 10.1096/fj.07-9610com. Epub 2007 Oct 10. PMID: 17928358.
Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways
Methylene blue (MB) and similar compounds have been utilized for a century in clinical settings to treat various ailments. Recent research demonstrates that MB extends the lifespan of human fibroblasts in culture by over 20 population doublings, primarily by delaying cellular aging. This effect is achieved through improved mitochondrial function, indicated by increased mitochondrial complex IV levels and enhanced oxygen consumption. MB also counters premature senescence caused by oxidative stressors like H2O2 and cadmium, while prompting the production of antioxidant enzymes. The interplay between MB and its reduced form, MBH2, within mitochondria appears crucial for its protective impact, potentially by minimizing mitochondrial-generated oxidative stress. This suggests MB might have utility in mitigating age-related mitochondrial dysfunction and even in addressing complex IV decline in conditions like Alzheimer’s disease.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17928358/.
Xue H, Thaivalappil A, Cao K. The Potentials of Methylene Blue as an Anti-Aging Drug. Cells. 2021 Dec 1;10(12):3379. doi: 10.3390/cells10123379. PMID: 34943887.
The Potentials of Methylene Blue as an Anti-Aging Drug
This review explores methylene blue (MB) as a potential anti-aging agent, highlighting its role in enhancing mitochondrial function, reducing oxidative stress, and modulating cellular signaling pathways. The authors discuss MB’s ability to improve skin health, cognitive function, and overall cellular longevity. These findings suggest that MB may offer therapeutic benefits in mitigating age-related decline.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/34943887/.
Bhurtel S, Bok E, Katila N, Kim J, Choi DY. Activation of Nrf2 by methylene blue is associated with the neuroprotection against MPP+ induced toxicity via ameliorating oxidative stress and mitochondrial dysfunction. Biochem Pharmacol. 2021 Oct;192:114719. doi: 10.1016/j.bcp.2021.114719. PMID: 34352280.
Activation of Nrf2 by methylene blue is associated with the neuroprotection against MPP+ induced toxicity via ameliorating oxidative stress and mitochondrial dysfunction
This study investigates the neuroprotective effects of methylene blue (MB) against MPP+-induced toxicity in neuronal cells. Researchers found that MB activates the Nrf2 pathway, leading to reduced oxidative stress and improved mitochondrial function. These results suggest that MB may serve as a therapeutic agent in neurodegenerative diseases characterized by oxidative damage.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/34352280/.
Kuliaviene I, Baniene R, Virketyte S, Kincius M, Jansen E, Gulbinas A, Kupcinskas L, Trumbeckaite S, Borutaite V. Methylene blue attenuates mitochondrial dysfunction of rat kidney during experimental acute pancreatitis. J Dig Dis. 2016 Mar;17(3):186-92. doi: 10.1111/1751-2980.12328. PMID: 26861116.
Methylene blue attenuates mitochondrial dysfunction of rat kidney during experimental acute pancreatitis
This study examines the effects of methylene blue (MB) on mitochondrial dysfunction in the kidneys of rats with experimentally induced acute pancreatitis. The findings indicate that MB treatment improves mitochondrial respiration and reduces oxidative stress in renal tissues. These results suggest that MB may have therapeutic potential in preventing renal complications associated with acute pancreatitis.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/26861116/.
Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis. 2010;20 Suppl 2:S439-52. doi: 10.3233/JAD-2010-100414. PMID: 20463399.
Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase
This review discusses the potential of methylene blue (MB) in treating Alzheimer’s disease by targeting mitochondrial dysfunction and cytochrome c oxidase activity. The authors highlight MB’s ability to enhance mitochondrial respiration, reduce oxidative stress, and inhibit tau aggregation. These properties position MB as a promising candidate for Alzheimer’s therapy.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20463399/.
Poudel SB, Frikha-Benayed D, Ruff RR, Yildirim G, Dixit M, Korstanje R, Robinson L, Miller RA, Harrison DE, Strong JR, Schaffler MB, Yakar S. Targeting mitochondrial dysfunction using methylene blue or mitoquinone to improve skeletal aging. Aging (Albany NY). 2024 Mar 25;16(6):4948-4964. doi: 10.18632/aging.205147. PMID: 38535998.
Targeting mitochondrial dysfunction using methylene blue or mitoquinone to improve skeletal aging
This study explores the effects of methylene blue (MB) and mitoquinone (MitoQ) on mitochondrial function and skeletal aging in mice. The authors report that both compounds enhance mitochondrial respiration, reduce oxidative damage, and improve bone density. These findings suggest that targeting mitochondrial dysfunction with MB or MitoQ may be beneficial in mitigating age-related skeletal deterioration.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/38535998/.
Varanasi LK, Jayasankar K, Allen SC, Jenkins JS, Gonzalez-Lima F, Wang H. Methylene blue enhances resilience and energy metabolism under mitochondrial stress in C. elegans. Redox Biol. 2017 Aug;14:260-271. doi: 10.1016/j.redox.2017.07.004. PMID: 28738167.
Methylene blue enhances resilience and energy metabolism under mitochondrial stress in C. elegans
This study examined the effects of methylene blue (MB) on energy metabolism and resilience under mitochondrial stress in Caenorhabditis elegans. The researchers found that MB improved survival rates, reduced oxidative stress, and enhanced mitochondrial respiration in stressed worms. These findings highlight MB’s potential as a therapeutic agent for mitochondrial dysfunction.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28738167/.
Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012 Jan;96(1):32-45. doi: 10.1016/j.pneurobio.2011.10.007. Epub 2011 Nov 3. PMID: 22067440; PMCID: PMC3265679.
Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue
This paper presents the first comprehensive exploration of methylene blue’s memory-enhancing and neuroprotective mechanisms in vivo. These mechanisms hold significant potential for a novel neurobiological strategy to enhance regular memory and address memory loss and neurodegeneration linked to mitochondrial dysfunction. Unlike conventional drug-receptor interactions, methylene blue’s impact relies on distinctive metabolic pathways, exhibiting a hormetic dose-response pattern with contrasting effects at low and high doses. At lower doses, methylene blue acts as a mitochondrial electron transporter, displaying remarkable antioxidant and cell respiration-boosting attributes that adaptably influence nervous system function. Notably, the memory-enhancing impacts are linked to memory consolidation improvement within specific neural networks. Moreover, methylene blue’s application at low doses demonstrates efficacy in safeguarding against mitochondrial dysfunction-associated neurodegeneration, owing to its unique self-oxidizing property and broad effects on various tissue oxidases. The findings underscore the potential of low-dose methylene blue as a safe and promising avenue for memory enhancement and the management of conditions marked by oxidative stress, memory loss, and neurodegeneration.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3265679/.
Auchter A, Williams J, Barksdale B, Monfils MH, Gonzalez-Lima F. Therapeutic benefits of methylene blue on cognitive impairment during chronic cerebral hypoperfusion. J Alzheimers Dis. 2014;42 Suppl 4:S525-35. doi: 10.3233/JAD-141527. PMID: 25079810.
Therapeutic benefits of methylene blue on cognitive impairment during chronic cerebral hypoperfusion
Chronic cerebral hypoperfusion, linked to mild cognitive impairment and Alzheimer’s disease risk, impacts mitochondrial function and memory consolidation. Enhancing mitochondrial function with drugs could potentially treat cerebral hypoperfusion-related cognitive issues. Methylene blue (MB), which crosses the blood-brain barrier and aids mitochondrial electron transport at low doses, has shown memory enhancement and neuroprotection. Rats with bilateral carotid occlusion or sham surgery were treated with MB or saline daily for a month, undergoing various behavioral tests. Carotid occlusion rats performed poorly in the visual water maze test, but daily MB improved their visual learning and memory deficits. MB could offer cognitive benefits for chronic cerebral hypoperfusion conditions like mild cognitive impairment, vascular dementia, and Alzheimer’s disease, as indicated by this pioneering study.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25079810/.
Tucker D, Lu Y, Zhang Q. From Mitochondrial Function to Neuroprotection-an Emerging Role for Methylene Blue. Mol Neurobiol. 2018 Jun;55(6):5137-5153. doi: 10.1007/s12035-017-0712-2. Epub 2017 Aug 24. PMID: 28840449; PMCID: PMC5826781.
From Mitochondrial Function to Neuroprotection-an Emerging Role for Methylene Blue
Methylene blue (MB) is a well-established drug with a history of use for malaria, methemoglobinemia, and carbon monoxide poisoning. Its recent resurgence in interest stems from its role in mitochondria, where it enhances mitochondrial activity and reduces oxidative stress. Additionally, MB exhibits potent neuroinflammation mitigation. Mitochondrial dysfunction plays a pivotal role in neurodegenerative disorders, making MB a promising therapy. In both in vitro and in vivo studies, MB has demonstrated efficacy in mitigating neurodegeneration and associated behaviors in models of stroke, Alzheimer’s, Parkinson’s, and traumatic brain injury. This review highlights MB’s potential for neuroprotection, focusing on its impact on mitochondrial function, neurogenesis, and age-related cognitive decline.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5826781/.
Alda M. Methylene Blue in the Treatment of Neuropsychiatric Disorders. CNS Drugs. 2019 Aug;33(8):719-725. doi: 10.1007/s40263-019-00641-3. PMID: 31144270.
Methylene Blue in the Treatment of Neuropsychiatric Disorders
Methylene blue, a well-established drug with complex pharmacology, boasts a multitude of clinical applications due to its diverse mechanisms of action. Notably, it has demonstrated antidepressant, anxiolytic, and neuroprotective properties in both animal and human studies, largely attributed to its stabilizing influence on mitochondrial function and its dose-dependent impact on reactive oxygen species generation. As a result, methylene blue shows promise as a proof-of-concept treatment for organic/neurodegenerative disorders and as a general neuroprotective agent. In psychiatry, its century-long history includes successful applications in treating psychotic and mood disorders and enhancing memory during fear-extinction training. Particularly encouraging outcomes have emerged from short- and long-term use in bipolar disorder, where methylene blue has delivered antidepressant and anxiolytic effects without inducing manic episodes. Long-term usage has led to improved stability and reduced residual symptoms, although caution is advised due to its monoamine oxidase A inhibitory effect.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/31144270/.
Medina DX, Caccamo A, Oddo S. Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011 Mar;21(2):140-9. doi: 10.1111/j.1750-3639.2010.00430.x. PMID: 20731659; PMCID: PMC2992595.
Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity
In a phase II clinical trial aimed at evaluating methylene blue (MB) as a potential Alzheimer’s disease (AD) therapy, noteworthy improvements in cognitive function were observed in AD patients after 6 months of MB treatment. However, the specific mechanisms of MB’s action concerning AD pathology remained unclear, as no prior preclinical mammalian studies had been published. To uncover MB’s effects on AD pathology and its mechanism of action, we conducted experiments using a mouse model (3xTg‐AD) that replicates age-related Aβ and tau accumulation along with cognitive decline. Our findings reveal that chronic dietary MB treatment reduces Aβ levels and enhances learning and memory in 3xTg‐AD mice. The mechanism underlying MB’s impact on Aβ pathology appears to involve increased Aβ clearance, demonstrated by the enhanced chymotrypsin- and trypsin-like activities of brain proteasomes induced by MB. This study marks the first report of MB’s ability to augment proteasome function and ameliorate AD-like pathology in vivo, providing compelling support for MB’s potential as an AD treatment and offering insight into its mechanism of action.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992595/.
Méndez M, Fidalgo C, Arias JL, Arias N. Methylene blue and photobiomodulation recover cognitive impairment in hepatic encephalopathy through different effects on cytochrome c-oxidase. Behav Brain Res. 2021 Apr 9;403:113164. doi: 10.1016/j.bbr.2021.113164. Epub 2021 Feb 4. PMID: 33549685.
Methylene blue and photobiomodulation recover cognitive impairment in hepatic encephalopathy through different effects on cytochrome c-oxidase
Mitochondrial dysfunction is a central factor in hepatic encephalopathy (HE), resulting in altered cytochrome c-oxidase (CCO) enzyme activity and reduced brain metabolism. In an HE animal model, we investigated the effects of intracranial methylene blue (MB) administration and transcranial photobiomodulation (PBM), both targeting CCO, on cognitive recovery. Rats were divided into five groups: sham-operated + saline (SHAM + SAL), HE + SAL, SHAM + MB, HE + MB, and HE + PBM. PBM involved daily transcranial exposure to 670 ± 10 nm LED light (9 J/cm2) for 7 days, while the MB and SAL groups received accumbens injections (2.2 μg/0.5 μL). Cognitive improvement was observed in the HE group treated with MB, accompanied by reduced CCO activity in the prefrontal cortex, dorsal striatum, and dorsal hippocampus. Comparing MB and PBM, both improved HE-related memory deficits, but PBM led to a more generalized decrease in CCO activity in the prefrontal and entorhinal cortices, dorsal striatum, and hippocampus compared to MB. These findings suggest that CCO alteration contributes to mitochondrial dysfunction and reduced brain metabolism in HE, and both MB and PBM can mitigate these effects.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0166432821000516.
Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011 Jan;31(1):93-117. doi: 10.1002/med.20177. PMID: 19760660; PMCID: PMC3005530.
Cellular and molecular actions of Methylene Blue in the nervous system
Methylene Blue (MB), introduced to biology by Ehrlich in the 19th century, has since found diverse applications in medicine and biology. Currently, it serves as the primary treatment for methemoglobinemias, is commonly used to address ifosfamide-induced encephalopathy, and is a standard diagnostic tool in surgical procedures. Recent research indicates potential benefits of MB in Alzheimer’s disease and memory enhancement. While the modulation of the cGMP pathway is considered its primary mechanism, newer studies highlight its multiple cellular and molecular targets. MB’s distinctive physicochemical properties, including its planar structure, redox chemistry, ionic charges, and light spectrum characteristics, largely dictate its biological effects and clinical applications. This review explores these physicochemical attributes and MB’s impact on various cellular and molecular targets, particularly in the context of the nervous system.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3005530/.
Rodriguez P, Singh AP, Malloy KE, Zhou W, Barrett DW, Franklin CG, Altmeyer WB, Gutierrez JE, Li J, Heyl BL, Lancaster JL, Gonzalez-Lima F, Duong TQ. Methylene blue modulates functional connectivity in the human brain. Brain Imaging Behav. 2017 Jun;11(3):640-648. doi: 10.1007/s11682-016-9541-6. PMID: 26961091; PMCID: PMC5018244.
Methylene blue modulates functional connectivity in the human brain
Methylene blue USP (MB) is a drug approved by the FDA and used in hospitals to treat specific types of poisoning and blood-related issues. Recent research on animals has shown that it might improve brain responses and memory, even in small amounts. However, we don’t fully understand how it affects the human brain.
To learn more, we gave healthy adults a low dose of MB or a placebo, without them knowing which one. We then used brain scans while they did tasks and while their brains were at rest, both before and after taking MB or the placebo. The results were interesting: when people took MB, blood flow in a brain network connected to tasks decreased during a task, and different parts of the brain that are related to how we perceive things and remember them became more connected when the brain was resting. This suggests that even a small amount of MB can change how our brains work when we’re doing things and when we’re not.
These findings are important because they show that MB could have potential benefits for brain function, and we need to look into this more, especially in different groups of people and those with brain-related conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5018244/.
Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol. 2014 Apr 15;88(4):584-93. doi: 10.1016/j.bcp.2013.11.010. Epub 2013 Dec 4. PMID: 24316434.
Mitochondrial respiration as a target for neuroprotection and cognitive enhancement
This paper emphasizes brain mitochondrial respiration as a key therapeutic target for enhancing neuroprotection and cognitive function. It suggests that advancing brain mitochondrial respiration should be a crucial focus in research and treatment not only for Alzheimer’s disease (AD) but also for various cognitive impairment and neurodegenerative conditions. Three distinct approaches for enhancing brain mitochondrial respiration are proposed: (a) pharmacological intervention using low-dose USP methylene blue to boost mitochondrial respiration and promote memory improvement and neuroprotection, (b) photobiomodulation through transcranial low-level light/laser therapy using near-infrared light with similar neurometabolic mechanisms as methylene blue, and (c) nutrition intervention to enhance mitochondrial respiration by increasing dietary ketone bodies. These interventions aim to improve oxidative energy metabolism while reducing the pro-oxidant tendencies of the nervous system, representing a promising bioenergetics-driven strategy for addressing cognitive impairment and neurodegeneration in AD and related neuropsychological disorders.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0006295213007417?via%3Dihub.
Zakaria A, Hamdi N, Abdel-Kader RM. Methylene Blue Improves Brain Mitochondrial ABAD Functions and Decreases Aβ in a Neuroinflammatory Alzheimer’s Disease Mouse Model. Mol Neurobiol. 2016 Mar;53(2):1220-1228. doi: 10.1007/s12035-014-9088-8. Epub 2015 Jan 20. PMID: 25601181.
Methylene Blue Improves Brain Mitochondrial ABAD Functions and Decreases Aβ in a Neuroinflammatory Alzheimer’s Disease Mouse Model
Phase II clinical trials with Methylene blue (MB) have demonstrated cognitive improvements in Alzheimer’s disease (AD) patients, aligning with its known antioxidant and mitochondrial protective properties. Recent findings also indicate MB’s capacity to reduce amyloid beta (Aβ) levels in AD models. Since Aβ can induce mitochondrial dysfunction through its interaction with the mitochondrial enzyme amyloid-binding alcohol dehydrogenase (ABAD), implicating a direct link between Aβ toxicity and AD-related mitochondrial dysfunction, this study aimed to investigate whether MB’s protective effects involve ABAD regulation. The study shows that MB enhances cell viability, reduces reactive oxygen species levels, and notably decreases Aβ oligomers in a lipopolysaccharide (LPS) mouse model. Furthermore, MB treatment reduces ABAD levels, and it has the potential to modulate both ABAD levels and function, as seen in the restoration of estradiol levels in mouse brains. These findings shed new light on potential mechanisms through which MB may act in AD.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25601181/.
Gonzalez-Lima F, Auchter A. Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Front Cell Neurosci. 2015 May 12;9:179. doi: 10.3389/fncel.2015.00179. PMID: 26029050; PMCID: PMC4428125.
Protection against neurodegeneration with low-dose methylene blue and near-infrared light
This paper delves into the neuroprotective effects of low-dose methylene blue and near-infrared light, emphasizing their shared cellular mechanism of action centered on stimulating mitochondrial respiration. Low-dose methylene blue, upon systemic administration, preferentially enters neuronal mitochondria and forms an electron cycling redox complex that enhances the mitochondrial electron transport chain, promoting energy metabolism and neuronal survival. Conversely, low-level near-infrared light, applied transcranially, delivers photons to cortical neurons, accepted by cytochrome oxidase, thereby increasing cellular respiration and cerebral blood flow. These interventions show promising potential for safeguarding against various neurodegenerative disorders, underpinned by their common cellular mechanisms, including energy transfer, hormetic dose-responses, and enhanced oxidative metabolic energy production, ultimately shielding nervous tissue from degeneration.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4428125/.
Rodriguez P, Zhou W, Barrett DW, Altmeyer W, Gutierrez JE, Li J, Lancaster JL, Gonzalez-Lima F, Duong TQ. Multimodal Randomized Functional MR Imaging of the Effects of Methylene Blue in the Human Brain. Radiology. 2016 Nov;281(2):516-526. doi: 10.1148/radiol.2016152893. Epub 2016 Jun 28. PMID: 27351678; PMCID: PMC5084971.
Multimodal Randomized Functional MR Imaging of the Effects of Methylene Blue in the Human Brain
In a prospective, double-blinded clinical trial approved by the institutional review board, twenty-six participants were administered either a low dose of methylene blue or a placebo. Functional MR imaging showed increased neural activity in the bilateral insular cortex during sustained attention tasks and in the prefrontal, parietal, and occipital cortex during short-term memory tasks after methylene blue administration. Additionally, methylene blue led to a 7% improvement in memory retrieval. These findings suggest that low-dose methylene blue can enhance neural activity related to sustained attention, short-term memory, and memory retrieval in the healthy human brain.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5084971/.
Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009 Oct 15;78(8):927-32. doi: 10.1016/j.bcp.2009.04.034. Epub 2009 May 9. PMID: 19433072.
Methylene blue and Alzheimer’s disease
Methylene blue (MB) has garnered increased scientific interest in its potential to slow down Alzheimer’s disease (AD) progression. MB, known for its inhibitory effects on the cGMP pathway, also impacts various cellular and molecular factors linked to AD development. It has demonstrated the ability to reduce amyloid plaques and neurofibrillary tangles, partially restore mitochondrial function and cellular metabolism, and influence neurotransmitter systems such as cholinergic, serotonergic, and glutamatergic, all implicated in AD pathogenesis. These multifaceted effects of MB are believed to underpin its potential benefits, leading to the exploration of novel MB-based AD treatments. This review summarizes MB’s actions on neurotransmitter systems and diverse cellular and molecular targets in relation to AD.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19433072/.
Xie L, Li W, Winters A, Yuan F, Jin K, Yang S. Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation. Front Cell Neurosci. 2013 May 3;7:56. doi: 10.3389/fncel.2013.00056. PMID: 23653592; PMCID: PMC3642497.
Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation
Methylene blue, a compound previously recognized for its potential neuroprotective qualities in experimental neurodegenerative disease models, has posed intriguing questions about its underlying mechanisms. While prior research has highlighted the various roles of macroautophagy in maintaining cellular balance and its protective effects during myocardial ischemia, our current study reveals that methylene blue shields HT22 hippocampal cells from serum deprivation-induced death by promoting macroautophagy. Remarkably, inhibition of macroautophagy reverses methylene blue’s neuroprotective effects. Furthermore, our investigation indicates a dose-dependent activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) signaling, rather than inhibition of the mammalian target of rapamycin signaling, at 12 and 24 hours following methylene blue treatment. Notably, the inhibition of AMPK blocks methylene blue-induced macroautophagy. Supporting our in vitro findings, we observed macroautophagy induction in the cortex and hippocampus of methylene blue-treated mouse brains. In summary, our study suggests that methylene blue’s neuroprotection is, at least partially, mediated through macroautophagy activation via the AMPK signaling pathway.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3642497/.
Patel PN. Methylene blue for management of Ifosfamide-induced encephalopathy. Ann Pharmacother. 2006 Feb;40(2):299-303. doi: 10.1345/aph.1G114. Epub 2006 Jan 3. PMID: 16391008.
Methylene blue for management of Ifosfamide-induced encephalopathy
The study aimed to assess methylene blue’s effectiveness in treating ifosfamide-induced encephalopathy. Data were collected from MEDLINE (1966-August 2005) and International Pharmaceutical Abstracts (1971-August 2005) using specific search terms. The analysis revealed several case reports and one retrospective chart review detailing methylene blue’s use for this condition, but no controlled clinical trials were identified. Methylene blue showed promise in rapidly alleviating encephalopathic symptoms in some patients, often within 10 minutes of administration, yet its overall efficacy was modest in most cases. Notably, patients who didn’t receive methylene blue also experienced symptom resolution within a similar timeframe, suggesting the possibility of spontaneous recovery from ifosfamide-induced encephalopathy. Consequently, while methylene blue could be considered as a treatment option, particularly for severe cases, its true utility remains uncertain due to the lack of controlled clinical trials and the potential for spontaneous symptom improvement.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16391008/.
Isaev NK, Genrikhs EE, Stelmashook EV. Methylene blue and its potential in the treatment of traumatic brain injury, brain ischemia, and Alzheimer’s disease. Rev Neurosci. 2024 Mar 27;35(5):585-595. doi: 10.1515/revneuro-2024-0007. PMID: 38530227.
Methylene blue and its potential in the treatment of traumatic brain injury, brain ischemia, and Alzheimer’s disease
Traumatic brain injury (TBI) and brain ischemia/reperfusion initiate neurodegenerative processes that can progress into severe brain atrophy and dementia, resembling Alzheimer’s disease (AD). Shared mechanisms, including oxidative stress, progressive inflammation, glial activation, blood-brain barrier dysfunction, and excessive autophagy, present numerous therapeutic targets for intervention. Methylene blue (MB) has shown promise as a neuroprotective agent due to its antiapoptotic, anti-inflammatory, and autophagy-activating effects, along with its ability to inhibit protein aggregation and support mitochondrial function, making it a potential treatment for neurodegenerative disorders, including TBI, ischemia, and AD.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/38530227/.
Gureev AP, Sadovnikova IS, Popov VN. Molecular mechanisms of the neuroprotective effect of methylene blue. Biochemistry (Mosc). 2022 Sep;87(9):940-956. doi: 10.1134/S0006297922090073. PMID: 36180986.
Molecular mechanisms of the neuroprotective effect of methylene blue
This review explores the neuroprotective properties of methylene blue (MB), focusing on its molecular mechanisms. MB enhances mitochondrial function, reduces oxidative stress, and modulates neurotransmitter systems, contributing to its neuroprotective effects. These findings suggest MB’s potential as a therapeutic agent for neurodegenerative diseases.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/36180986/.
Rodriguez P, Singh AP, Malloy KE, Zhou W, Barrett DW, Franklin CG, Altmeyer WB, Gutierrez JE, Li J, Heyl BL, Lancaster JL, Gonzalez-Lima F, Duong TQ. Methylene blue modulates functional connectivity in the human brain. Brain Imaging Behav. 2017 Jun;11(3):640-648. doi: 10.1007/s11682-016-9541-6. PMID: 26961091.
Methylene blue modulates functional connectivity in the human brain
This randomized controlled trial investigated the effects of methylene blue (MB) on brain functional connectivity using fMRI. Results showed that MB administration increased connectivity in brain regions associated with memory and attention. These findings suggest MB’s potential role in enhancing cognitive functions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/26961091/.
Mori T, Koyama N, Segawa T, Maeda M, Maruyama N, Kinoshita N, Hou H, Tan J, Town T. Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. J Biol Chem. 2014 Oct 31;289(44):30303-30317. doi: 10.1074/jbc.M114.568212. PMID: 25157105.
Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice
This study examined the effects of methylene blue (MB) on Alzheimer’s disease pathology in transgenic mice. MB inhibited β-secretase activity, reduced amyloid plaque formation, and improved cognitive performance in the mice. These results indicate MB’s potential as a therapeutic agent for Alzheimer’s disease.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25157105/.
Miclescu A, Sharma HS, Martijn C, Wiklund L. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med. 2010 Nov;38(11):2199-206. doi: 10.1097/CCM.0b013e3181f26b0c. PMID: 20711066.
Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions
This study investigated methylene blue’s (MB) protective effects on the blood-brain barrier during ischemia/reperfusion injury. Findings showed that MB preserved the integrity of the blood-brain barrier and reduced neuronal damage. These results suggest MB’s potential in mitigating brain injuries caused by ischemic events.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20711066/.
Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012 Jan;96(1):32-45. doi: 10.1016/j.pneurobio.2011.10.007. PMID: 22067440.
Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue
This review explores how methylene blue (MB) enhances memory and provides neuroprotection by influencing neurometabolic pathways. MB improves mitochondrial function, leading to increased energy production in neurons. These findings suggest potential therapeutic applications for MB in neurodegenerative diseases.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22067440/.
Deng Y, Wang R, Li S, Zhu X, Wang T, Wu J, Zhang J. Methylene blue reduces incidence of early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery: An open-label randomized controlled clinical trial. J Clin Anesth. 2021 Feb;68:110108. doi: 10.1016/j.jclinane.2020.110108. PMID: 33091706.
Methylene blue reduces incidence of early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery: An open-label randomized controlled clinical trial
This study investigated the effect of methylene blue (MB) on early postoperative cognitive disorders in elderly patients undergoing major non-cardiac surgery. Researchers found that MB administration significantly reduced the incidence of postoperative cognitive dysfunction compared to the control group. These findings suggest that MB may be beneficial in preserving cognitive function following surgery in elderly patients.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/33091706/.
Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, Gonzalez-Lima F. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. Am J Psychiatry. 2014 Oct;171(10):1091-8. doi: 10.1176/appi.ajp.2014.13101407. PMID: 25018057.
Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia
This study examined the impact of methylene blue (MB) on fear extinction and contextual memory in adults with claustrophobia. Participants who received MB after exposure therapy sessions showed enhanced fear extinction and improved retention of contextual memory compared to those who received a placebo. These results suggest that MB may augment the effectiveness of exposure therapy for anxiety disorders.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25018057/.
Nair A. Using intravenous methylene blue to reduce postoperative cognitive disorders in elderly patients—a methodical approach. J Clin Anesth. 2021 Aug;71:110206. doi: 10.1016/j.jclinane.2021.110206. PMID: 33601280.
Using intravenous methylene blue to reduce postoperative cognitive disorders in elderly patients—a methodical approach
This article discusses a methodical approach to using intravenous methylene blue (MB) to mitigate postoperative cognitive disorders in elderly patients. The author emphasizes the importance of appropriate dosing, timing, and patient selection to maximize the neuroprotective benefits of MB. The commentary highlights the potential of MB as a therapeutic agent in reducing cognitive decline following surgery.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/33601280/.
Zheng F, Fang P, Chang J, Chen M, Zhong Q, Chen T, Chen C, Zhang Z. Methylene blue protects against sevoflurane-induced cognitive dysfunction by suppressing Drp1 deSUMOylation in aged mice. Neurochem Res. 2020 Apr;45(4):956-963. doi: 10.1007/s11064-020-02976-6. PMID: 32008150.
Methylene blue protects against sevoflurane-induced cognitive dysfunction by suppressing Drp1 deSUMOylation in aged mice
This study explored the protective effects of methylene blue (MB) against sevoflurane-induced cognitive dysfunction in aged mice. Researchers found that MB administration suppressed Drp1 deSUMOylation, a process implicated in mitochondrial dysfunction and neuronal damage. The findings suggest that MB may offer neuroprotection by maintaining mitochondrial integrity during anesthesia exposure.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/32008150/.
Hochgräfe K, Sydow A, Matenia D, Cadinu D, Könen S, Petrova O, Pickhardt M, Goll P, Morellini F, Mandelkow E, Mandelkow EM. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun. 2015 May 10;3:25. doi: 10.1186/s40478-015-0204-4. PMID: 25958115.
Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau
This study investigated the effects of preventive methylene blue (MB) treatment on cognitive function in mice expressing human Tau protein associated with neurodegenerative diseases. Researchers found that MB treatment preserved cognitive abilities and reduced Tau pathology in the brain. These results suggest that MB may have therapeutic potential in preventing cognitive decline related to Tau aggregation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25958115/.
Auchter AM, Barrett DW, Monfils MH, Gonzalez-Lima F. Methylene blue preserves cytochrome oxidase activity and prevents neurodegeneration and memory impairment in rats with chronic cerebral hypoperfusion. Front Cell Neurosci. 2020 May 20;14:130. doi: 10.3389/fncel.2020.00130. PMID: 32508596.
Methylene blue preserves cytochrome oxidase activity and prevents neurodegeneration and memory impairment in rats with chronic cerebral hypoperfusion
This study investigated the effects of methylene blue (MB) on cytochrome oxidase activity, neurodegeneration, and memory function in a rat model of chronic cerebral hypoperfusion. Researchers found that MB treatment preserved cytochrome oxidase activity, reduced neuronal loss, and prevented memory impairment. These findings suggest that MB may offer neuroprotective benefits in conditions involving reduced cerebral blood flow.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/32508596/.
Choi HJ, Han M, Jung B, Hong YR, Shin S, Lim S, Lee EH, Kim YK, Park J. Methylene blue delivery mediated by focused ultrasound-induced blood-brain barrier disruption reduces neural damage and amyloid-beta plaques by AQP-4 upregulation. Biomedicines. 2022 Dec 8;10(12):3191. doi: 10.3390/biomedicines10123191. PMID: 36551947.
Methylene blue delivery mediated by focused ultrasound-induced blood-brain barrier disruption reduces neural damage and amyloid-beta plaques by AQP-4 upregulation
This study explored the use of focused ultrasound (FUS) to enhance methylene blue (MB) delivery across the blood-brain barrier (BBB) in a mouse model of Alzheimer’s disease. The combination of FUS and MB treatment led to upregulation of aquaporin-4 (AQP-4), reduction in amyloid-beta plaques, and decreased neural damage. These results suggest that FUS-mediated MB delivery may be a promising strategy for treating neurodegenerative diseases.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/36551947/.
Paban V, Manrique C, Filali M, Maunoir-Regimbal S, Fauvelle F, Alescio-Lautier B. Therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in a transgenic mouse model. Neuropharmacology. 2014 Jan;76 Pt A:68-79. doi: 10.1016/j.neuropharm.2013.06.033. PMID: 23891615.
Therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in a transgenic mouse model
This study assessed both therapeutic and preventive effects of methylene blue (MB) on Alzheimer’s disease pathology using a transgenic mouse model. Researchers observed that MB treatment reduced amyloid-beta accumulation, improved cognitive performance, and decreased neuroinflammation. These findings indicate that MB may serve as both a treatment and preventive measure for Alzheimer’s disease.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23891615/.
Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, Boullon FJ. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. 2004 Feb;77(2):496-9. doi: 10.1016/S0003-4975(03)01510-8. PMID: 14759425.
Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery
The discovery of nitric oxide as a mediator in cardiac postoperative vasoplegia has prompted the investigation of inhibitory drugs like methylene blue, which have shown promise in isolated cases. This study aimed to assess the incidence and prognosis of postoperative vasoplegic syndrome and evaluate the impact of intravenous methylene blue on mortality. In total, 638 cardiac surgery patients were enrolled, with 8.8% meeting vasoplegia criteria, resulting in a higher mortality rate (10.7% vs. 3.6%). Patients treated with methylene blue exhibited reduced morbidity and mortality (0% vs. 21.4%), and the syndrome’s duration was shorter (less than 6 hours vs. over 48 hours in some cases). In conclusion, vasoplegic postoperative syndrome affected 8.8% of patients, leading to poorer outcomes, but methylene blue administration significantly improved mortality rates.
You can read the full article at https://www.annalsthoracicsurgery.org/article/S0003-4975(03)01510-8/fulltext.
Otero Luna AV, Johnson R, Funaro M, Canarie MF, Pierce RW. Methylene Blue for Refractory Shock in Children: A Systematic Review and Survey Practice Analysis. Pediatr Crit Care Med. 2020 Jun;21(6):e378-e386. doi: 10.1097/PCC.0000000000002295. PMID: 32453920.
A Systematic Review and Survey Practice Analysis
In the challenging realm of pediatric medicine, where shock resistant to conventional treatments poses grave risks to young patients, the potential role of methylene blue remains a largely uncharted territory. Our mission was to delve into this unexplored domain, gathering and summarizing existing literature while shedding light on the practices of physicians when it comes to employing methylene blue as a lifesaving measure for children in refractory shock. Our systematic search combed through databases from their inception up to 2019, unveiling 24 pertinent studies that, despite their limited quantity and sometimes questionable quality, hinted at the safety and efficacy of methylene blue in elevating mean arterial blood pressure among pediatric patients enduring refractory shock of various origins. Simultaneously, we probed the minds of U.S.-based pediatric critical care physicians through an electronic survey, revealing a striking schism: 40% reported using methylene blue, while 43% had never contemplated its use. The reasons for this divide were multifaceted, spanning from a lack of familiarity with the drug to uncertainties about dosing and insufficient empirical backing. This duality of practice patterns underscores the pressing need for rigorous studies to definitively assess methylene blue’s safety and efficacy in the treatment of pediatric shock, ultimately guiding physicians towards more informed and effective clinical decisions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/32453920/.
Zhao CC, Zhai YJ, Hu ZJ, Huo Y, Li ZQ, Zhu GJ. Efficacy and safety of methylene blue in patients with vasodilatory shock: A systematic review and meta-analysis. Front Med (Lausanne). 2022 Sep 26;9:950596. doi: 10.3389/fmed.2022.950596. PMID: 36237547; PMCID: PMC9552293.
Efficacy and safety of methylene blue in patients with vasodilatory shock
This systematic review and meta-analysis aimed to assess the efficacy and safety of methylene blue (MB) in vasodilatory shock patients. After analyzing 15 studies involving 832 patients, it was found that MB, when administered alongside vasopressors, significantly lowered mortality rates and reduced vasopressor requirements. MB also improved hemodynamics by increasing mean arterial pressure, heart rate, and peripheral vascular resistance, while lowering the incidence of renal failure and lactate levels. No serious side effects were observed. Further research is needed to validate these results, but concomitant MB and vasopressor administration appears promising for vasodilatory shock management.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9552293/.
Gachot B, Bedos JP, Veber B, Wolff M, Regnier B. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med. 1995 Dec;21(12):1027-31. doi: 10.1007/BF01700666. PMID: 8750129.
Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock
This prospective, open-label, single-dose study aimed to investigate the acute effects of methylene blue (MB), an inhibitor of the L-arginine nitric oxide pathway, in six patients with severe septic shock treated in the medical ICU of a university hospital. The study found that MB increased mean arterial pressure, mean pulmonary artery pressure, systemic vascular resistance index, and pulmonary vascular resistance index while decreasing PaO2/FIO2 without significant changes in other hemodynamic parameters. The effects on oxygenation may limit its use in patients with adult respiratory distress syndrome. The study suggests that MB induces vasoconstriction in septic shock patients, and larger studies are needed to assess its impact on patient outcomes.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8750129/.
Daemen-Gubbels CR, Groeneveld PH, Groeneveld AB, van Kamp GJ, Bronsveld W, Thijs LG. Methylene blue increases myocardial function in septic shock. Crit Care Med. 1995 Aug;23(8):1363-70. doi: 10.1097/00003246-199508000-00009. PMID: 7634806.
Methylene blue increases myocardial function in septic shock
This open-label, nonrandomized clinical trial investigated the role of nitric oxide in circulatory changes in human septic shock. Nine consecutive patients with septic shock were studied in an intensive care unit. After initial resuscitation, they received a 20-minute infusion of 2 mg/kg of methylene blue, an inhibitor of nitric oxide action. The results showed that methylene blue temporarily increased mean arterial pressure and cardiac index, along with changes in vascular resistance and arterial compliance. Oxygen delivery and uptake also improved. These findings suggest that nitric oxide may contribute to the circulatory alterations seen in septic shock after initial resuscitation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7634806/.
Ibarra-Estrada M, Kattan E, Aguilera-González P, Sandoval-Plascencia L, Rico-Jauregui U, Gómez-Partida CA, Ortiz-Macías IX, López-Pulgarín JA, Chávez-Peña Q, Mijangos-Méndez JC, Aguirre-Avalos G, Hernández G. Early adjunctive methylene blue in patients with septic shock: a randomized controlled trial. Crit Care. 2023 Mar 13;27(1):110. doi: 10.1186/s13054-023-04397-7. PMID: 36915146; PMCID: PMC10010212.
Early adjunctive methylene blue in patients with septic shock: a randomized controlled trial
In this single-center randomized controlled trial, researchers investigated the potential benefits of methylene blue (MB) as an early adjunctive therapy for septic shock. They randomized 91 patients with septic shock according to Sepsis-3 criteria into two groups: MB and placebo. The study found that MB, when initiated within 24 hours, significantly reduced the time to vasopressor discontinuation, increased vasopressor-free days at 28 days, and shortened ICU and hospital stays without adverse effects. While days on mechanical ventilation and mortality rates were similar, these findings suggest the potential of MB as an adjuvant therapy, warranting further investigation in larger randomized clinical trials.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10010212/.
Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010 Jul;30(7):702-15. doi: 10.1592/phco.30.7.702. PMID: 20575634.
Methylene blue for the treatment of septic shock
This single-center randomized controlled trial aimed to investigate the potential benefits of early adjunctive methylene blue (MB) in septic shock patients. Among the 91 participants, with 45 in the MB group and 46 in the placebo group, MB administration initiated within 24 hours significantly reduced the time to vasopressor discontinuation, increased vasopressor-free days at 28 days, and led to shorter ICU and hospital stays without adverse effects. Days on mechanical ventilation and mortality rates were similar between the groups. These findings support the need for further research on MB’s role in septic shock through larger randomized clinical trials.
You can read the abstract of this article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10010212/.
Luis-Silva F, Menegueti MG, Sepeda CDR, Petroski-Moraes BC, Sato L, Peres LM, Becari C, Basile-Filho A, Evora PRB, Martins-Filho OA, Auxiliadora-Martins M. Effect of methylene blue on hemodynamic and metabolic response in septic shock patients. Medicine (Baltimore). 2022 Jan 21;101(3):e28599. doi: 10.1097/MD.0000000000028599. PMID: 35060528; PMCID: PMC8772761.
Effect of methylene blue on hemodynamic and metabolic response in septic shock patients
Methylene blue (MB) has been utilized to raise blood pressure in septic shock by affecting guanylate cyclase and nitric oxide synthase activity. This case series involving six septic shock patients within 72 hours of diagnosis aimed to demonstrate the benefits of MB in the early phase of septic shock. After fluid replacement, norepinephrine, and vasopressin, patients received a loading dose of MB followed by a 48-hour maintenance dose. Results showed a reduction in vasopressor dose and lactate levels after the loading dose of MB, with sustained effects during the maintenance phase. Interleukin 6 and interleukin 8 levels, initially elevated during septic shock, progressively decreased following MB infusion, suggesting a role for MB in reducing inflammation. While these findings are promising, further studies are needed to confirm their validity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9875988/.
Habib AM, Elsherbeny AG, Almehizia RA. Methylene Blue for Vasoplegic Syndrome Postcardiac Surgery. Indian J Crit Care Med. 2018 Mar;22(3):168-173. doi: 10.4103/ijccm.IJCCM_494_17. PMID: 29657374; PMCID: PMC5879859.
Vasoplegic Syndrome Postcardiac Surgery
In this retrospective observational historical control-matched study, the objective was to assess the efficacy of methylene blue (MB) as an alternative treatment for vasoplegia after cardiopulmonary bypass (CPB). Patients who received MB for post-CPB vasoplegia from 2010 to 2015 were compared to historical controls from 2004 to 2009. The study included 28 matched patients in each group, and findings revealed that MB administration led to a significantly quicker improvement in vasoplegia (Ti), reduced mortality at day 30, decreased cardiac surgical Intensive Care Unit (CSICU) morbidity, and shorter hospital stays. The use of MB was associated with rapid hemodynamic recovery, reduced reliance on vasopressors, lower ICU mortality, decreased incidence of renal failure, and shorter length of stay post-cardiac surgery.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5879859/.
Rodriguez P, Jiang Z, Huang S, Shen Q, Duong TQ. Methylene blue treatment delays progression of perfusion-diffusion mismatch to infarct in permanent ischemic stroke. Brain Res. 2014 Nov 7;1588:144-9. doi: 10.1016/j.brainres.2014.09.007. Epub 2014 Sep 8. PMID: 25218555; PMCID: PMC4907336.
Methylene blue treatment delays progression of perfusion-diffusion mismatch to infarct in permanent ischemic stroke
Stroke is a major global health concern, and low-dose methylene blue (MB), known for its safe use in treating methemoglobinemia and cyanide poisoning, was investigated for its potential neuroprotective effects in rats with permanent middle cerebral artery occlusion. Serial MRI assessments showed that MB notably extended the perfusion-diffusion mismatch, mildly improved blood flow in underperfused areas, and sustained ATP production. However, it did not alter the final infarct volume, and there were no dose-dependent effects observed. These findings suggest that MB may offer neuroprotection by maintaining ATP production and enhancing blood flow in at-risk brain tissue, making it a promising candidate for clinical stroke trials due to its established safety profile.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4907336/.
Gillman PK. Methylene blue is a potent monoamine oxidase inhibitor. Can J Anaesth. 2008 May;55(5):311-2; author reply 312. doi: 10.1007/BF03017212. PMID: 18451123.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/18451123/.
Oxenkrug GF, Sablin SO, Requintina PJ. Effect of methylene blue and related redox dyes on monoamine oxidase activity; rat pineal content of N-acetylserotonin, melatonin, and related indoles; and righting reflex in melatonin-primed frogs. Ann N Y Acad Sci. 2007 Dec;1122:245-52. doi: 10.1196/annals.1403.017. PMID: 18077577.
Effect of methylene blue and related redox dyes on monoamine oxidase activity; rat pineal content of N-acetylserotonin, melatonin, and related indoles; and righting reflex in melatonin-primed frogs
This study explored the mechanisms underlying the antidepressant effects of methylene blue (MB). While previous research suggested that MB might work by inhibiting nitric oxide-induced stimulation of N-methyl-D-aspartate receptors, this study investigated additional mechanisms. It found that MB and related redox dyes, including toluidine blue O (TBO), thionine (TN), brilliant cresyl blue (BCB), and toluylene blue (TB), acted as reversible competitive inhibitors of monoamine oxidase type A (MAO-A) and monoamine oxidase type B (MAO-B), with a strong preference for MAO-A. These dyes also increased the levels of N-acetylserotonin (NAS) and melatonin in the pineal gland, consistent with the stimulation of melatonin biosynthesis observed with selective MAO-A inhibition. Furthermore, these dyes exhibited antidepressant-like activity in frogs, indicating a potential role of MAO-A inhibition in mediating the clinical antidepressant effect of MB through NAS stimulation and melatonin production.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/18077577/.
Delport A, Harvey BH, Petzer A, Petzer JP. The monoamine oxidase inhibition properties of selected structural analogues of methylene blue. Toxicol Appl Pharmacol. 2017 Jun 15;325:1-8. doi: 10.1016/j.taap.2017.03.026. Epub 2017 Apr 1. PMID: 28377303.
The monoamine oxidase inhibition properties of selected structural analogues of methylene blue
Methylene blue (MB), a thionine dye, is known for its potent inhibition of monoamine oxidase (MAO) A, contributing to its antidepressant effects and its potential for serotonin toxicity (ST) when combined with serotonergic drugs. This study explored the MAO inhibition properties of five MB analogues: neutral red, Nile blue, new methylene blue, cresyl violet, and 1,9-dimethyl methylene blue. Like MB, these analogues exhibited specific MAO-A inhibition, with cresyl violet, Nile blue, and 1,9-dimethyl methylene blue showing even greater potency than MB. Nile blue also demonstrated strong MAO-B inhibition. These findings suggest that non-thionine MB analogues, such as cresyl violet and Nile blue, should be considered as potential high-potency MAO-A inhibitors with a risk of ST, similar to phenothiazines like MB, in pharmacological studies.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0041008X17301400.
Alda M. Methylene Blue in the Treatment of Neuropsychiatric Disorders. CNS Drugs. 2019 Aug;33(8):719-725. doi: 10.1007/s40263-019-00641-3. PMID: 31144270.
Methylene Blue in the Treatment of Neuropsychiatric Disorders
Methylene blue is a versatile and well-established drug with a range of clinical applications, driven by its multifaceted mechanisms of action. Notably, it has demonstrated antidepressant, anxiolytic, and neuroprotective properties supported by both animal and human studies. Its capacity to stabilize mitochondrial function and regulate reactive oxygen species production holds significant promise in treating organic/neurodegenerative disorders and providing neuroprotection. In psychiatry, methylene blue has been used successfully for over a century, proving effective in treating psychotic and mood disorders and enhancing memory in fear-extinction training. Notably, it has shown promise in the short- and long-term management of bipolar disorder, offering antidepressant and anxiolytic benefits without the risk of inducing manic episodes. While generally well-tolerated, caution is advised due to its inhibitory effect on monoamine oxidase A.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/31144270/.
Alda M, McKinnon M, Blagdon R, Garnham J, MacLellan S, O’Donovan C, Hajek T, Nair C, Dursun S, MacQueen G. Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. British Journal of Psychiatry. 2017 Jan;210(1):54-60. doi: 10.1192/bjp.bp.115.173930. Epub 2016 Jun 9. PMID: 27284082.
Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study
In a double-blind crossover study, low-dose methylene blue (15 mg, ‘placebo’) and an active dose (195 mg) were investigated in bipolar disorder patients already receiving lamotrigine treatment. Over a 6-month trial, the active dose of methylene blue significantly alleviated depression symptoms measured by the Montgomery-Åsberg Depression Rating Scale and Hamilton Rating Scale for Depression, as well as reducing anxiety symptoms assessed by the Hamilton Rating Scale for Anxiety. Mania symptoms remained stable, and although methylene blue had no significant effect on cognitive symptoms, it was well-tolerated with mild and temporary side effects. These findings suggest that methylene blue, as an adjunctive treatment, can improve residual depression and anxiety symptoms in bipolar disorder patients.
You can read the full article at https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/methylene-blue-treatment-for-residual-symptoms-of-bipolar-disorder-randomised-crossover-study/D3ACE2C595AADA7AF7410A8EF7BAF6D5.
Delport A, Harvey BH, Petzer A, Petzer JP. Methylene blue and its analogues as antidepressant compounds. Metab Brain Dis. 2017 Oct;32(5):1357-1382. doi: 10.1007/s11011-017-0081-6. Epub 2017 Jul 31. PMID: 28762173.
Methylene blue and its analogues as antidepressant compounds
Methylene Blue (MB) has a wide range of medical applications, including its established use in treating methemoglobinemias and ifosfamide-induced encephalopathy. Recently, MB has gained attention for its potential as an antimalarial agent and as a treatment for neurodegenerative conditions like Alzheimer’s disease. Notably, MB has shown antidepressant and anxiolytic properties in pre-clinical models and has demonstrated promise in clinical trials for schizophrenia and bipolar disorder. It functions as an inhibitor of monoamine oxidase A (MAO-A), a known target for antidepressant effects, and it also inhibits nitric oxide synthase (NOS) and guanylate cyclase, which are relevant in mood and anxiety disorders. Furthermore, MB’s ability to restore mitochondrial function and mitigate redox imbalances contributes to its therapeutic potential. The use of MB in depression alongside neurodegenerative disorders like Alzheimer’s and Parkinson’s disease is a promising approach. Analogues of MB with similar properties may offer novel multi-target strategies for treating depression, potentially with fewer adverse effects.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28762173/.
Delport A, Harvey BH, Petzer A, Petzer JP. Methylene Blue Analogues with Marginal Monoamine Oxidase Inhibition Retain Antidepressant-like Activity. ACS Chem Neurosci. 2018 Dec 19;9(12):2917-2928. doi: 10.1021/acschemneuro.8b00042. Epub 2018 Jul 25. PMID: 29976053.
Methylene Blue Analogues with Marginal Monoamine Oxidase Inhibition Retain Antidepressant-like Activity
Methylene blue (MB) boasts a wide range of medical applications, with particular interest in its antidepressant-like effects in animals and potential for mood disorder treatment in clinical trials. While its antidepressant properties may arise from various mechanisms, including modulation of the nitric oxide cyclic guanosine monophosphate (NO-cGMP) cascade, mitochondrial respiration enhancement, and antioxidant effects, MB is also a potent inhibitor of monoamine oxidase (MAO) A. This dual nature, while beneficial for antidepressant effects, has raised concerns about adverse effects like serotonin toxicity. To address this, a study synthesized five new MB analogues with lower MAO-A inhibitory activity and found that these analogues exhibited antidepressant-like properties in rats without affecting locomotor activity. These results suggest that these novel MB analogues may provide effective antidepressant compounds with reduced MAO-A-related adverse effects, offering potential as a new class of antidepressants.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29976053/.
Naylor GJ, Smith AH, Connelly P. A controlled trial of methylene blue in severe depressive illness. Biol Psychiatry. 1987 May;22(5):657-9. doi: 10.1016/0006-3223(87)90194-6. PMID: 3555627.
A controlled trial of methylene blue in severe depressive illness
In a 3-week trial, a daily dose of 15 mg of methylene blue was compared to a placebo for the treatment of severe depressive illness. The study was carefully designed to minimize both placebo response bias and observer bias. Results indicated that patients who received methylene blue showed significantly greater improvement compared to those on placebo. This suggests that a daily dose of 15 mg of methylene blue may be a potent antidepressant, warranting further clinical evaluation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/3555627/.
Xiong ZM, O’Donovan M, Sun L, Choi JY, Ren M, Cao K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci Rep. 2017 May 30;7(1):2475. doi: 10.1038/s41598-017-02419-3. PMID: 28559565; PMCID: PMC5449383.
Anti-Aging Potentials of Methylene Blue for Human Skin Longevity
Oxidative stress is the major cause of skin aging that includes wrinkles, pigmentation, and weakened wound healing ability. Application of antioxidants in skin care is well accepted as an effective approach to delay the skin aging process. Methylene blue (MB), a traditional mitochondrial-targeting antioxidant, showed a potent ROS scavenging efficacy in cultured human skin fibroblasts derived from healthy donors and from patients with progeria, a genetic premature aging disease. In comparison with other widely used general and mitochondrial-targeting antioxidants, we found that MB was more effective in stimulating skin fibroblast proliferation and delaying cellular senescence. The skin irritation test, performed on an in vitro reconstructed 3D human skin model, indicated that MB was safe for long-term use, and did not cause irritation even at high concentrations. Application of MB to this 3D skin model further demonstrated that MB improved skin viability, promoted wound healing and increased skin hydration and dermis thickness. Gene expression analysis showed that MB treatment altered the expression of a subset of extracellular matrix proteins in the skin, including upregulation of elastin and collagen 2A1, two essential components for healthy skin. Altogether, our study suggests that MB has a great potential for skin care.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5449383/.
Xiong ZM, Mao X, Trappio M, Arya C, Kordi JE, Cao K. Ultraviolet radiation protection potentials of Methylene Blue for human skin and coral reef health. Sci Rep. 2021 May 28;11(1):10871. doi: 10.1038/s41598-021-89970-2. PMID: 34050204; PMCID: PMC8163870.
Ultraviolet radiation protection potentials of Methylene Blue for human skin and coral reef health
Methylene blue (MB) is a century-old medicinal compound and laboratory dye, recently recognized for its potent antioxidant properties in combating ROS-induced cellular aging in human skin. Leveraging MB’s molecular structure and light absorption characteristics, this study investigates its potential as a sunscreen active ingredient for UV radiation protection. The research demonstrates that MB treatment reduces DNA damage and cell death caused by UVB irradiation in primary human keratinocytes. Compared to Oxybenzone, a commonly used chemical sunscreen ingredient with known ecological risks, MB exhibits superior UVB absorption capability at the same concentrations and effectively prevents UVB-induced DNA damage while clearing UVA-induced cellular ROS. Importantly, unlike Oxybenzone, MB-containing seawater does not adversely affect the growth of the coral species Xenia umbellata. This study suggests that MB holds promise as a coral reef-friendly sunscreen active ingredient, offering broad-spectrum protection against both UVA and UVB radiation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8163870/.
Samalavicius NE, Poskus T, Gupta RK, Lunevicius R. Long-term results of single intradermal 1 % methylene blue injection for intractable idiopathic pruritus ani: a prospective study. Tech Coloproctol. 2012 Aug;16(4):295-9. doi: 10.1007/s10151-012-0846-1. Epub 2012 Jun 6. PMID: 22669483.
Long-term results of single intradermal 1 % methylene blue injection for intractable idiopathic pruritus ani: a prospective study
This study aimed to evaluate the efficacy and side effects of intradermal methylene blue injection in the perianal skin of patients suffering from chronic refractory idiopathic pruritus ani (IPA). Ten IPA patients, unresponsive to standard treatments and care, received intradermal injections of 1% methylene blue solution into the itching perianal area. Symptoms resolved within 4 weeks in all cases, with numbness and tattooing disappearing within 3-4 weeks. No skin necrosis or anaphylaxis occurred during the study, and while anal itching recurred in 8 patients, 4 noted milder symptoms upon recurrence, while 6 out of 10 patients reported significant improvement or resolution of pruritus ani. The procedure showed a 20% success rate within a 60-month follow-up period, demonstrating positive effects on IPA with mild sensory side effects.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22669483/.
Scigliano G, Scigliano GA. Methylene blue in covid-19. Med Hypotheses. 2021 Jan;146:110455. doi: 10.1016/j.mehy.2020.110455. Epub 2020 Dec 10. PMID: 33341032; PMCID: PMC7728423.
Methylene blue in covid-19
SARS-CoV-2 infection generally begins in the respiratory tract where it can cause bilateral pneumonia. The disease can evolve into acute respiratory distress syndrome and multi-organ failure, due to viral spread in the blood and an excessive inflammatory reaction including cytokine storm. Antiviral and anti-cytokine drugs have proven to be poorly or in-effective in stopping disease progression, and mortality or serious chronic damage is common in severely ill cases. The low efficacy of antiviral drugs is probably due to late administration, when the virus has triggered the inflammatory reaction and is no longer the main protagonist. The relatively poor efficacy of anti-cytokine drugs is explained by the fact that they act on one or a few of the dozens of cytokines involved, and because other mediators of inflammation – reactive oxygen and nitrogen species – are not targeted. When produced in excess, reactive species cause extensive cell and tissue damage. The only drug known to inhibit the excessive production of reactive species and cytokines is methylene blue, a low-cost dye with antiseptic properties used effectively to treat malaria, urinary tract infections, septic shock, and methaemoglobinaemia. We propose testing methylene blue to contrast Covid-related acute respiratory distress syndrome, but particularly suggest testing it early in Covid infections to prevent the hyper-inflammatory reaction responsible for the serious complications of the disease.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7728423/.
Dabholkar N, Gorantla S, Dubey SK, Alexander A, Taliyan R, Singhvi G. Repurposing methylene blue in the management of COVID-19: Mechanistic aspects and clinical investigations. Biomed Pharmacother. 2021 Oct;142:112023. doi: 10.1016/j.biopha.2021.112023. Epub 2021 Aug 10. PMID: 34399199; PMCID: PMC8352658.
Repurposing methylene blue in the management of COVID-19: Mechanistic aspects and clinical investigations
The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), responsible for COVID-19, was declared a pandemic by the World Health Organization in March 2020. This virus primarily infects the respiratory tract, particularly the lungs, sometimes progressing to acute respiratory distress syndrome and multi-organ failure. Current COVID-19 treatments involve antiviral and anti-cytokine drugs, but their efficacy is limited as they can’t simultaneously inhibit free radical and cytokine production. Methylene blue (MB) has recently garnered attention as a potential treatment. MB, a well-established therapeutic agent with FDA approval for other conditions, offers the added benefit of affordability and safety when used at low doses (< 2 mg/kg). This review explores MB’s applicability in COVID-19 management, delving into its mechanisms and detailing relevant clinical studies, thereby examining its potential role in COVID-19 treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8352658/.
Gendrot M, Andreani J, Duflot I, Boxberger M, Le Bideau M, Mosnier J, Jardot P, Fonta I, Rolland C, Bogreau H, Hutter S, La Scola B, Pradines B. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int J Antimicrob Agents. 2020 Dec;56(6):106202. doi: 10.1016/j.ijantimicag.2020.106202. Epub 2020 Oct 16. PMID: 33075512; PMCID: PMC7566888.
Methylene blue inhibits replication of SARS-CoV-2 in vitro
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, leading to coronavirus disease 2019 (COVID-19). Currently, there is no recommended antiviral treatment against SARS-CoV-2, necessitating the urgent search for effective antiviral drugs. Methylene blue, known for its in vitro antiviral activity in photodynamic therapy and its antibacterial, antifungal, and antiparasitic properties, exhibited significant in vitro activity against SARS-CoV-2 at very low micromolar concentrations, with an EC50 (median effective concentration) of 0.30 ± 0.03 μM and an EC90 (90% effective concentration) of 0.75 ± 0.21 μM at a multiplicity of infection (MOI) of 0.25. These values are lower than those obtained for hydroxychloroquine (1.5 μM and 3.0 μM) and azithromycin (20.1 μM and 41.9 μM). Methylene blue concentrations in human blood support its potential as a promising drug for COVID-19 treatment, warranting further in vivo evaluation in animal models and prospective clinical studies to confirm its effectiveness.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7566888/.
Arentz J, von der Heide HJ. Evaluation of methylene blue based photodynamic inactivation (PDI) against intracellular B-CoV and SARS-CoV2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a Covid19 infection. Photodiagnosis Photodyn Ther. 2022 Mar;37:102642. doi: 10.1016/j.pdpdt.2021.102642. Epub 2021 Dec 2. PMID: 34863949; PMCID: PMC8635689.
Evaluation of methylene blue based photodynamic inactivation (PDI) against intracellular B-CoV and SARS-CoV2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a Covid19 infection
Local antiviral photodynamic inactivation (PDI) has the potential to reduce viral load in the nose and throat during early-phase COVID-19 infections. In this study, low-dose methylene blue (MB) was explored as a photosensitizer, and LED light was used instead of a laser. Initial experiments with BCoV-infected cells showed that 0.001% MB sensitizer, combined with LED irradiation for 2.5 or 10 minutes, resulted in a significant reduction in viral load (logarithmic reduction factor ≥ 5.29). In contrast, dark conditions with MB sensitizer alone showed residual viruses. In SARS-CoV-2 experiments with VERO E6 infected cells, a minimum concentration of 0.0001% MB and a minimum radiation intensity of 20,000 lx led to a 99.99% reduction in intracellular and extracellular viruses after one minute of exposure. These findings suggest a potential PDI therapy option for early-phase COVID-19 using MB and LED light.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8635689/.
Gama CRB, Pombo MAG, Nunes CP, Gama GF, Mezitis SGE, Suchmacher Neto M, Guimarães OR, Geller M, Oliveira L, de Souza da Fonseca A, Sitnoveter A, Goldwasser G, Cunha KS, Darrigo Junior LG. Treatment of Recurrent Urinary Tract Infection Symptoms with Urinary Antiseptics Containing Methenamine and Methylene Blue: Analysis of Etiology and Treatment Outcomes. Res Rep Urol. 2020 Dec 15;12:639-649. doi: 10.2147/RRU.S279060. PMID: 33365282; PMCID: PMC7751791.
Treatment of Recurrent Urinary Tract Infection Symptoms with Urinary Antiseptics Containing Methenamine and Methylene Blue: Analysis of Etiology and Treatment Outcomes
This prospective, double-blind, randomized study compared the efficacy and safety of two urinary antiseptic combinations, methenamine 120mg + methylene blue 20mg (Group A) and acriflavine 15mg + methenamine 250mg + methylene blue 20mg + Atropa belladonna L. 15mg (Group B), in treating recurrent cystitis. Both groups showed improvement in UTI symptoms assessed by the UTISA questionnaire after 3 days of treatment, with no significant difference in efficacy. However, Group A had fewer treatment-related adverse events compared to Group B, indicating that the combination of methenamine + methylene blue resulted in a more favorable safety profile.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7751791/.
Cagno V, Medaglia C, Cerny A, Cerny T, Zwygart AC, Cerny E, Tapparel C. Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci Rep. 2021 Jul 12;11(1):14295. doi: 10.1038/s41598-021-92481-9. PMID: 34253743; PMCID: PMC8275569.
Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro
Methylene blue, an FDA and EMA approved drug known for its safety, demonstrates broad-spectrum virucidal activity, particularly when exposed to UV light. This study investigated its effectiveness against influenza virus H1N1 and SARS-CoV-2, both as a preventive and therapeutic measure. Methylene blue displayed antiviral activity at low micromolar concentrations, even without UV activation, suggesting its potential for clinical trials as a preventive or therapeutic agent against these infections. The drug’s mechanisms of action, including genomic RNA degradation, were also explored, emphasizing its multifaceted antiviral properties.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8275569/.
Shaw JD, Miller S, Plourde A, Shaw DL, Wustrack R, Hansen EN. Methylene Blue-Guided Debridement as an Intraoperative Adjunct for the Surgical Treatment of Periprosthetic Joint Infection. J Arthroplasty. 2017 Dec;32(12):3718-3723. doi: 10.1016/j.arth.2017.07.019. Epub 2017 Jul 21. PMID: 28811108.
Methylene Blue-Guided Debridement as an Intraoperative Adjunct for the Surgical Treatment of Periprosthetic Joint Infection
Current methods for identifying infected tissue in periprosthetic joint infection (PJI) are insufficient. This prospective study assessed the use of methylene blue-guided surgical debridement as a novel technique in PJI. Sixteen total knee arthroplasty patients with PJI underwent this procedure, and samples from methylene blue-stained and unstained tissues were analyzed using various methods. The results showed that more bacteria and higher neutrophil counts were observed in methylene blue-stained tissue, suggesting its potential as a visual guide for surgical debridement in PJI treatment. Clinical success, defined as infection eradication and infection-free survival, was also evaluated, with some patients experiencing new infections at follow-up.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28811108/.
Ansari MA, Fatima Z, Hameed S. Antifungal Action of Methylene Blue Involves Mitochondrial Dysfunction and Disruption of Redox and Membrane Homeostasis in C. albicans. Open Microbiol J. 2016 Feb 25;10:12-22. doi: 10.2174/1874285801610010012. PMID: 27006725; PMCID: PMC4780517.]
Antifungal Action of Methylene Blue Involves Mitochondrial Dysfunction and Disruption of Redox and Membrane Homeostasis in C. albicans
Candida albicans is a well-known cause of infections, particularly in immunocompromised individuals, with manifestations ranging from superficial to systemic. This study investigated the antifungal mechanism of Methylene blue (MB) against C. albicans and clinical isolates. The research demonstrated MB’s efficacy against C. albicans, including non-albicans species, and revealed that its antifungal action was not reliant on major drug efflux pumps but likely involved mitochondrial inhibition, as evidenced by increased sensitivity on non-fermentable carbon sources. MB also disrupted membrane integrity, reduced ergosterol levels, and inhibited the transition from yeast to hyphal forms, a significant virulence attribute in Candida infections. Overall, this study highlights MB as a promising antifungal agent for combating Candida infections.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4780517/.
Steinmann E, Gravemann U, Friesland M, Doerrbecker J, Müller TH, Pietschmann T, Seltsam A. Two pathogen reduction technologies–methylene blue plus light and shortwave ultraviolet light–effectively inactivate hepatitis C virus in blood products. Transfusion. 2013 May;53(5):1010-8. doi: 10.1111/j.1537-2995.2012.03858.x. Epub 2012 Aug 21. PMID: 22905868.
Two pathogen reduction technologies–methylene blue plus light and shortwave ultraviolet light–effectively inactivate hepatitis C virus in blood products
The contamination of blood products with hepatitis C virus (HCV) poses a risk of liver diseases. To combat this, pathogen reduction methods like methylene blue (MB) plus visible light and shortwave ultraviolet (UVC) light irradiation have been developed to inactivate viruses in plasma and platelet concentrates (PCs). Previous studies only used model viruses for HCV, but this research employed a cell culture-derived HCV infection system. The results show that both MB plus light and UVC irradiation effectively inactivated HCV in plasma and PCs, with HCV being more sensitive to these methods than the model virus, bovine viral diarrhea virus (BVDV). This suggests that these pathogen reduction technologies have the potential to significantly reduce transfusion-transmitted HCV infections.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22905868/.
10. Li Z, Lang Y, Sakamuru S, Samrat S, Trudeau N, Kuo L, Rugenstein N, Tharappel A, D’Brant L, Koetzner CA, Hu S, Zhang J, Huang R, Kramer LD, Butler D, Xia M, Li H. Methylene blue is a potent and broad-spectrum inhibitor against Zika virus in vitro and in vivo. Emerg Microbes Infect. 2020 Dec;9(1):2404-2416. doi: 10.1080/22221751.2020.1838954. PMID: 33078696; PMCID: PMC7646565.
Methylene blue is a potent and broad-spectrum inhibitor against Zika virus in vitro and in vivo
Methylene blue, an FDA-approved drug, has been identified as a potent and broad-spectrum antiviral against Zika virus (ZIKV) and Dengue virus (DENV) in both in vitro and in vivo studies. This research revealed that methylene blue can effectively disrupt the interactions between the viral protease NS3 and its co-factor NS2B, inhibit viral protease activity, hinder viral growth, protect mini-brain organoids from ZIKV infection, and reduce viremia in a mouse model. Its antiviral mechanism encompasses both entry and post-entry steps, reducing virus production and inhibiting processed NS3 protein production. With its established safety profile, methylene blue presents a promising therapeutic option for managing flavivirus infections, particularly ZIKV, and other related pathogens.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7646565/.
Caffarel-Salvador E, Kearney MC, Mairs R, Gallo L, Stewart SA, Brady AJ, Donnelly RF. Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds. Pharmaceutics. 2015 Sep 28;7(4):397-412. doi: 10.3390/pharmaceutics7040397. PMID: 26426040; PMCID: PMC4695826.
Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds
Photodynamic therapy involves delivery of a photosensitising drug that is activated by light of a specific wavelength, resulting in generation of highly reactive radicals. This activated species can cause destruction of targeted cells. Application of this process for treatment of microbial infections has been termed “photodynamic antimicrobial chemotherapy” (PACT). In the treatment of chronic wounds, the delivery of photosensitising agents is often impeded by the presence of a thick hyperkeratotic/necrotic tissue layer, reducing their therapeutic efficacy. Microneedles (MNs) are an emerging drug delivery technology that have been demonstrated to successfully penetrate the outer layers of the skin, whilst minimising damage to skin barrier function. Delivering photosensitising drugs using this platform has been demonstrated to have several advantages over conventional photodynamic therapy, such as, painless application, reduced erythema, enhanced cosmetic results and improved intradermal delivery. The aim of this study was to physically characterise dissolving MNs loaded with the photosensitising agent, methylene blue and assess their photodynamic antimicrobial activity. Dissolving MNs were fabricated from aqueous blends of Gantrez® AN-139 co-polymer containing varying loadings of methylene blue. A height reduction of 29.8% was observed for MNs prepared from blends containing 0.5% w/w methylene blue following application of a total force of 70.56 N/array. A previously validated insertion test was used to assess the effect of drug loading on MN insertion into a wound model. Staphylococcus aureus, Escherichia coli and Candida albicans biofilms were incubated with various methylene blue concentrations within the range delivered by MNs in vitro (0.1–2.5 mg/mL) and either irradiated at 635 nm using a Paterson Lamp or subjected to a dark period. Microbial susceptibility to PACT was determined by assessing the total viable count. Kill rates of >96%, were achieved for S. aureus and >99% for E. coli and C. albicans with the combination of PACT and methylene blue concentrations between 0.1 and 2.5 mg/mL. A reduction in the colony count was also observed when incorporating the photosensitiser without irradiation, this reduction was more notable in S. aureus and E. coli strains than in C. albicans.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695826/.
12. Zheng X, Ma X, Li T, Shi W, Zhang Y. Effect of different drugs and drug combinations on killing stationary phase and biofilms recovered cells of Bartonella henselae in vitro. BMC Microbiol. 2020 Apr 10;20(1):87. doi: 10.1186/s12866-020-01777-9. PMID: 32276590; PMCID: PMC7149919.
Effect of different drugs and drug combinations on killing stationary phase and biofilms recovered cells of Bartonella henselae in vitro
Bartonella henselae, a Gram-negative bacterium transmitted through cat scratches and ectoparasites, can lead to various clinical diseases in humans, including local lymphadenopathy and severe conditions like persistent bacteremia and endocarditis. The current treatment for persistent B. henselae infections faces challenges regarding effectiveness. To address this, the study evaluated 14 antibiotics and 25 antibiotic combinations against stationary phase and biofilm-recovered B. henselae cells. Notably, ciprofloxacin, gentamicin, and nitrofurantoin showed high activity, while clofazimine and miconazole performed poorly. Combinations like azithromycin/ciprofloxacin, azithromycin/methylene blue, rifampin/ciprofloxacin, and rifampin/methylene blue effectively eliminated stationary phase B. henselae within a day, and methylene blue and rifampin displayed strong activity against biofilm B. henselae after 6 days. These findings hold promise for developing more effective treatments for persistent Bartonella infections in the future.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7149919/.
Khanal A, Bui MP, Seo SS. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J Breast Cancer. 2014 Mar;17(1):18-24. doi: 10.4048/jbc.2014.17.1.18. Epub 2014 Mar 28. PMID: 24744793; PMCID: PMC3988338.
Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy
Photodynamic therapy (PDT) is gaining traction in breast cancer treatment due to its localized selectivity and reduced toxicity compared to radiotherapy and chemotherapy. PDT involves using photosensitizer drugs loaded into various nanomaterials along with light exposure. However, a key challenge with PDT is the limited capacity of nanomaterials to encapsulate anticancer drugs at high doses, resulting in reduced treatment efficacy. This study proposes the use of poly(N-isopropylacrylamide) (PNIPAM) microgels for encapsulating methylene blue, an anticancer drug, for potential breast cancer treatment in the MCF-7 cell line. The research involved developing biocompatible microgels based on PNIPAM and its anionically functionalized counterpart (PNIPAM-co-PAA) for drug encapsulation. The findings suggest that core PNIPAM microgels outperformed core/shell PNIPAM-co-PAA microgels in drug loading and retention, leading to enhanced photodynamic efficacy against MCF-7 cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3988338/.
Sanchala D, Bhatt LK, Pethe P, Shelat R, Kulkarni YA. Anticancer activity of methylene blue via inhibition of heat shock protein 70. Biomed Pharmacother. 2018 Nov;107:1037-1045. doi: 10.1016/j.biopha.2018.08.095. Epub 2018 Aug 24. PMID: 30257315.
Anticancer activity of methylene blue via inhibition of heat shock protein 70
Heat shock proteins 70 (Hsp70) and 90 (Hsp90) are vital for the survival and proliferation of lung cancer cells. This study compared the anticancer potential of methylene blue (MB), an Hsp70 inhibitor, novobiocin (NB), a known Hsp90 inhibitor, and their combination. In vitro, MB showed lower cell viability compared to NB, and the combination of MB and NB further reduced cell viability. MB was more potent than NB in inhibiting cancer cell growth. In vivo, MB effectively inhibited Hsp70, reduced tumor biomarkers, and improved lung histopathology in a lung carcinogenesis mouse model induced by benzo[a]pyrene. These findings highlight MB’s significant anticancer activity via Hsp70 inhibition in lung cancer.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0753332218343944.
Dos Santos AF, Terra LF, Wailemann RA, Oliveira TC, Gomes VM, Mineiro MF, Meotti FC, Bruni-Cardoso A, Baptista MS, Labriola L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer. 2017 Mar 15;17(1):194. doi: 10.1186/s12885-017-3179-7. PMID: 28298203; PMCID: PMC5353937.
Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells
Breast cancer remains a significant cause of mortality in women, often due to incomplete primary treatment efficacy in eliminating all cancer cells, leading to recurrence. Photodynamic therapy (PDT), which involves visible light activation in the presence of methylene blue (MB) and oxygen, presents a promising adjunct therapy to chemotherapy and surgery. In this study, MB-PDT demonstrated varying cell-killing potential among different breast cell lines, with malignant cells being more susceptible than non-malignant ones. The cell death mechanisms induced by MB-PDT appeared to involve alternative pathways rather than classical apoptosis. This research highlights the potential of MB-PDT as an effective adjunct therapy to enhance the eradication of microscopic residual disease in breast tumors, reducing the risk of recurrence.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/28298203/.
Roldan CJ, Chung M, Feng L, Bruera E. Methylene Blue for the Treatment of Intractable Pain From Oral Mucositis Related to Cancer Treatment: An Uncontrolled Cohort. J Natl Compr Canc Netw. 2021 Jan 4;19(5):521-527. doi: 10.6004/jnccn.2020.7651. PMID: 33395626.
Methylene Blue for the Treatment of Intractable Pain From Oral Mucositis Related to Cancer Treatment: An Uncontrolled Cohort
Oral mucositis is a distressing complication of cancer therapy, often causing severe pain and negatively impacting patients’ oral health, nutrition, and overall quality of life. This retrospective study assessed the efficacy and safety of methylene blue (MB) oral rinse in relieving oral pain associated with mucositis in cancer patients who did not respond to conventional treatments. The analysis included 281 patients with various cancer types, and the results demonstrated a significant reduction in pain scores after MB oral rinse treatment. Most patients achieved pain relief within the first few doses, with minimal adverse effects reported. This study suggests that MB oral rinse is an effective and safe option for managing refractory oral mucositis pain in cancer patients.
You can read the full article at https://jnccn.org/view/journals/jnccn/19/5/article-p521.xml.
Müller O, Lu G, Jahn A, Mockenhaupt FP. How worthwhile is methylene blue as a treatment of malaria? Expert Rev Anti Infect Ther. 2019 Jul;17(7):471-473. doi: 10.1080/14787210.2019.1634545. Epub 2019 Jun 25. PMID: 31237457.
How worthwhile is methylene blue as a treatment of malaria?
Malaria remains a significant cause of illness and death, particularly in sub-Saharan Africa, where progress in reducing the malaria burden has stagnated since 2015. Historically, success in malaria control was attributed to widespread use of insecticide-treated mosquito nets and the introduction of artemisinin-based combination therapy (ACT). However, resistance to artemisinins and ACT has emerged in Southeast Asia, posing a threat to global malaria control efforts. To combat this, researchers have proposed adding a third antimalarial drug (triple therapy) to standard ACT. Two promising triple therapy regimens are currently under investigation in clinical trials in Southeast Asia. Additionally, the addition of a single dose of the gametocytocidal drug primaquine (PQ) to standard ACT has been shown to reduce the transmission of drug-resistant parasites. While concerns about PQ’s potential for hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency have limited its use, a low dose of PQ is now recommended by the World Health Organization (WHO) for uncomplicated falciparum malaria, even without G6PD testing. However, concerns about PQ’s toxicity remain, necessitating further investigation of alternative drugs. Methylene blue (MB), a versatile medical dye, has a long history of use, including as an antimalarial drug since 1891. Recent research has highlighted MB’s potential as a partner drug for ACT, given its efficacy against various malaria parasites, strong gametocytocidal effects, and synergy with artemisinin derivatives. Clinical studies have demonstrated the effectiveness and safety of MB in malaria treatment, with high cure rates and minimal adverse events. MB’s impact on gametocytes of P. falciparum has been confirmed in clinical trials. Although MB may cause urine discoloration and mild gastrointestinal side effects, it has not been associated with serious adverse events, making it a viable alternative to PQ for inclusion in combination therapies. MB has the potential to enhance malaria treatment, reduce transmission, and mitigate the risk of artemisinin and ACT resistance. Further research is needed to optimize MB formulations and conduct multicountry phase III studies to validate its efficacy and safety in diverse endemic regions and populations, especially considering the various types of G6PD deficiency. The development of MB-based combination therapy is considered highly cost-effective in the context of global health efforts to combat malaria.
You can read the full article at https://www.tandfonline.com/doi/full/10.1080/14787210.2019.1634545.
Meissner PE, Mandi G, Coulibaly B, Witte S, Tapsoba T, Mansmann U, Rengelshausen J, Schiek W, Jahn A, Walter-Sack I, Mikus G, Burhenne J, Riedel KD, Schirmer RH, Kouyaté B, Müller O. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006 Oct 8;5:84. doi: 10.1186/1475-2875-5-84. PMID: 17026773; PMCID: PMC1617109.
Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine
Developing safe, effective, and affordable drug combinations to combat malaria in Africa is a public health priority. Methylene blue (MB) shares similarities in its mode of action with chloroquine (CQ) and has demonstrated selective inhibition of Plasmodium falciparum glutathione reductase. In 2004, an uncontrolled dose-finding study was conducted with the MB-CQ combination in 435 young children suffering from uncomplicated falciparum malaria in Burkina Faso, where CQ monotherapy had shown a high clinical failure rate in 2003. During the study, three serious adverse events (SAE) were recorded, with one likely attributable to the study medication. Analysis showed no dose-specific effects on safety, and by day 14, the overall clinical and parasitological failure rates were 10% and 24%, respectively. While MB appears to have antimalarial efficacy, the CQ-MB combination was found to be ineffective for treating malaria in Africa.
You can read the full article https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1617109/.
Lu G, Nagbanshi M, Goldau N, Mendes Jorge M, Meissner P, Jahn A, Mockenhaupt FP, Müller O. Efficacy and safety of methylene blue in the treatment of malaria: a systematic review. BMC Med. 2018 Apr 25;16(1):59. doi: 10.1186/s12916-018-1045-3. PMID: 29690878; PMCID: PMC5979000.
Efficacy and safety of methylene blue in the treatment of malaria: a systematic review
Methylene blue (MB) was the first synthetic antimalarial to be discovered and was used during the late 19th and early 20th centuries against all types of malaria. MB has been shown to be effective in inhibiting Plasmodium falciparum in culture, in the mouse model and in rhesus monkeys. MB was also shown to have a potent ex vivo activity against drug-resistant isolates of P. falciparum and P. vivax. In preclinical studies, MB acted synergistically with artemisinin derivates and demonstrated a strong effect on gametocyte reduction in P. falciparum. MB has, thus, been considered a potentially useful partner drug for artemisinin-based combination therapy (ACT), particularly when elimination is the final goal. The aim of this study was to review the scientific literature published until early 2017 to summarise existing knowledge on the efficacy and safety of MB in the treatment of malaria.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5979000/.
Schirmer RH, Coulibaly B, Stich A, Scheiwein M, Merkle H, Eubel J, Becker K, Becher H, Müller O, Zich T, Schiek W, Kouyaté B. Methylene blue as an antimalarial agent. Redox Report. 2003;8(5):272-5. doi: 10.1179/135100003225002899. PMID: 14962363.
Methylene blue as an antimalarial agent
Methylene blue has intrinsic antimalarial activity and it can act as a chloroquine sensitizer. In addition, methylene blue must be considered for preventing methemoglobinemia, a serious complication of malarial anemia. As an antiparasitic agent, methylene blue is pleiotropic: it interferes with hemoglobin and heme metabolism in digestive organelles, and it is a selective inhibitor of Plasmodium falciparum glutathione reductase. The latter effect results in glutathione depletion which sensitizes the parasite for chloroquine action.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/14962363/.
Dormoi J, Amalvict R, Gendrot M, Pradines B. Methylene Blue-Based Combination Therapy with Amodiaquine Prevents Severe Malaria in an Experimental Rodent Model. Pharmaceutics. 2022 Sep 24;14(10):2031. doi: 10.3390/pharmaceutics14102031. PMID: 36297466; PMCID: PMC9611243.
Methylene Blue-Based Combination Therapy with Amodiaquine Prevents Severe Malaria in an Experimental Rodent Model
Untreated malaria can rapidly progress to severe forms within 24 hours, and drug resistance poses a global threat to malaria control efforts, necessitating the development of new chemotherapy approaches. This study contributes novel data on the use of methylene blue (MB) in combination therapy with common antimalarial drugs, including mefloquine (MQ) and amodiaquine (AQ), for the treatment of malaria and cerebral malaria. Using a C57BL6/J mouse model, Plasmodium berghei ANKA infection was initiated on Day 0, and treatment commenced on Day 3 when parasitemia reached nearly 1%, involving AQ, MQ, or MB alone or in combination with AQ or MQ. Late-stage treatment with AQ, MQ, or MB alone failed to prevent cerebral malaria. However, MB-based combination therapies remained effective even when initiated late. Significant differences in survival rates were observed, with MB-AQ providing complete protection against cerebral malaria, while MB-MQ demonstrated partial protection. These findings suggest that MB in combination with AQ holds promise as a candidate for preventing cerebral malaria.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9611243/.
Pushparajah Mak RS, Liebelt EL. Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatr Emerg Care. 2021 Sep 1;37(9):474-477. doi: 10.1097/PEC.0000000000002526. PMID: 34463662.
Methylene Blue: An Antidote for Methemoglobinemia and Beyond
Methylene blue, a centuries-old medicinal treatment, is primarily known for countering acquired methemoglobinemia (MetHB). Recently, it has gained recognition for its effectiveness in treating ifosfamide neurotoxicity and refractory vasoplegic shock, expanding its applications beyond MetHB. Methylene blue’s mechanism of action, rooted in its oxidizing properties, will be explored in this review, along with its use in acquired MetHB and ifosfamide neurotoxicity. The review will also assess its role in critically ill pediatric and adult patients with refractory vasoplegic shock, along with discussions on dosing and potential adverse effects.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/34463662/.
Iolascon A, Bianchi P, Andolfo I, Russo R, Barcellini W, Fermo E, Toldi G, Ghirardello S, Rees D, Van Wijk R, Kattamis A, Gallagher PG, Roy N, Taher A, Mohty R, Kulozik A, De Franceschi L, Gambale A, De Montalembert M, Forni GL, Harteveld CL, Prchal J; SWG of red cell and iron of EHA and EuroBloodNet. Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol. 2021 Dec 1;96(12):1666-1678. doi: 10.1002/ajh.26340. Epub 2021 Sep 23. PMID: 34467556; PMCID: PMC9291883.
Recommendations for diagnosis and treatment of methemoglobinemia
Methemoglobinemia is a rare condition characterized by the conversion of divalent ferro‐iron in hemoglobin (Hb) to ferri‐iron in methemoglobin (MetHb). It can arise from inherited or acquired factors, with acquired forms being more common, often linked to exposure to substances causing Hb oxidation. Inherited forms stem from autosomal recessive CYB5R3 gene variants or autosomal dominant globin gene variants, collectively known as HbM disease. This review is based on a systematic literature search and offers recommendations for key signs, diagnosis methods, clinical management across different age groups, and therapeutic approaches for methemoglobinemia. The recommendations were achieved through a consensus reached by an expert panel with over 75% agreement on all questions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9291883/.
Cefalu JN, Joshi TV, Spalitta MJ, Kadi CJ, Diaz JH, Eskander JP, Cornett EM, Kaye AD. Methemoglobinemia in the Operating Room and Intensive Care Unit: Early Recognition, Pathophysiology, and Management. Adv Ther. 2020 May;37(5):1714-1723. doi: 10.1007/s12325-020-01282-5. Epub 2020 Mar 19. PMID: 32193811; PMCID: PMC7467467.
Methemoglobinemia in the Operating Room and Intensive Care Unit: Early Recognition, Pathophysiology, and Management
This review aims to provide insights into the acquired and hereditary causes of methemoglobinemia, recommend sensitive diagnostic tests, and equip critical care clinicians with the knowledge to promptly identify and manage methemoglobinemia cases. The review methodology involved searching internet sources for relevant articles, encompassing case reports, case series, observational, longitudinal, and surveillance studies. Common methemoglobinemia culprits include oxidizing reactions triggered by substances like cocaine-derived anesthetics (e.g., benzocaine, lidocaine), antibiotics (e.g., dapsone, sulfonamides), and gases (e.g., nitric oxide). Furthermore, the review underscores the superior capability of CO-oximetry compared to standard pulse oximetry for methemoglobinemia detection. Lastly, it highlights effective treatment options, including intravenous administration of methylene blue, ascorbic acid, and riboflavin. The manuscript offers comprehensive insights into the occurrence and management of methemoglobinemia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7467467/.
Other Peptides
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








