GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
- Overall Health Benefits of Melanotan 2
- Key Takeaways
- What is Melanotan 2?
- How Melanotan 2 Works
- Chemical Structure of Melanotan 2
- Research on Melanotan 2
- Associated Side Effects of Melanotan 2
- Melanotan 2 Injections
- Melanotan 2 Tanning Injections
- Melanotan 2 Nasal Spray
- Melanotan 2 Bodybuilding
- Melanotan 2 Dosage for ED
- Where to Inject Melanotan 2
- Melanotan 2 vs PT 141
- Melanotan 2 Rosacea
- Melanotan 2 Dosage
- Melanotan 2 vs 1
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
- Overall Health Benefits of Melanotan 2
- Key Takeaways
- What is Melanotan 2?
- How Melanotan 2 Works
- Chemical Structure of Melanotan 2
- Research on Melanotan 2
- Associated Side Effects of Melanotan 2
- Melanotan 2 Injections
- Melanotan 2 Tanning Injections
- Melanotan 2 Nasal Spray
- Melanotan 2 Bodybuilding
- Melanotan 2 Dosage for ED
- Where to Inject Melanotan 2
- Melanotan 2 vs PT 141
- Melanotan 2 Rosacea
- Melanotan 2 Dosage
- Melanotan 2 vs 1
- FAQ
- Reference
Overall Health Benefits of Mitochondrial ORF of the twelve S c (MOTS-c)
The twelve S c (MOTS-c) benefits include improved metabolic function, enhanced mitochondrial biogenesis, increased insulin sensitivity, reduced inflammation, better endurance, and protection against age-related decline. Additionally, it supports cognitive function, promotes muscle regeneration, reduces oxidative stress, aids in weight management, enhances physical performance, and improves overall longevity.
- Treats obesity [1-12]
- Improves blood sugar levels and treats symptoms of diabetes [13-18]
- Improves heart health [19-24]
- Fights bone loss [25-27]
- Increases life expectancy [28-34]
- Improves exercise tolerance [35-51]
- Fights bacterial infection [52]
Overall Health Benefits of Melanotan 2
Melanotan 2 benefits include enhanced tanning, reduced UV exposure risk, and potential appetite suppression. Additionally, it may improve sexual function and increase energy levels.
- Treats erectile dysfunction [1-9]
- Helps lose weight [10-17]
- Prevents cancer [18-19]
- Prevents heart disease [20-22]
- Promotes healthy brain [23-28]
- Treats inflammatory disorders [29-30]
- Improves blood sugar levels and treats symptoms of diabetes [31-33]
- Treats alcohol abuse disorder [34-37]
Key Takeaways
- Enhanced Tanning: Melanotan 2 significantly darkens skin by stimulating melanin production, providing a tanned appearance with less sun exposure.
- Reduced UV Exposure Risk: By promoting natural tanning, Melanotan 2 decreases the need for prolonged UV exposure, potentially lowering the risk of skin damage and skin cancer.
- Appetite Suppression: Some users report a reduction in appetite, which can aid in weight management.
- Improved Sexual Function: Melanotan 2 has been noted to enhance libido and sexual arousal, particularly in men.
- Increased Energy Levels: Users often experience heightened energy and vitality, contributing to overall well-being.
What is Melanotan 2?
Melanotan 2 (MT-2) is a synthetic analog of the peptide hormone α-melanocyte-stimulating hormone (α-MSH), primarily developed to stimulate melanin production, which darkens skin pigmentation. Besides its use for achieving a tanned appearance, MT-2 is known for its effects on sexual health, as it can induce erections in men with erectile dysfunction and enhance sexual arousal in women. Additionally, MT-2 has been noted for its potential to reduce appetite and promote fat loss, further adding to its diverse applications in both aesthetic and therapeutic contexts.
How Melanotan 2 Works
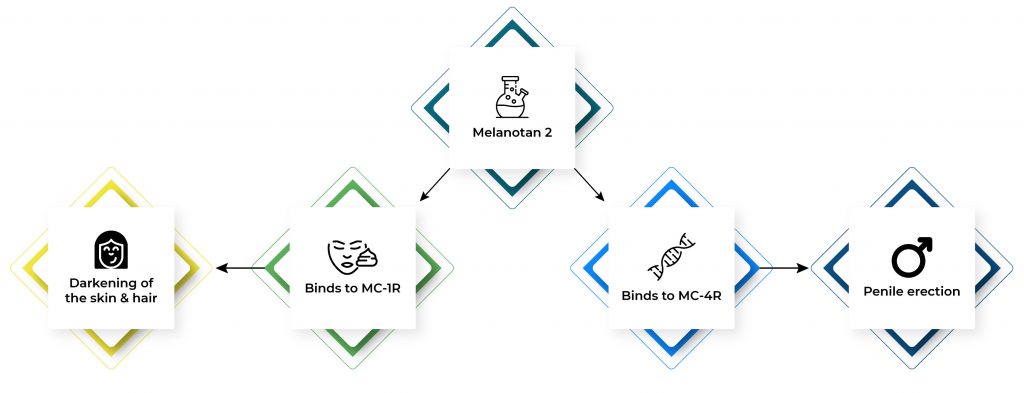
Melanotan 2 is similar to the melanocyte-stimulating hormone, a substance found in your body that is responsible for the production of skin-darkening pigments called melanin. It works by binding with melanocortin receptors. Melanotan 2 binds to MC-1R to stimulate the darkening of the skin and hair. It also stimulates penile erection by binding to MC-4R.
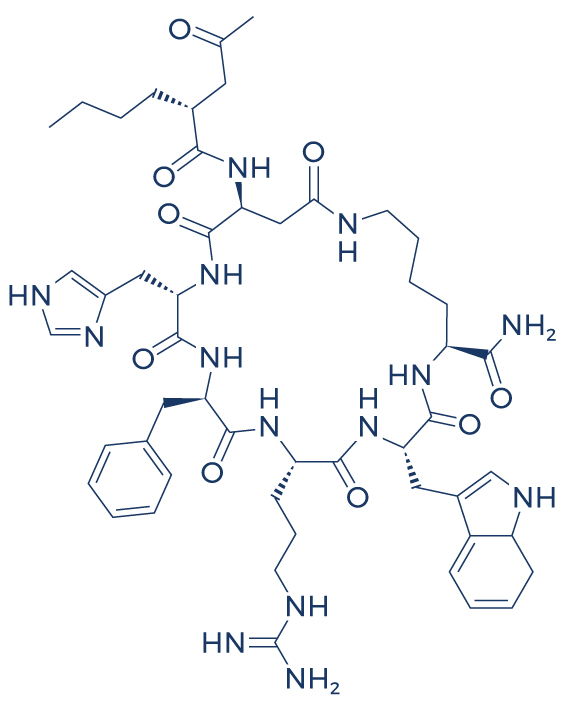
Chemical Structure of Melanotan 2
Research on Melanotan 2
A. Treats Erectile Dysfunction

Melanotan 2 treats erectile dysfunction by binding to the melanocortin 4 receptor (MC-4R) in the brain, which is involved in regulating sexual arousal. Activation of MC-4R increases nerve signaling associated with sexual function, leading to enhanced blood flow to the penis and the ability to achieve and maintain an erection. This mechanism makes Melanotan 2 a potential option for men who experience difficulty with conventional erectile dysfunction treatments.
- In men with psychogenic erectile dysfunction, MT-2 treatment successfully resulted in penile erections. [1]
- In men with erectile dysfunction (ED), MT-2 treatment led to increased sexual desire and penile erection even without any sexual stimulation. [2-3]
- In rats, MT-2 induced erections sufficient for sexual intercourse. [4]
- In rabbits, intravenous injection of MT-2 resulted in increased pressure within the erectile tissue. [5]
- In normal male volunteers, administration of MT-2 produced spontaneous, penile erections after 1-5 hours. [6]
- A rat study showed that MT-2 was effective for skin tanning and could be used to prevent sunlight-induced skin cancers. [7]
- In men with psychogenic erectile dysfunction, MT-2 was effective in inducing an erection. [8]
- In male rats, MT-2 injection successfully induced penile erection. [9]
B. Helps Lose Weight
Melanotan 2 aids in weight loss by activating melanocortin receptors that influence energy balance and appetite regulation. Specifically, MT-2 stimulates the MC-4R receptor, which has been linked to reduced appetite and increased energy expenditure. This combination can lead to decreased caloric intake and enhanced fat metabolism, contributing to gradual fat loss when combined with a healthy lifestyle.
- In obese rats, MT-2 treatment resulted in increased fat breakdown. [10]
- In rats, peripheral MT-2 treatment led to weight loss. [11]
- A study showed that MT-2 could safely reduce fat mass without inducing apoptosis (programmed cell death) in rats. [12]
- A study showed that continued MT-2 application consistently induced weight and fat loss in rat subjects. [13]
- In rats, MT-2 treatment effectively reduced body mass without long-term food restrictions. [14]
- A rat study also found that MT-2 treatment decreased food intake and increased energy expenditure. [15]
- A study suggested that MT-2 treatment could be effective in treating obesity by stimulating leptin and melanocortin pathways in the brain.[16-17]
C. Prevents Cancer
Melanotan 2 may help prevent skin cancer by stimulating melanin production, which provides a protective barrier against ultraviolet (UV) radiation from the sun. Increased melanin in the skin absorbs and disperses UV rays, reducing cellular DNA damage that can lead to cancerous changes. By promoting a natural tan without the need for excessive sun exposure, Melanotan 2 helps lower the risk of UV-induced skin cancers, including melanoma.
- A study suggested that MT-2 application could prevent sunlight-induced skin cancer. [18]
- In healthy volunteers, it was found that MT-1 was safe and effective for skin tanning when combined with solar UV light, and has protective effects against sunlight-induced skin cancer. [19]
D. Prevents Heart Disease
Melanotan 2 may help prevent heart disease indirectly by promoting fat loss, reducing inflammation, and improving lipid profiles. By binding to melanocortin receptors, it can influence metabolic pathways that lower body fat and improve insulin sensitivity, both of which are crucial in reducing cardiovascular risk. Additionally, MT-2’s anti-inflammatory effects may help lower chronic inflammation, a known contributor to heart disease, making it potentially beneficial for cardiovascular health over time.
- In mice, MT-2 treatment had therapeutic benefits in pre-established atherosclerosis (plaque formation within the heart arteries) by limiting inflammation and promoting vascular endothelial function. [20]
- In patients with coronary heart disease, it was found that they have altered levels of MT-2 in the heart. [21]
- In an animal model of myocardial ischemia (inadequate blood flow to the heart), MT-2 treatment improved heart function by reducing oxidative stress and programmed cell death. [22]
E. Promotes Healthy Brain
Melanotan 2 may promote brain health by activating melanocortin receptors, particularly MC-4R, which plays a role in neuroprotection and cognitive function. This activation can help reduce inflammation and oxidative stress in the brain, supporting cell survival and potentially slowing neurodegenerative processes. Additionally, MT-2’s effects on energy balance and appetite regulation may indirectly benefit brain health by promoting a balanced metabolic state, which is essential for optimal cognitive function and mental clarity.
- In rats, it was shown that treatment with melanocortin receptor agonists like melanotan could regulate the activity of central dopamine neurons in the brain. [23]
- In a mouse model of stroke, MT-2 treatment improved working memory. [24]
- In rats, it was found that MT-2 levels were impaired in the group with cognitive dysfunction. [25]
- A study reported that MT-2 may potentially treat mental disorders such as depression and anxiety. [26]
- In rats, MT-2 promoted nerve regeneration in the brain and protected against injury. [27]
- In a maternal immune activation (MIA) mouse model of autism, MT-2 administration reversed the negative behaviors associated with autism spectrum disorder (ASD). [28]
F. Treats Inflammatory Disorders
Melanotan 2 may help treat inflammatory disorders by modulating immune response through its action on melanocortin receptors, particularly MC-1R. By activating these receptors, Melanotan 2 can reduce the production of pro-inflammatory cytokines and enhance anti-inflammatory pathways, which may help alleviate inflammation and protect tissues from damage. This effect supports its potential in managing chronic inflammatory conditions and improving immune system balance.
- In a mouse model of stroke, MT-2 treatment inhibited brain inflammation. [29]
- In mice with plaque build-up within the heart, MT-2 inhibited plaque inflammation and promoted vascular endothelial function. [30]
G. Improves Blood Sugar and Treats Symptoms of Diabetes
Melanotan 2 may improve blood sugar regulation and help treat symptoms of diabetes by activating melanocortin receptors that influence energy balance, glucose metabolism, and insulin sensitivity. By binding to these receptors, Melanotan 2 can enhance insulin action, reduce blood glucose levels, and promote fat loss, which collectively reduce metabolic strain. These effects can help improve overall glucose control and may alleviate some diabetes-related symptoms, supporting better metabolic health.
- In rats, injection of MT-2 reduced food intake and body weight and improved insulin sensitivity. [31]
- In rats fed with a high-fat diet, MT-2 reversed diabetes by suppressing the hypothalamic-pituitary-adrenal axis. [32]
- In mice, MT-2 and leptin administration induced increased glucose uptake in skeletal muscles. [33]
H. Treats Alcohol Abuse Disorder
Melanotan 2 may help treat Alcohol Use Disorder by influencing the brain’s reward pathways, particularly through its action on melanocortin receptors like MC-4R. By modulating these receptors, Melanotan 2 can reduce cravings and the reinforcing effects of alcohol, potentially lowering consumption. Its impact on mood and anxiety levels may also contribute to reducing dependence on alcohol as a coping mechanism, offering a novel approach to managing addiction.
- In male rats who were subjected to long-term voluntary alcohol consumption, MT-2 reduced alcohol intake. [34]
- In mice, MT-2 administration reduced binge-like ethanol drinking. [35]
- In a mouse model of alcohol abuse disorder, low-dose MT-2 reduced binge-ethanol drinking. [36]
- In rats, MT-2 administration through injections at the border of the central amygdala nucleus and the basolateral amygdala resulted in reduced alcohol intake while increasing their water intake. [37]
Associated Side Effects of Melanotan 2
Melanotan 2 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on melanotan 2. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of melanotan 2. Despite this, it was listed as a side effect associated with melanotan 2 even though these associated side effects are very uncommon.
Side effects associated with melanotan 2 may include the following:
- Agitation
- Darkening of the skin
- Facial flushing
- Fatigue
- Increased blood pressure
- Increased heart rate
- Increased moles and freckles
- Increased sweating
- Loss of appetite
- Nausea
- Spontaneous erections
- Vomiting
Melanotan 2 Injections
Melanotan 2 injections are a synthetic analog of the hormone alpha-melanocyte-stimulating hormone (α-MSH), which stimulates melanogenesis, the process responsible for pigmentation of the skin. Originally developed to help prevent skin cancer by promoting tanning without exposure to harmful UV rays, Melanotan 2 has gained popularity for its ability to provide a deep, even tan that can be achieved relatively quickly compared to natural sunbathing.
In addition to its tanning effects, Melanotan 2 injections have been associated with several secondary benefits. Many users report a decrease in appetite, which can aid in weight management efforts. Furthermore, there is evidence to suggest that Melanotan 2 can enhance sexual arousal and libido, particularly in men, making it a subject of interest for research into treatments for sexual dysfunction.
Despite its benefits, Melanotan 2 injections come with potential risks and side effects. Common side effects include nausea, flushing, and increased blood pressure. There are also concerns about the long-term safety of its use, as well as the legality and quality of products available on the market, since Melanotan 2 is not approved by regulatory authorities in many countries. As with any supplement or medication, it is crucial to consult with a healthcare provider before starting Melanotan 2 to ensure it is safe and appropriate for individual use.
Melanotan 2 Tanning Injections
Melanotan 2 tanning injections are a synthetic hormone that stimulates melanin production in the skin, leading to a darker, tanned appearance. This peptide mimics the action of melanocyte-stimulating hormone (MSH), which naturally occurs in the body and regulates skin pigmentation. By increasing melanin levels, Melanotan 2 offers an alternative to traditional tanning methods, reducing the need for excessive sun exposure or UV tanning beds, which can pose significant health risks.
One of the primary benefits of Melanotan 2 injections is their ability to provide a deep, natural-looking tan with minimal sun exposure. This not only enhances cosmetic appearance but also helps protect the skin from harmful UV radiation by boosting its natural defense mechanism. Users often find that their tans are more even and longer-lasting compared to those achieved through sunbathing or artificial tanning methods. However, it’s important to note that while Melanotan 2 can reduce the need for UV exposure, it does not eliminate the risk of skin cancer and other sun-related skin damage entirely.
Despite its popularity for cosmetic tanning, Melanotan 2 injections come with potential side effects and risks. Common side effects include nausea, facial flushing, and increased freckling or darkening of existing moles. More serious concerns involve the lack of regulation and quality control in the production of Melanotan 2, as it is not approved by major health authorities like the FDA. As a result, users must exercise caution, seek medical advice before use, and ensure they source the product from reputable suppliers to minimize health risks.
Melanotan 2 Nasal Spray
Melanotan 2 nasal spray offers an alternative method of administration for those seeking the benefits of this synthetic peptide without the need for injections. This nasal delivery system allows for easier and more convenient usage, particularly appealing to individuals who are uncomfortable with needles. By using the nasal spray, users can achieve the desired effects of Melanotan 2, such as enhanced tanning and potential appetite suppression, in a less invasive manner.
The convenience of Melanotan 2 nasal spray extends beyond just ease of use; it also provides a more controlled dosage. With each spray, users can administer a precise amount of the peptide, reducing the risk of overuse or inconsistent dosing. This control can lead to more consistent and predictable results, making it a preferred option for many seeking the aesthetic and potential health benefits of Melanotan 2.
Additionally, the nasal spray method may offer quicker absorption into the bloodstream compared to traditional injections. The nasal mucosa’s rich blood supply allows for rapid uptake, potentially leading to faster onset of effects. As a result, users might experience the benefits of Melanotan 2, such as improved skin pigmentation and increased libido, more promptly. This swift action, combined with the convenience and controlled dosing, makes Melanotan 2 nasal spray an attractive option for many users.
Melanotan 2 Bodybuilding
Melanotan 2 has gained popularity in the bodybuilding community due to its ability to enhance physical appearance by promoting a darker, more even tan. This is particularly appealing for bodybuilders who aim to showcase their muscle definition and vascularity during competitions. A well-tanned body can highlight muscle contours, making the physique appear more defined and aesthetically pleasing under stage lighting.
Beyond its tanning effects, Melanotan 2 is also believed to aid in appetite suppression, which can be beneficial during cutting phases. Bodybuilders often follow strict diets to reduce body fat while maintaining muscle mass, and appetite control can be a significant challenge. By curbing hunger, Melanotan 2 helps bodybuilders adhere to their dietary plans more effectively, thus supporting their overall fitness and competition goals.
Additionally, Melanotan 2 may contribute to improved energy levels and enhanced sexual function, both of which can positively impact a bodybuilder’s overall quality of life. Increased energy can lead to more effective and productive training sessions, while improved sexual health can contribute to mental well-being. However, it’s essential for users to be aware of potential side effects and consult with healthcare professionals before incorporating Melanotan 2 into their regimen.
Melanotan 2 Dosage for ED
Melanotan 2 (MT2) has gained attention for its potential to address erectile dysfunction (ED). Typically, the starting dosage for treating ED with Melanotan 2 is around 0.025 to 0.05 mg, administered via subcutaneous injection. This low initial dose helps users gauge their body’s response and minimize potential side effects. Gradual dose adjustments can be made based on individual tolerance and effectiveness, with some users finding optimal results at doses ranging from 0.1 to 0.2 mg.
It’s crucial to administer Melanotan 2 in a controlled manner to avoid adverse reactions such as nausea, flushing, or increased blood pressure. The peptide works by stimulating melanocortin receptors, which not only promote skin tanning but also influence sexual arousal and erectile function. For those using MT2 for ED, the timing of the injection is essential; administering it approximately an hour before anticipated sexual activity can enhance its efficacy.
Consultation with a healthcare professional is recommended before starting Melanotan 2 to ensure safe usage and proper dosage. While anecdotal evidence supports its benefits for ED, clinical research is still limited. Users should approach Melanotan 2 with caution, being mindful of the legal status and potential risks associated with its use.
Where to Inject Melanotan 2
When injecting Melanotan 2, the most common and recommended site is the subcutaneous layer of fat. This layer is easily accessible, and injections here are generally less painful and have a lower risk of complications. The abdomen, around 2 inches away from the navel, is a preferred area because it provides a substantial fat layer that absorbs the peptide efficiently.
Rotating injection sites is crucial to avoid tissue damage and minimize discomfort. Apart from the abdomen, other suitable areas include the thighs and upper arms. Ensuring that injections are administered in different spots each time can help prevent localized reactions and potential skin irritation. It’s important to clean the injection site thoroughly with an alcohol swab before injecting to reduce the risk of infection.
Proper injection technique and hygiene are vital for the safe and effective use of Melanotan 2. Using a clean, new needle for each injection and following a step-by-step process can ensure consistent results and minimize side effects. Consulting with a healthcare professional before starting Melanotan 2 is advisable to receive personalized guidance and address any concerns regarding the injection process.
Melanotan 2 vs PT 141
Melanotan 2 and PT-141 are both peptides with distinct applications, though they share some similarities in their effects. Melanotan 2 primarily functions as a synthetic analog of the melanocyte-stimulating hormone (MSH), which promotes skin tanning by increasing melanin production. It is often used by individuals seeking a deeper tan with reduced sun exposure, which may help mitigate the risk of UV-related skin damage. Additionally, Melanotan 2 has been reported to have appetite-suppressing effects and can enhance sexual function, particularly in men.
In contrast, PT-141, also known as Bremelanotide, was developed as a successor to Melanotan 2 with a focus on sexual health. While it shares a similar mechanism in terms of melanocortin receptor activation, PT-141 is specifically designed to enhance sexual desire and arousal. It works by stimulating the central nervous system rather than influencing skin pigmentation. This peptide is used primarily for treating sexual dysfunction and is noted for its potential to improve libido and sexual performance in both men and women.
Both peptides have garnered attention for their unique benefits, but they are utilized for different purposes. Melanotan 2 is favored for its tanning and appetite-suppressing effects, along with secondary sexual benefits, whereas PT-141 is targeted at addressing sexual dysfunction and boosting libido. Users should consider their specific goals and consult with a healthcare provider to determine which peptide may be more appropriate for their needs.
Melanotan 2 Rosacea
Melanotan 2, a peptide known for its tanning effects, can potentially impact individuals with rosacea. Rosacea is a chronic skin condition characterized by redness, flushing, and visible blood vessels, primarily affecting the face. The increased melanin production stimulated by melanotan ii may lead to uneven pigmentation, which could exacerbate the redness and flushing associated with rosacea. Therefore, those with this condition should approach the use of melanotan ii with caution.
While some people use melanotan ii to achieve a tan with less sun exposure, its effects on rosacea are not well-documented. The peptide’s influence on skin color might not be uniform, which can potentially make rosacea symptoms more noticeable or worsen their appearance. This is particularly concerning for individuals who already struggle with skin sensitivity and inflammation.
Consulting with a dermatologist before starting melanotan ii is crucial for those with rosacea. A healthcare professional can provide personalized advice and alternatives to help manage rosacea symptoms effectively while considering the potential risks and benefits of such treatments.
Melanotan 2, known for its tanning effects, also goes by the name melanotan ii. For individuals with rosacea, the use of melanotan ii should be approached with caution. The uneven pigmentation caused by melanotan ii can make rosacea symptoms more apparent. Before using melanotan ii, consulting with a dermatologist is essential to understand its potential impacts.
Those considering melanotan ii for tanning should be aware of its possible effects on rosacea. The uneven pigmentation from melanotan ii might worsen rosacea symptoms. Therefore, it’s important to seek medical advice before starting melanotan ii.
Melanotan 2 Dosage
Melanotan ii dosage varies depending on individual goals and tolerance. Generally, a common starting dose of melanotan ii is around 0.25 mg to 0.5 mg, administered via subcutaneous injection. It’s important to gradually increase the melanotan ii dosage to find the optimal level that achieves desired tanning effects while minimizing potential side effects.
For tanning purposes, a typical dosing regimen of melanotan ii involves` initial daily injections for a period of 5-7 days, followed by a maintenance phase with less frequent melanotan ii dosing. The maintenance dose of melanotan ii is usually lower, around 0.5 mg to 1 mg per week, to sustain skin pigmentation. Consistency in melanotan ii dosing is key to achieving and maintaining the desired tan.
It’s crucial to consult with a healthcare professional before starting melanotan ii to ensure the melanotan ii dosage is appropriate and safe for your specific health profile. Overuse or improper dosing of melanotan ii can lead to unwanted side effects, such as nausea or skin reactions, so professional guidance helps in achieving optimal results while minimizing risks.
Melanotan 2 vs 1
Melanotan 2 and Melanotan 1 are both peptides designed to enhance skin pigmentation, but they differ in their mechanisms and effects. Melanotan 1, also known as afamelanotide, primarily functions as a synthetic analog of the naturally occurring hormone alpha-melanocyte-stimulating hormone (α-MSH). Its main use is to stimulate melanin production, leading to a tan that helps protect the skin from UV damage. It has been primarily developed for medical use, including the treatment of certain skin conditions like erythropoietic protoporphyria.
In contrast, Melanotan 2, a more potent derivative of Melanotan 1, is known for its ability to induce tanning with greater effectiveness. Melanotan II stimulates melanogenesis, the process that produces melanin, and also acts on melanocortin receptors that influence other physiological processes. Besides enhancing pigmentation, Melanotan II has been reported to offer additional benefits such as appetite suppression, increased libido, and improved energy levels. However, these additional effects are not its primary function and can vary between individuals.
While both peptides aim to increase skin pigmentation, Melanotan II is often preferred for its broader range of effects, although it may come with a higher risk of side effects and is not FDA-approved for general use. Melanotan II remains more focused on therapeutic uses and is subject to stricter regulations. Users should be aware of the legal status and potential health risks associated with Melanotan II before considering their use. Melanotan II has gained popularity in certain circles despite the regulatory concerns. Those interested in using Melanotan II should consult healthcare professionals and consider all potential risks. The use of Melanotan II should be approached with caution and awareness of the legal implications.
FAQ
What are the side effects of Melanotan 2 tan?
Side effects of Melanotan 2 can include nausea, flushing, increased libido, darkening of moles and freckles, darkened skin, and potential allergic reactions. Some users may also experience increased blood pressure and headaches. Additionally, there is a concern about the risk of developing skin cancer due to the darkened skin and the darkening of moles and freckles. Users should be aware of the potential for developing skin cancer as a side effect. Overall, it’s important to consider the risk of developing skin cancer when using Melanotan 2, especially due to the darkened skin.
Are melanotan injections safe?
Melanotan injections, including melanotan tanning injection, are not universally considered safe for all skin types, as they are not approved by many regulatory bodies and lack comprehensive safety data. Users with different skin types may experience various side effects from melanotan tanning injection, and there is a risk of contamination or improper dosing. Consulting a healthcare professional before using melanotan tanning injection is advised, especially for those with sensitive skin types.
Is melanotan nasal spray safe?
Melanotan nasal spray shares similar safety concerns with injections, as melanotan products are not widely approved or regulated. Potential side effects of melanotan products include nasal irritation, headaches, and systemic effects similar to those seen with injections, including melanotan induced priapism. Safety data is limited, and melanotan induced priapism is one of the more serious concerns. Therefore, users should be aware of the risk of melanotan induced priapism associated with melanotan products and consult healthcare providers for more information.
How often should you use nasal tanning spray?
Usage guidelines for nasal tanning spray vary depending on the product and individual response. Generally, it is recommended to follow the manufacturer’s instructions, often involving daily or weekly application of melanotan ii injection, but consulting with a healthcare professional is essential, especially if you are breast feeding. When using melanotan ii injection, it is crucial to adhere strictly to the recommended dosage and schedule. Additionally, individuals, particularly those who are breast feeding, should be aware of potential side effects and consult with a healthcare professional before beginning melanotan ii injection. If you are breast feeding, make sure to discuss with your doctor the suitability of using melanotan ii injection.
Do nasal tanners work without sun?
Nasal tanners can stimulate melanin production even without sun exposure, leading to some degree of tanning. However, the extent of tanning may be less pronounced compared to tanning achieved with sun exposure or UV light. Melanotan II injection resulting in a more pronounced effect can be considered by those looking for a stronger tan. Unlike nasal tanners, melanotan II injection resulting from melanotan use may provide a deeper and more sustained tan. It is important to research and understand the potential side effects and proper usage of melanotan II injection resulting in enhanced melanin production through melanotan use. Proper education on melanotan use is essential to ensure safety and effectiveness, especially for individuals at high risk. For those at high risk, the use of these products should be approached with caution. Understanding the implications and potential dangers is crucial for high risk individuals considering melanotan use.
What are the negative effects of nasal tanners?
Negative effects of nasal tanners can include nasal irritation, headaches, dizziness, and potential systemic side effects similar to those of injectable forms. Long-term safety data is lacking for this unregulated product, and misuse can increase risks to human skin, especially those looking for more melanin. It is essential to consider the impact on human skin and energy homeostasis when evaluating the safety of these unregulated products, particularly for those seeking more melanin. Continuous misuse of this unregulated product can lead to adverse effects not just internally but also on human skin, affecting energy homeostasis and highlighting the risks for individuals aiming to achieve more melanin. Overall, the impact on energy homeostasis should be a key consideration when assessing the safety of these products.
Does Melanotan actually work?
Melanotan is effective in stimulating melanin production, leading to enhanced tanning of the skin. However, results can vary, and its use comes with potential risks and side effects, including squamous cell carcinoma as documented in case reports. It is important to use it under medical supervision to mitigate risks such as squamous cell carcinoma. Case reports highlight the necessity for medical oversight. Individuals should be aware of the potential for squamous cell carcinoma and other health concerns when using Melanotan, as evidenced by numerous case reports.
What are the side effects of melanin?
Melanin itself generally does not cause side effects, as it is a natural pigment in the body. However, excessive melanin production can lead to hyperpigmentation, dark spots, and other skin discolorations. These conditions are usually cosmetic rather than harmful, but in rare cases, they may indicate a rare cause of potentially serious side effects. While melanin-related conditions are often benign, it’s important to be aware that a rare cause of potentially serious side effects can occur. Therefore, monitoring any changes in skin pigmentation is crucial to catch any potentially serious side effects early on.
Which peptide is best for tanning?
Melanotan 2 is often considered the most effective peptide for sunless tanning due to its broader range of effects, including enhanced melanin production and additional benefits such as appetite suppression and increased libido. Melanotan 1 also promotes sunless tanning but lacks some of the additional benefits. The way melanotan II works is by stimulating the production of melanin, leading to a darker skin tone. Additionally, the peptide melanotan II works for sunless tanning includes appetite suppression and increased libido, making it a popular choice for many. Overall, understanding how melanotan II works can help users make informed decisions about their sunless tanning options.
What is Melanotan 2?
Melanotan 2 is a synthetic analogue of the alpha-melanocyte-stimulating hormone (α-MSH). It is designed to stimulate the production of melanin in the skin, leading to a darker tan. For the general population, melanocortin receptor agonists, such as Melanotan 2, are often used to achieve a tanned appearance without the need for extensive sun exposure. The use of melanocortin receptor agonists like Melanotan 2 can provide an alternative to traditional tanning methods for the general population. By acting as melanocortin receptor agonists, these compounds enhance melanin production efficiently, offering a potential benefit to the general population seeking a tanned look.
How is Melanotan 2 administered?
Melanotan 2 is typically administered via subcutaneous injection, which involves injecting the peptide under the skin. Some users also use nasal sprays as an alternative method of administration. Proper technique and hygiene are important to avoid infections and ensure effective dosing, as improper administration could increase the risk of systemic toxicity. It’s crucial to be aware of potential systemic toxicity associated with the peptide to prevent adverse effects. Monitoring for signs of systemic toxicity, including those that could relate to melanoma, can help mitigate any health risks related to Melanotan 2 use. Additionally, being cautious about melanoma-related symptoms is important when using Melanotan 2 to avoid any potential complications.
What is the recommended dosage of Melanotan 2?
Dosages of superpotent cyclic melanotropic peptide Melanotan 2 can vary, but users often start with a low dose and gradually increase it to achieve the desired tanning effect. A common starting dose is around 0.25 mg of superpotent cyclic melanotropic peptide, with incremental increases over time. It is crucial to follow dosing guidelines of superpotent cyclic melanotropic peptide to minimize side effects. Melanoma concerns should be taken seriously when using such peptides, as the risk of melanoma may increase. Always consult with a healthcare professional about the potential links between melanotropic peptides and melanoma. Monitoring for signs of melanoma is important when using Melanotan 2. Users should be aware of melanoma symptoms and seek medical advice if any suspicious changes occur.
How long does it take to see results from Melanotan 2?
Results from Melanotan 2 can vary among individuals, but some users may start to see new moles or tanning effects within a few days to a week. Full tanning results typically develop over several weeks of consistent use. The speed of results depends on factors like skin type, dosage, and how effectively the product interacts with muscle cells. Individual responses may vary based on how the body’s muscle cells react to the compound. Additionally, the overall impact on the skin, including the formation of new moles, can be influenced by the condition of muscle cells, which can affect the efficacy of the tanning process.
Can Melanotan 2 be used to treat any medical conditions?
Melanotan 2 has potential applications in treating conditions like erectile dysfunction and certain types of skin disorders, including actinic keratoses. The pituitary gland, which plays a crucial role in hormone regulation, might be influenced by Melanotan 2 in these contexts. However, its use for these purposes is not widely approved and should only be considered under medical supervision. Research is ongoing to explore its therapeutic benefits, its effects on actinic keratoses, and its impact on the pituitary gland.
What are the benefits of using Melanotan 2?
The primary benefit of Melanotan 2 is its ability to induce a tan without extensive sun exposure, potentially reducing the risk of UV-induced skin damage. Melanotan I may also provide some protection against sunburn. Additionally, melanotan i has been studied for its potential effects on appetite and sexual function.
How does Melanotan 2 affect melanin production?
Melanotan 2 binds to melanocortin receptors in the skin, stimulating the production of melanin by melanocytes. This increase in melanin leads to a darker skin tone. According to a case report, the peptide essentially mimics the body’s natural tanning process without the need for UV exposure. Another case report supports this by highlighting the effectiveness of Melanotan 2 in achieving a tan-like effect. The peptide’s action is consistent with the findings reported in the case report.
Is there a risk of dependency with Melanotan 2?
There is no evidence to suggest that Melanotan i 2 causes chemical dependency. However, users may become psychologically reliant on the aesthetic results provided by melanotan i. It is important to use melanotan i responsibly and under guidance to avoid misuse.
Can Melanotan 2 be used alongside other tanning products?
Melanotan i can be used in conjunction with other tanning products, such as topical tanning lotions and bronzers, to enhance the overall effect. However, caution should be exercised to avoid excessive UV exposure. Combining methods, including melanotan i, should be done thoughtfully to prevent skin damage. Additionally, melanotan i should be used responsibly to ensure safe and effective tanning.
How should Melanotan 2 be stored?
Melanotan 2 should be stored in a cool, dry place, typically in the refrigerator, to maintain its stability and potency. Improper storage can lead to an adverse effect on the peptide’s effectiveness. It should be kept away from light and heat to avoid any adverse effects on its quality. Proper storage is essential to ensure the peptide remains effective and to prevent any adverse effects from occurring.
Can Melanotan 2 be used by everyone?
Melanotan 2 is not suitable for everyone, particularly those with certain medical conditions or allergies. It is important to consult with a healthcare provider before starting Melanotan 2, especially for individuals with a history of skin cancer or other skin-related issues, including those with skin types iii. Those with skin types iii should be particularly cautious, as the effects of Melanotan 2 may vary. Always discuss any concerns related to skin types iii with your healthcare provider before beginning treatment.
What precautions should be taken when using Melanotan 2?
When using Melanotan 2, it is important to follow dosing instructions carefully, maintain hygiene to prevent infections and skin diseases, and monitor for any adverse reactions related to skin diseases. Users should also use sun protection measures as Melanotan 2 does not eliminate the risk of UV damage and associated skin diseases.
Can Melanotan 2 be used to prevent sunburn?
While Melanotan 2 increases melanin levels, which can offer some protection against sunburn, it should not be relied upon solely for sun protection. The food and drug administration has not approved Melanotan 2 for this purpose, so sunscreen and other protective measures are still necessary to prevent sunburn and skin damage. It’s important to consider that the food and drug administration regulates products that are meant to protect against sunburn, and using approved methods remains crucial.
What are the legal considerations for using Melanotan 2?
The legal status of Melanotan II varies by country, and it is not approved by regulatory agencies like the FDA for tanning purposes. Users should be aware of the legal implications and obtain Melanotan II through legitimate sources if it is legal in their area. If you’re wondering how does Melanotan II work, it’s important to consider the legal context and safety of its use. Understanding how Melanotan II works can help you make informed decisions if it is permitted in your area.
How does Melanotan 2 differ from natural tanning?
Melanotan 2 induces tanning by stimulating melanin production without the need for UV exposure, whereas natural tanning relies on UV radiation to increase melanin. This can potentially reduce the risk of skin damage associated with natural tanning.
Can Melanotan 2 cause changes in mole appearance?
Some users report changes in the appearance of existing moles or the development of new moles while using Melanotan 2. Any significant changes should be evaluated by a healthcare provider to rule out potential skin issues.
What is the shelf life of Melanotan 2?
The shelf life of Melanotan 2 depends on its storage conditions. When stored properly in a refrigerator, it can remain stable for several months. Users should always check expiration dates and storage instructions.
Can Melanotan 2 be used to treat vitiligo?
Melanotan 2 has been explored as a potential treatment for vitiligo, a condition characterized by loss of skin pigment. While some studies show promising results, its use for vitiligo is not yet widely accepted or approved and should be pursued under medical supervision.
How does Melanotan 2 affect sexual function?
Melanotan 2 has been reported to have aphrodisiac effects and may improve erectile function in some users. This effect is thought to be related to its action on melanocortin receptors. Further research is needed to fully understand its impact on sexual health.
What should I do if I experience side effects from Melanotan 2?
If you experience side effects from Melanotan 2, such as nausea, changes in mole appearance, or other concerning symptoms, discontinue use immediately and consult a healthcare provider. Monitoring and addressing side effects promptly is crucial for safe use.
Reference
Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J Urol. 1998 Aug;160(2):389-93. PMID: 9679884.
Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study
We conducted a double-blind, placebo-controlled crossover study with ten men suffering from psychogenic erectile dysfunction, examining the efficacy of Melanotan-II, a cyclic alpha-melanocyte-stimulating hormone analogue. When compared to a placebo, Melanotan-II induced clinically apparent erections in 8 out of 10 participants, with a significantly longer duration of tip rigidity greater than 80%. Although some transient side effects such as nausea and decreased appetite were reported more frequently with Melanotan-II, they were manageable, indicating its potential as an effective treatment for psychogenic erectile dysfunction at a 0.025 mg/kg dose.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/9679884/.
Wessells H, Levine N, Hadley ME, Dorr R, Hruby V. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res. 2000 Oct;12 Suppl 4:S74-9. doi: 10.1038/sj.ijir.3900582. PMID: 11035391.
Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II
In a study involving 20 men with both psychogenic and organic erectile dysfunction (ED), we assessed the effects of Melanotan II, a non-selective melanocortin receptor agonist, in a double-blind, placebo-controlled crossover design. Our results showed that Melanotan II induced penile erection in 17 of the 20 men without sexual stimulation, with an average duration of 41 minutes of tip rigidity exceeding 80%. Additionally, increased sexual desire was reported more frequently after Melanotan II doses compared to the placebo. Although some side effects like nausea and yawning were noted, especially at a 0.025 mg/kg dose, Melanotan II demonstrated significant potential as a potent initiator of penile erection for men with ED, prompting further investigation of melanocortin agonists and antagonists in this context.
You can read the abstract of the article at https://www.nature.com/articles/3900582.
Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000 Oct 1;56(4):641-6. doi: 10.1016/s0090-4295(00)00680-4. PMID: 11018622.
Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction
In a double-blind, placebo-controlled crossover study involving ten subjects with erectile dysfunction and organic risk factors, we evaluated Melanotan II, a synthetic melanotropic initiator of erection. Melanotan II induced subjectively reported erections in 12 of 19 injections compared to just 1 of 21 doses of placebo, with responders having a mean rigidity score of 6.9 on a scale of 0 to 10. The average duration of tip rigidity exceeding 80% was significantly higher with Melanotan II (45.3 minutes) compared to placebo (1.9 minutes). Furthermore, sexual desire increased substantially after Melanotan II administration. Although some side effects like nausea and stretching/yawning occurred more frequently with Melanotan II, the results suggest that this compound’s erectogenic properties are not limited to psychogenic erectile dysfunction, highlighting its potential for men with various organic risk factors and the need for further exploration of centrally acting agents in addressing disorders of sexual desire.
You can read the abstract of the article at https://www.goldjournal.net/article/S0090-4295(00)00680-4/fulltext.
Giuliano F, Clément P, Droupy S, Alexandre L, Bernabé J. Melanotan-II: Investigation of the inducer and facilitator effects on penile erection in anaesthetized rat. Neuroscience. 2006;138(1):293-301. doi: 10.1016/j.neuroscience.2005.11.008. Epub 2005 Dec 19. PMID: 16360286.
Melanotan-II: Investigation of the inducer and facilitator effects on penile erection in anaesthetized rat
In anesthetized rats, we investigated the effects of melanotan-II, a non-specific melanocortin receptor agonist, on erections and its potential sites of action. When administered intravenously (0.1, 0.3, and 1 mg/kg) or directly into the paraventricular nucleus of the hypothalamus (0.1 and 1 microg), melanotan-II demonstrated a dose-dependent ability to induce erections, resulting in more frequent and faster onset of erectile events. Rats treated with melanotan-II at the L6-S1 level in the lower spinal cord showed higher-amplitude erectile responses compared to those receiving a vehicle. Cavernous nerve stimulation-triggered erections were facilitated by intravenous melanotan-II (1 mg/kg) but not by direct injection into the corpus cavernosum (1 microg). The facilitator effect of melanotan-II was unaffected by spinalization or nerve transections but was abolished after the removal of the lumbar paravertebral sympathetic chain. This suggests that melanotan-II can influence erection through both central and peripheral melanocortin pathways, depending on its mode of administration.
You can read the abstract of the article at https://www.ibroneuroscience.org/article/S0306-4522(05)01288-1/fulltext.
Vemulapalli R, Kurowski S, Salisbury B, Parker E, Davis H. Activation of central melanocortin receptors by MT-II increases cavernosal pressure in rabbits by the neuronal release of NO. Br J Pharmacol. 2001;134(8):1705-1710. doi:10.1038/sj.bjp.0704437.
Activation of central melanocortin receptors by MT-II increases cavernosal pressure in rabbits by the neuronal release of NO
In this study, the researchers explored the mechanisms by which systemic administration of Melanotan-II (MT-II) increases intracavernosal pressure in anesthetized rabbits. MT-II had no effect on electrical field stimulation-induced relaxations of rabbit corpus cavernosal strips in vitro. However, intravenous injection of MT-II (66 and 133 microg kg(-1)) dose-dependently increased cavernosal pressure. The non-selective melanocortin receptor antagonist SHU 9119 (3 microg kg(-1), i.v.) didn’t affect baseline cavernosal pressure but blocked the MT-II-induced increases. A cocktail injection into the corpus cavernosum also elevated cavernosal pressure. The role of the nitric oxide (NO)-cyclic GMP-dependent pathway was investigated, and bilateral transection of the pudendal nerves and inhibition of NO synthase with L-NAME abolished the MT-II-induced pressure increases, suggesting that central melanocortin receptor activation by MT-II increases cavernosal pressure through the neuronal release of NO.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1572913/.
Dorr RT, Lines R, Levine N, Brooks C, Xiang L, Hruby VJ, Hadley ME. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58(20):1777-84. doi: 10.1016/0024-3205(96)00160-9. PMID: 8637402.
Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study
The study by Dorr RT, Lines R, Levine N, Brooks C, Xiang L, Hruby VJ, Hadley ME, published in Life Sciences in 1996, evaluates Melanotan-II, a superpotent cyclic melanotropic peptide, in a pilot phase-I clinical study. Melanotan-II is an analog of the naturally occurring melanocortin peptide hormone α-melanocyte-stimulating hormone (α-MSH) and has been studied for its effects on melanogenesis and potential as a tanning agent without the need for sun exposure. The phase-I trial aimed to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of Melanotan-II in human volunteers. The findings indicate that Melanotan-II effectively stimulates melanogenesis, leading to increased pigmentation. Moreover, the study provided initial data on the safety profile of Melanotan-II, marking an important step in exploring its potential therapeutic applications beyond tanning, such as protecting against skin cancers associated with UV exposure. This early clinical research underscores the significance of melanocortin receptors in skin physiology and opens the door for further studies on Melanotan-II and related peptides in various therapeutic areas.
For the full article https://www.sciencedirect.com/science/article/pii/0024320596001609
Lan EL, Ugwu SO, Blanchard J, Fang X, Hruby VJ, Sharma S. Preformulation studies with melanotan-II: a potential skin cancer chemopreventive peptide. J Pharm Sci. 1994 Aug;83(8):1081-4. doi: 10.1002/jps.2600830805. PMID: 7983590.
Preformulation studies with melanotan-II: a potential skin cancer chemopreventive peptide
Melanotan-II (1) is a cyclic heptapeptide analog of alpha-melanocyte-stimulating hormone (alpha-MSH) that induces skin tanning and is under investigation for preventing sunlight-induced skin cancers. The study determined the dissociation constants of 1 using potentiometric titration and UV spectrophotometry, estimating pKa1 (histidine) at 6.54 and pKa2 (arginine) at 11.72. Apparent partition coefficients (PC) were measured at different pH values with n-octanol and isooctane as the nonpolar phases. At pH 7.35, PC(octanol) was 2.82, and delta log PC was 1.05. With a rat bioavailability of 4.6%, these findings suggest that 1 could be a suitable candidate for oral delivery, providing essential data for developing an appropriate dosage form.
You can read the abstract of the article at https://jpharmsci.org/article/S0022-3549(15)49547-4/pdf.
Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J Urol. 1998 Aug;160(2):389-93. PMID: 9679884.
Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study
The study aimed to assess the erectogenic effects of Melanotan-II, a cyclic alpha-melanocyte-stimulating hormone analogue, in men with psychogenic erectile dysfunction. In a double-blind, placebo-controlled crossover trial with ten participants, real-time RigiScan monitoring was employed to compare the erectogenic properties of Melanotan-II and a placebo. Eight out of ten men treated with Melanotan-II experienced clinically apparent erections, with a mean duration of tip rigidity greater than 80% lasting 38.0 minutes, compared to 3.0 minutes with the placebo (p=0.0045). Some transient side effects, such as nausea, stretching, yawning, and decreased appetite, were reported more frequently with Melanotan-II than with the placebo, but none required treatment. In conclusion, Melanotan-II proved to be a potent initiator of erections in men with psychogenic erectile dysfunction, with manageable side effects at a dose of 0.025 mg/kg.
You can read the abstract of the article https://pubmed.ncbi.nlm.nih.gov/9679884/.
Available from https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2003.tb03166.x.
Li G, Zhang Y, Wilsey JT, Scarpace PJ. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004 Jul;182(1):123-32. doi: 10.1677/joe.0.1820123. PMID: 15225137.
Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression
The impact of chronic activation of the central melanocortin (MC) system by melanotan II (MTII) was studied in Sprague-Dawley rats fed a regular diet (CH) and those with diet-induced obesity (DIO). Over a six-day period, MTII infusion significantly reduced body weight and visceral fat in both diet groups and significantly decreased calorie intake. The anorexic response to MTII was consistent in both CH and DIO rats. MTII also led to sustained increased oxygen consumption in DIO rats and delayed response in CH rats. Additionally, MTII lowered insulin and cholesterol levels in both groups and enhanced brown adipose tissue uncoupling protein 1 (UCP1) expression. Despite reduced hypothalamic MC3/MC4 receptor expression in DIO rats, MTII’s anorexic and thermogenic effects remained, suggesting its potential in obesity management.
You can read the full article at https://joe.bioscientifica.com/view/journals/joe/182/1/123.xml.
Strader AD, Shi H, Ogawa R, Seeley RJ, Reizes O. The effects of the melanocortin agonist (MT-II) on subcutaneous and visceral adipose tissue in rodents. J Pharmacol Exp Ther. 2007 Sep;322(3):1153-61. doi: 10.1124/jpet.107.123091. Epub 2007 Jun 13. PMID: 17567964.
The effects of the melanocortin agonist (MT-II) on subcutaneous and visceral adipose tissue in rodents
The study examined the peripheral effects of the synthetic melanocortin agonist melanotan-II (MT-II) in rodents on low-fat and high-fat diets. MT-II treatment resulted in weight and body fat loss in high-fat diet-induced obese (DIO) mice while maintaining the weight of low-fat-fed mice. MT-II-treated DIO mice experienced a reduction in both visceral and subcutaneous adipose tissue compared to Vehicle (ad libitum) controls. Pair-fed DIO mice, though losing an equivalent amount of body weight as MT-II-treated mice, retained more adipose tissue. These findings highlight the complexity of analyzing weight-reducing compounds, suggesting that MT-II’s effects extend beyond reduced food intake to involve other mechanisms. The results were consistent in DIO rats, showing a generalized reduction in adipose tissue with MT-II treatment.
You can read the abstract of the article at https://jpet.aspetjournals.org/content/322/3/1153.long.
Choi YH, Li C, Hartzell DL, Lin J, Della-Fera MA, Baile CA. MTII administered peripherally reduces fat without invoking apoptosis in rats. Physiol Behav. 2003 Jul;79(2):331-7. doi: 10.1016/s0031-9384(03)00118-5. PMID: 12834806.
MTII administered peripherally reduces fat without invoking apoptosis in rats
The study focused on the melanocortin (MC) system, an essential component of leptin signaling related to obesity. It investigated the impact of Melanotan II (MTII), an MC3/4-receptor agonist, on adipose apoptosis and other metabolic factors in rats. MTII treatment significantly reduced food and water intake, body weight gain, and body temperature compared to control groups. It notably decreased the m ass of retroperitoneal and epididymal white adipose tissue but not through apoptosis. While both MTII and pair-fed (PF) rats had reduced brown fat weight, muscle mass remained unaffected. Serum free fatty acid concentrations were elevated in the PF group. These findings suggest that MTII reduces fat mass primarily by enhancing lipid mobilization, not apoptosis.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0031938403001185?via%3Dihub.
Zhang Y, Collazo R, Gao Y, Li G, Scarpace PJ. Intermittent MTII application evokes repeated anorexia and robust fat and weight loss. Peptides. 2010;31(4):639-643. doi:10.1016/j.peptides.2009.12.019.
Intermittent MTII application evokes repeated anorexia and robust fat and weight loss
The study aimed to overcome the transient anorectic effects and tachyphylaxis associated with central melanocortin (MC) analog, Melanotan II (MTII), by employing intermittent therapy. F344/BN rats received MTII or vehicle infusions into the lateral ventricle via a mini pump for 14 days. Half of the MTII-infused rats received a break with vehicle, then MTII reintroduction, while the rest had continuous MTII treatment. Initial MTII application induced a 30% reduction in food intake, which diminished within 5 days. Reapplication of MTII after the off period led to a new robust anorectic response. Body weights decreased significantly in both MTII groups, and gene expressions and phosphorylation in fat tissues were altered. In conclusion, intermittent MTII therapy preserved anorectic effects but did not prevent tachyphylaxis, while constant MTII application led to a stable but blunted food response after the initial decline. Both treatments resulted in significant weight and adiposity reductions, potentially through enhanced fatty acid oxidation in specific fat tissues.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2860181/.
Côté I, Sakarya Y, Kirichenko N, et al. Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction. Can J Physiol Pharmacol. 2017;95(2):206-214. doi:10.1139/cjpp-2016-0290.
Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction
Melanotan II (MTII) is a potent appetite suppressor that rapidly reduces body mass. Many studies have focused on the initial effects of MTII, but this research examined its chronic impact on energy balance. MTII was infused into the lateral ventricle of the brain in rats for 40 days. While food intake returned to normal levels, body mass remained reduced in both MTII groups. Additionally, they had lower adiposity compared to the control group. This suggests that MTII can chronically reduce body mass without requiring long-term caloric restriction, and a combination of food restriction and ongoing pharmacological approaches may help address obesity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5572812/.
Maria M. Glavas, Sandra E. Joachim, Shin J. Draper, M. Susan Smith, Kevin L. Grove, Melanocortinergic Activation by Melanotan II Inhibits Feeding and Increases Uncoupling Protein 1 Messenger Ribonucleic Acid in the Developing Rat, Endocrinology, Volume 148, Issue 7, 1 July 2007, Pages 3279–3287, https://doi.org/10.1210/en.2007-0184.
Melanocortinergic Activation by Melanotan II Inhibits Feeding and Increases Uncoupling Protein 1 Messenger Ribonucleic Acid in the Developing Rat
The study explored the melanocortin system in the early postnatal period in rats. It investigated the effects of the melanocortin receptor agonist melanotan II (MTII) on body weight, energy expenditure, and hypothalamic neuropeptide Y (NPY) expression. MTII was administered to rat pups at different postnatal stages, leading to hypothalamic activation, reduced body weight gain, decreased stomach weight, and increased energy expenditure, but it didn’t affect NPY expression. This suggests that MTII can influence food intake and energy expenditure in early postnatal rats, even before the full development of their hypothalamic feeding neurocircuitry, and these effects don’t seem to be mediated by changes in NPY expression.
You can read the full article at https://academic.oup.com/endo/article/148/7/3279/2502072?login=false.
-
The Role of Leptin-Melanocortin System and Human Weight Regulation: Lessons from Experiments of Nature
Common obesity is a complex condition influenced by genetic predisposition and an environment characterized by abundant calories and sedentary lifestyles. Recent advances have identified genetic mutations contributing to obesity in both humans and animal models. Rare conditions, such as leptin deficiency, leptin receptor deficiency, proopiomelanocortin deficiency (POMC), and melanocortin 4 receptor (MC4R) deficiency, result in severe obesity. Common obesity may also involve variants in genes like POMC and melanocortin 3 receptor. Additionally, defects in thyrosine kinase B (TrkB) and brain-derived neurotrophic factor (BDNF) have been associated with obesity. While single gene mutations can cause obesity, most cases result from intricate interactions between various genetic variants and environmental factors that promote fat accumulation, offering potential insights for future therapeutic interventions.
You can read the abstract of the article at https://www.researchgate.net/publication/24019710_The_Role_of_Leptin-Melanocortin_System_and_Human_Weight_Regulation_Lessons_from_Experiments_of_Nature.
Bjørbaek C, Hollenberg AN. Leptin and melanocortin signaling in the hypothalamus. Vitam Horm. 2002;65:281-311. doi: 10.1016/s0083-6729(02)65068-x. PMID: 12481551.
Leptin and melanocortin signaling in the hypothalamus
The intricate regulation of body weight in humans relies on the interplay between food consumption and energy expenditure. Leptin, an adipocyte-secreted hormone, plays a pivotal role in orchestrating these processes, and mutations in the genes for leptin and its receptor can result in severe obesity. Leptin primarily exerts its effects through target neurons in the hypothalamus, where it influences food intake, energy expenditure, and neuroendocrine functions. Critical neuropeptides, including alpha-melanocyte-stimulating hormone (alpha-MSH) derived from proopiomelanocortin (POMC), are regulated by leptin in the hypothalamus. Disruptions in POMC or central melanocortin receptors, much like mutations in the leptin pathway, can lead to obesity in humans. Therefore, understanding the mechanisms underlying leptin and melanocortin signaling in the hypothalamus is essential for unraveling the complexities of weight regulation in humans.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5572812/.
Lan EL, Ugwu SO, Blanchard J, Fang X, Hruby VJ, Sharma S. Preformulation studies with melanotan-II: a potential skin cancer chemopreventive peptide. J Pharm Sci. 1994 Aug;83(8):1081-4. doi: 10.1002/jps.2600830805. PMID: 7983590.
Preformulation studies with melanotan-II: a potential skin cancer chemopreventive peptide
Melanotan-II (1) is a cyclic heptapeptide analog of alpha-melanocyte-stimulating hormone (alpha-MSH) known for its skin-tanning properties and under investigation for preventing sunlight-induced skin cancers. To understand its characteristics, dissociation constants were determined using potentiometric titration and ultraviolet spectrophotometry, revealing estimated pKa values of 6.54 (histidine) and 11.72 (arginine). The apparent partition coefficient (PC) was measured at various pH levels using n-octanol and isooctane as the nonpolar phase, with PC(octanol) and delta log PC at pH 7.35 measuring 2.82 and 1.05, respectively. These findings, combined with a 4.6% bioavailability in rats, suggest that 1 could be a viable candidate for oral delivery, providing valuable insights for developing an appropriate dosage form.
You can read the abstract of the article at https://jpharmsci.org/article/S0022-3549(15)49547-4/pdf.
Dorr RT, Ertl G, Levine N, Brooks C, Bangert JL, Powell MB, Humphrey S, Alberts DS. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch Dermatol. 2004 Jul;140(7):827-35. doi: 10.1001/archderm.140.7.827. PMID: 15262693.
Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers
Three phase 1 clinical trials investigated the safety of combining melanotan-1 (MT-1) therapy with UV-B light or sunlight exposure. These open-label studies involved various MT-1 doses, UV-B exposure to specific body areas, and sunlight exposure. Results showed successful tanning and improved sunburn protection with MT-1 treatment, suggesting its safe combination with UV-B light or sunlight for enhanced tanning effects. No pathological issues were observed at exposed sites, and MT-1’s side effects were minor, mainly nausea and facial flushing. This indicates that MT-1 can be used synergistically with light exposure for tanning safely.
You can read the full article at https://jamanetwork.com/journals/jamadermatology/fullarticle/480676.
Rinne P, Silvola JM, Hellberg S, Ståhle M, Liljenbäck H, Salomäki H, Koskinen E, Nuutinen S, Saukko P, Knuuti J, Saraste A, Roivainen A, Savontaus E. Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice. Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1346-54. doi: 10.1161/ATVBAHA.113.302963. Epub 2014 May 1. PMID: 24790139.
Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice
The study aimed to investigate whether melanocortin peptides, known for their anti-inflammatory and vascular benefits, could reduce atherosclerotic plaque inflammation and improve vasoreactivity in atherosclerotic mice. Melanotan II (MT-II) treatment in high-fat diet-fed atherosclerotic mice didn’t affect body weight, cholesterol levels, or lesion size but significantly reduced plaque inflammation, polarized lesional macrophages toward an anti-inflammatory state, and decreased systemic inflammation. Moreover, MT-II improved aortic vasoreactivity, enhancing endothelium-dependent relaxations and sensitivity to nitric oxide-mediated vasodilation. These findings suggest that activating the melanocortin system has therapeutic potential for atherosclerosis by mitigating inflammation and enhancing vascular function, offering a novel treatment approach.
You can read the full article at https://www.ahajournals.org/doi/10.1161/ATVBAHA.113.302963?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003 Aug;35(1):40-4. doi: 10.1034/j.1600-079x.2003.00051.x. PMID: 12823612.
The melatonin receptor subtype MT2 is present in the human cardiovascular system
The study found that the MT2 melatonin receptor subtype is expressed in various tissues, including human coronary arteries, aorta, and left ventricular specimens from both healthy donors and patients with cardiomyopathy or coronary heart disease. This indicates the presence of MT2 receptors in human vasculature. Notably, the expression levels of MT2 receptors varied among individuals, suggesting heterogeneity in receptor expression patterns, which may be altered in coronary heart disease.
You can read the full article at https://onlinelibrary.wiley.com/doi/abs/10.1034/j.1600-079X.2003.00051.x?sid=nlm%3Apubmed.
Han D, Wang Y, Chen J, Zhang J, Yu P, Zhang R, Li S, Tao B, Wang Y, Qiu Y, Xu M, Gao E, Cao F. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J Pineal Res. 2019 Aug;67(1):e12571. doi: 10.1111/jpi.12571. Epub 2019 Apr 12. PMID: 30903623.
Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury
A study conducted on mice investigated the melatonin receptors responsible for melatonin’s protective effects on myocardial ischemia/reperfusion (MI/R) injury. While both melatonin receptor subtypes, MT1 and MT2, were found in normal myocardium, MT2 was upregulated after MI/R. Melatonin administration reduced myocardial injury, improved cardiac function, and alleviated oxidative and nitrative stress, endoplasmic reticulum stress, and mitochondrial injury in MI/R. These effects were dependent on MT2, not MT1. Cardiomyocyte-specific overexpression of MT2 also mitigated MI/R injury and improved cardiac function, further supporting MT2 as the key receptor responsible for melatonin’s cardioprotective effects. Targeting MT2 may hold promise in treating ischemic heart disease.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/jpi.12571.
Lindblom J, Kask A, Hägg E, Härmark L, Bergström L, Wikberg J. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol Res. 2002 Feb;45(2):119-24. doi: 10.1006/phrs.2001.0913. PMID: 11846623.
Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain
Previous research has demonstrated that melanocortin peptides influence dopaminergic neurotransmission. This study aimed to investigate whether chronic exposure to melanocortin receptor agonists leads to sustained dopamine release, subsequently affecting dopamine receptor subtypes. Through autoradiographic analysis, it was found that a two-week intracerebroventricular infusion of the melanocortin receptor agonist melanotan-II resulted in alterations in dopamine D(1)-like and D(2)-like receptor binding in various regions of the rat brain. D(1)-like receptor binding increased in the nucleus accumbens and the caudate putamen but decreased in the substantia nigra (reticular part), while D(2)-like receptor binding decreased in the caudate putamen but increased in the periaqueductal grey, substantia nigra (compact part), and the ventral tegmental area. These findings suggest that chronic melanocortin receptor agonist infusion can modify the activity of dopaminergic neurons in specific brain regions, supporting the hypothesis that melanocortin peptides may regulate central dopamine neuron activity.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S1043661801909132?via%3Dihub.
Wang W, Guo DY, Lin YJ, Tao YX. Melanocortin Regulation of Inflammation. Front Endocrinol (Lausanne). 2019;10:683. Published 2019 Oct 9. doi:10.3389/fendo.2019.00683.
Melanocortin Regulation of Inflammation
Adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormones (α-, β-, γ-MSH), collectively known as melanocortins, along with their corresponding receptors (melanocortin receptors), constitute an ancient modulatory system. Initially used for treating rheumatoid arthritis in 1949, ACTH was believed to exert anti-inflammatory effects through the hypothalamus-pituitary-adrenal axis and glucocorticoid-dependent mechanisms. Over subsequent decades, research has unveiled the complex physiology and pharmacology of the melanocortin system. Current knowledge indicates that ACTH, α-, β-, and γ-MSHs possess glucocorticoid-independent anti-inflammatory and immunomodulatory effects by activating melanocortin receptors in the brain or peripheral immune cells. This review provides a brief overview of the melanocortin system and emphasizes the role of melanocortins in regulating immune functions based on in vitro, in vivo, preclinical, and clinical studies, also summarizing their potential therapeutic applications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6794349/.
Bahna SG, Sathiyapalan A, Foster JA, Niles LP. Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: therapeutic implications for Alzheimer’s disease. Neurosci Lett. 2014 Jul 25;576:84-7. doi: 10.1016/j.neulet.2014.05.056. Epub 2014 Jun 5. PMID: 24909617.
Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: therapeutic implications for Alzheimer’s disease
We previously demonstrated that clinically relevant concentrations of valproic acid (VPA) elevate the expression of the G protein-coupled melatonin MT1 receptor in rat C6 glioma cells and both MT1 and MT2 receptors in the rat hippocampus. The MT2 receptor, particularly abundant in the hippocampus and implicated in synaptic plasticity and cognitive function, exhibits reduced expression in Alzheimer’s patients. In this study, we investigated whether VPA-induced upregulation of MT1 and MT2 receptors, observed in earlier molecular studies, is localized to specific hippocampal regions associated with cognitive function. In situ hybridization of rat brain slices, following chronic VPA treatment, revealed a significant increase in MT2 receptor mRNA in the CA1, CA2, CA3, and dentate gyrus regions of the hippocampus. However, MT1 receptor expression was not detected in the hippocampus by in situ hybridization. The notable induction of melatonin MT2 receptor expression by VPA in hippocampal regions linked to learning, memory, and neural stem cell proliferation suggests a potential therapeutic strategy combining VPA with melatonin or other MT2 agonists for neurodegenerative disorders like Alzheimer’s disease.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S030439401400456X?via%3Dihub.
Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci. 2014;39(1):6-21. doi:10.1503/jpn.130009.
Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology
Melatonin (MLT) is a multifaceted neurohormone with implications for various physiological processes and potential links to diseases such as neurodegenerative disorders, circadian disturbances, mood disorders, insomnia, type 2 diabetes, and pain. Synthesized by the pineal gland, melatonin acts through two G-protein coupled receptors, MT1 (MEL1a) and MT2 (MEL1b). While extensive research has explored the physiopathological effects of MLT, fewer studies have delved into the specific roles of MT1 and MT2 receptors. This review focuses on the current understanding of the involvement of MT2 receptors in brain functions. Examining studies on MT2 receptor ligands in sleep, anxiety, neuropsychiatric diseases, and psychopharmacology, including genetic investigations on the MTNR1B gene, reveals their roles in sleep disorders, anxiety, depression, Alzheimer’s disease, and pain. Selective MT2 receptor agonists demonstrate hypnotic and anxiolytic properties. Although research on the psychopharmacological role of MT2 receptors is limited, the development of novel selective ligands and further preclinical studies may unveil the receptor’s potential in mental health treatment, addressing current therapeutic gaps.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3868666/.
The potent melanocortin receptor agonist melanotan-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat. Ter Laak, M.P., Brakkee, J.H., Adan, R.A., Hamers, F.P., Gispen, W.H. Eur. J. Pharmacol. (2003).
The potent melanocortin receptor agonist melanotan-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat
The study explored the neurotrophic and neuroprotective potential of melanotan-II, an alpha-melanocyte-stimulating hormone analog and potent melanocortin receptor agonist. Using the sciatic nerve crush model, the research found that melanotan-II significantly improved sensory function recovery after a sciatic nerve crush lesion in rats when administered at a dose of 20 microg kg(-1) every 48 hours subcutaneously, but not at doses of 2 or 50 microg kg(-1). Additionally, melanotan-II exhibited neuroprotective properties by partially shielding the nerve from toxic neuropathy induced by cisplatin. These findings indicate, for the first time, the efficacy of melanotan-II in promoting nerve regeneration and providing neuroprotection.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S001429990202945X?via%3Dihub.
Minakova E, Lang J, Medel-Matus JS, Gould GG, Reynolds A, Shin D, Mazarati A, Sankar R. Melanotan-II reverses autistic features in a maternal immune activation mouse model of autism. PLoS One. 2019 Jan 10;14(1):e0210389. doi: 10.1371/journal.pone.0210389. PMID: 30629642; PMCID: PMC6328175.
Melanotan-II reverses autistic features in a maternal immune activation mouse model of autism
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition marked by challenges in social interactions, communication difficulties, and repetitive behaviors. ASD is more prevalent in males, with a ratio of 4:1 compared to females. Current treatments for ASD are limited due to its multifaceted causes involving both genetic and environmental factors. Oxytocin has shown promise in addressing social deficits associated with ASD. In this study, the melanocortin receptor 4 agonist Melanotan-II (MT-II) was explored as a pharmacological approach to enhance oxytocinergic activity. Using a maternal immune activation (MIA) mouse model of autism, adult male mice with autism-like features were treated with MT-II, resulting in the restoration of social behavioral metrics. Normal mice treated with MT-II did not exhibit significant changes in social behavior, anxiety, or repetitive behaviors, though weight loss was observed. These findings highlight MT-II as a potential therapeutic agent for improving autism-like behavioral deficits in the MIA mouse model.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328175/.
Tsai TH, Lin CJ, Chua S, Chung SY, Yang CH, Tong MS, Hang CL. Melatonin attenuated the brain damage and cognitive impairment partially through MT2 melatonin receptor in mice with chronic cerebral hypoperfusion. Oncotarget. 2017 Aug 22;8(43):74320-74330. doi: 10.18632/oncotarget.20382. Erratum in: Oncotarget. 2020 Sep 22;11(38):3558. PMID: 29088788; PMCID: PMC5650343.
Melatonin attenuated the brain damage and cognitive impairment partially through MT2 melatonin receptor in mice with chronic cerebral hypoperfusion
Vascular cognitive impairment (VCI) encompasses cognitive decline associated with conditions such as aging, hypertension, and diabetes mellitus. Chronic cerebral hypoperfusion (CHP) is a contributing factor, leading to oxidative and inflammatory reactions. This study investigated the impact and mechanisms of melatonin in a CHP mice model. Behavioral assessments revealed cognitive impairment in CHP mice, which melatonin improved; however, the addition of an MT2 receptor antagonist reversed this improvement. Immunohistochemical staining showed that melatonin ameliorated white matter lesions and gliosis in CHP mice, and the antagonist worsened these effects. Similar results were observed for the expression of oxidative and inflammatory markers at the mRNA and protein levels. The findings suggest that melatonin, partially through the MT2 receptor, is effective against CHP-induced brain damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5650343/.
Rinne P, Silvola JM, Hellberg S, Ståhle M, Liljenbäck H, Salomäki H, Koskinen E, Nuutinen S, Saukko P, Knuuti J, Saraste A, Roivainen A, Savontaus E. Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice. Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1346-54. doi: 10.1161/ATVBAHA.113.302963. Epub 2014 May 1. PMID: 24790139.
Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice
The study aimed to investigate the potential anti-inflammatory and vasoprotective effects of melanocortin peptides in atherosclerotic mice. Low-density lipoprotein receptor-deficient mice on a high-fat diet were treated with the melanocortin analog melanotan II (MT-II). The results showed that MT-II treatment did not affect body weight, composition, or plasma cholesterol levels but significantly reduced fluorodeoxyglucose uptake in atherosclerotic plaques, indicating decreased inflammation. MT-II also induced a shift in lesional macrophages towards an anti-inflammatory M2 phenotype and attenuated systemic inflammation. Moreover, MT-II-treated mice exhibited improved aortic vasoreactivity compared to controls. These findings suggest that pharmacological activation of the melanocortin system may offer therapeutic benefits in established atherosclerosis by reducing plaque inflammation and enhancing vascular endothelial function.
You can read the full article at https://www.ahajournals.org/doi/10.1161/ATVBAHA.113.302963?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Banno R, Arima H, Sato I, Hayashi M, Goto M, Sugimura Y, Murase T, Oiso Y. The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats. Peptides. 2004 Aug;25(8):1279-86. doi: 10.1016/j.peptides.2004.05.007. PMID: 15350695.
The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats
The impact of peripheral administration of melanotan II (MTII), a melanocortin agonist, on insulin sensitivity and glucose tolerance was investigated in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. The subcutaneous administration of MTII through osmotic mini-pumps led to reduced food intake and body weight in OLETF rats. In insulin tolerance tests on day 9, the MTII group exhibited increased insulin sensitivity compared to the ad libitum or pair-fed group. Additionally, in glucose tolerance tests on days 11 and 23, the MTII group showed significantly lower glucose values than the ad libitum group, indicating improved glucose tolerance. Overall, MTII administration enhanced insulin sensitivity and positively influenced glucose tolerance in OLETF rats.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0196978104002050?via%3Dihub.
Zhou J, Zhang J, Luo X, Li M, Yue Y, Laudon M, Jia Z, Zhang R. Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur J Pharmacol. 2017 Oct 5;812:225-233. doi: 10.1016/j.ejphar.2017.07.001. Epub 2017 Jul 4. PMID: 28687198.
Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats
In type 2 diabetes mellitus (T2DM), excessive glucocorticoid (GC) adversely affects insulin sensitivity, β-cell function, and glucose metabolism. The study investigated the impact of the MT1/MT2 melatonin agonist Neu-P11 on glucose and lipid metabolism in T2DM rats. Administering Neu-P11 to T2DM rats for four weeks alleviated hyperglycemia, glucose intolerance, and insulin resistance, while also regulating food intake and water consumption. Neu-P11 enhanced GC receptor expression, reduced 11β-hydroxysteroid dehydrogenase 1 activity in the hippocampus, and influenced glucose metabolism-related gene expression in adipose tissue. The treatment improved metabolic profiles and insulin sensitivity by normalizing the hyperactivated hypothalamic-pituitary-adrenal axis in T2DM, suggesting its potential in managing obesity and T2DM.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299917304466?via%3Dihub.
Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009 Dec;58(12):2757-65. doi: 10.2337/db09-0638. Epub 2009 Sep 14. PMID: 19752162; PMCID: PMC2780865.
Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues
This study aimed to elucidate the contributions of the leptin receptor and melanocortin receptors (MCRs) in specific medial hypothalamic nuclei to the regulation of peripheral glucose uptake. In mice, injections of leptin or the MCR agonist MT-II into the ventromedial hypothalamus (VMH) increased glucose uptake in skeletal muscle, brown adipose tissue (BAT), and the heart. Leptin injections into the arcuate nucleus (ARC) selectively increased BAT glucose uptake, while injections into the dorsomedial hypothalamus (DMH) or paraventricular hypothalamus (PVH) had no effect. The MCR antagonist SHU9119 abolished the effects of leptin in the VMH. MT-II injections into the VMH or intracerebroventricularly increased glucose uptake in skeletal muscle, BAT, and the heart. In the PVH, MT-II increased BAT glucose uptake, while injections into the DMH or ARC had no effect. These findings highlight distinct roles of medial hypothalamic nuclei in mediating leptin- and MT-II-induced glucose uptake in peripheral tissues.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2780865/.
Vengeliene V, Noori HR, Spanagel R. Activation of Melatonin Receptors Reduces Relapse-Like Alcohol Consumption. Neuropsychopharmacology. 2015 Dec;40(13):2897-906. doi: 10.1038/npp.2015.143. Epub 2015 May 21. PMID: 25994077; PMCID: PMC4864625.
Activation of Melatonin Receptors Reduces Relapse-Like Alcohol Consumption
The study investigated the potential of targeting the melatonergic system as a novel approach to treat alcohol addiction. Using a rat model of long-term voluntary alcohol consumption with repeated abstinence phases, the researchers analyzed circadian drinking patterns. The novel antidepressant drug agomelatine, acting as a potent MT1 and MT2 receptor agonist and a 5-HT2C antagonist, was tested for its antirelapse effects. Melatonin and the 5-HT2C antagonist SB242084 were also examined. All drugs showed a reduction in relapse-like drinking, with agomelatine and melatonin demonstrating similar effects, suggesting that agomelatine’s impact is mainly through its melatonergic activity. These findings suggest that targeting the melatonergic system may induce a circadian phase advance, potentially mitigating relapse behavior in alcohol-dependent individuals.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4864625/.
Olney JJ, Sprow GM, Navarro M, Thiele TE. The protective effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), against binge-like ethanol drinking are facilitated by deletion of the MC3 receptor in mice. Neuropeptides. 2014;48(1):47-51. doi:10.1016/j.npep.2013.11.001.
The protective effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), against binge-like ethanol drinking are facilitated by deletion of the MC3 receptor in mice
Recent research has linked the melanocortin (MC) system to the regulation of voluntary ethanol consumption, with melanotan-II (MTII), a nonselective melanocortin receptor (MCR) agonist, reducing voluntary ethanol consumption in mice. While previous studies indicated that central MTII administration reduces ethanol drinking by signaling through MC4R, the present study aimed to explore the potential role of MC3R in binge-like ethanol intake using the “drinking in the dark” (DID) model. Results showed that MC3R-/- mice were more sensitive to the protective effects of MTII, suggesting that MC3Rs may counteract these effects. The findings imply that selective MC3R antagonists could be considered for therapeutic interventions in addressing excessive ethanol drinking.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3946855/.
Navarro M, Carvajal F, Lerma-Cabrera JM, Cubero I, Picker MJ, Thiele TE. Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2015 Aug;39(8):1425-33. doi: 10.1111/acer.12774. Epub 2015 Jun 24. PMID: 26108334; PMCID: PMC4515169.
Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice
In this study, the researchers explored the potential synergistic effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), and the nonselective opioid receptor antagonist, naltrexone (NAL), in reducing binge-like ethanol (EtOH) drinking. Given the co-localization of proopiomelanocortin (POMC)-derived melanocortin and opioid peptides in the brain, the study aimed to determine if combining MTII and NAL could enhance their individual efficacy. Results indicated that both NAL and MTII independently reduced binge-like EtOH drinking, and their co-administration significantly increased the effectiveness of NAL. This suggests that activating MC signaling, in combination with opioid receptor antagonism, might offer a novel approach for treating alcohol abuse disorders and improving existing therapies based on NAL.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4515169/.
York DA, Boghossian S, Park-York M. Melanocortin activity in the amygdala influences alcohol intake. Pharmacol Biochem Behav. 2011 Mar;98(1):112-9. doi: 10.1016/j.pbb.2010.12.010. Epub 2010 Dec 15. PMID: 21167196.
Melanocortin activity in the amygdala influences alcohol intake
Melanocortins, known to influence alcohol intake through actions in the hypothalamus via melanocortin MC4 receptors, were investigated for their role in regulating alcohol consumption in both alcohol-preferring (P) and non-preferring (NP) rats, particularly in amygdala regions expressing these receptors. Injections at the central amygdala nucleus and basolateral amygdala border revealed that the MC3/MC4R agonist MTII reduced alcohol and food intake but increased water intake. The selective MC4R antagonist HS014 increased food and water intake, while the MC3/MC4R antagonist SHU9119 had little effect on alcohol intake but increased food and water intake. Interestingly, when SHU9119-induced food intake was prevented by pair-feeding, it led to a significant and sustained decrease in alcohol intake, accompanied by increased water intake, specifically in alcohol-preferring rats. These findings suggest that melanocortin activity in the amygdala can influence the selective preference for water and alcohol independently of its effects on food intake.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0091305710003862?via%3Dihub.
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

