Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Melanotan 1
- Key Takeaways
- What is Melanotan 1?
- How Melanotan 1 Works
- Chemical Structure of Melanotan 1
- Research on Melanotan 1
- Melanotan 1 Side Effects
- Melanotan 1 Injections
- Melanotan 1 vs 2
- Melanotan 1 Dosage
- What is Afamelanotide?
- Afamelanotide Price
- Melanotan 1 Peptide
- Skin Sites Darkened by Melanotan 1 Administration
- Effects of Melanotan 1 on Sun-Exposed Sites
- Utilizing Melanotan 1 on More Skin Sites for Enhanced Results
- Effects of Melanotan 1 on Irradiated Neck Site
- Efficacy of Melanotan 1 Application on Buttock Site
- FAQ
- Reference
Table of Contents
- Overall Health Benefits of Melanotan 1
- Key Takeaways
- What is Melanotan 1?
- How Melanotan 1 Works
- Chemical Structure of Melanotan 1
- Research on Melanotan 1
- Melanotan 1 Side Effects
- Melanotan 1 Injections
- Melanotan 1 vs 2
- Melanotan 1 Dosage
- What is Afamelanotide?
- Afamelanotide Price
- Melanotan 1 Peptide
- Skin Sites Darkened by Melanotan 1 Administration
- Effects of Melanotan 1 on Sun-Exposed Sites
- Utilizing Melanotan 1 on More Skin Sites for Enhanced Results
- Effects of Melanotan 1 on Irradiated Neck Site
- Efficacy of Melanotan 1 Application on Buttock Site
- FAQ
- Reference
Overall Health Benefits of Melanotan 1
Melanotan 1 benefits include enhanced skin pigmentation, reduced sunburn risk, and potential suppression of appetite, though it should be used cautiously due to possible side effects like nausea and increased pigmentation in non-desired areas.
- Treats various skin disorders [1-17]
- Prevents heart disease [18-21]
- Fights inflammation [22-29]
- Lowers blood pressure [30]
- Improves cognitive function [31-35]
- Promotes recovery following a stroke [36]
- Promotes fat loss [37-38]
Key Takeaways
- Skin Pigmentation: Melanotan 1 enhances skin pigmentation by stimulating melanin production, providing a tan-like effect.
- Sunburn Protection: It reduces the risk of sunburn through increased melanin, which absorbs UV radiation.
- Appetite Suppression: Some users report appetite suppression as a potential side effect.
- Caution Required: Use with caution due to potential side effects such as nausea and darkening of non-targeted areas.
- Administration: Melanotan 1 is typically self-administered via injections and is not approved for medical use in many countries, remaining controversial in some regions due to safety concerns.
What is Melanotan 1?
Melanotan 1, also known as afamelanotide, is a medication used for the treatment of light-related skin conditions and prevention of skin cancer. This peptide is also used for body tanning and can be given through nasal sprays and injections.
How Melanotan 1 Works
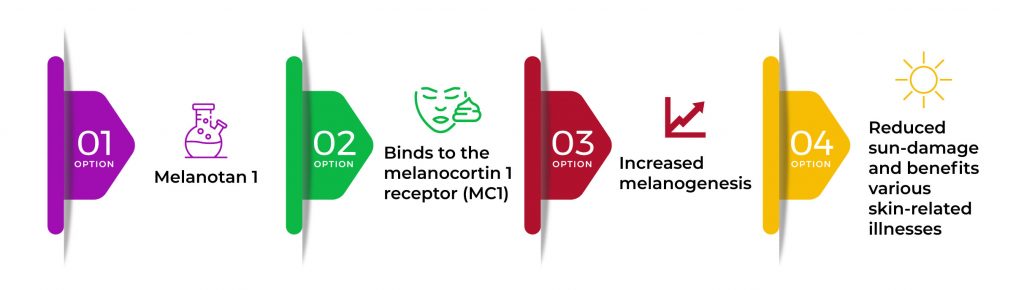
Melanotan 1 primarily increases the process of melanin production known as melanogenesis. It does this by binding to the melanocortin 1 receptor (MC1). This process reduces sun damage to the skin exposed to ultraviolet rays and also creates beneficial effects on a broad range of skin-related illnesses.
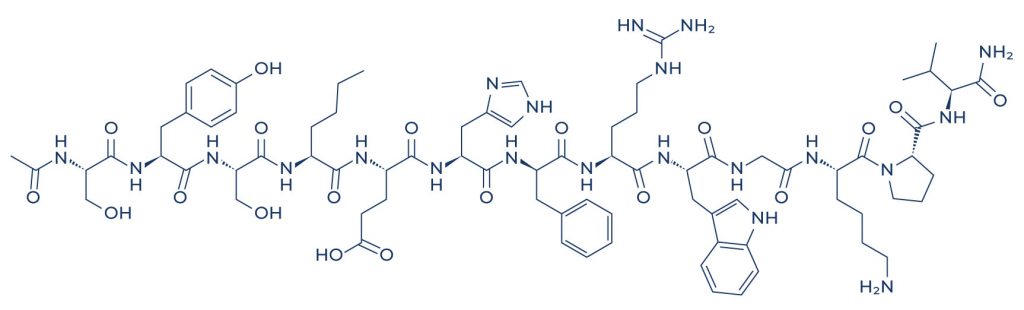
Chemical Structure of Melanotan 1
Research on Melanotan 1
A. Treats various Skin Disorders

Melanotan 1 helps treat various skin disorders by increasing melanin production, which enhances skin pigmentation and provides a natural barrier against UV radiation. This protective effect is beneficial for individuals with conditions like vitiligo, where pigmentation is reduced, as well as for those at high risk of UV-induced skin damage. By stimulating the melanocortin 1 receptor (MC1), Melanotan 1 can improve pigmentation balance and reduce symptoms associated with skin disorders that are sensitive to sun exposure.
- Results from several key Phase II and III clinical trials found that melanotan 1 can help treat erythropoietic protoporphyria, a painful skin condition caused by sensitivity to sunlight. [1-6]
- In patients with generalized vitiligo (pale white skin patches), the administration of afamelanotide resulted in faster and deeper repigmentation. [7-8]
- In patients with mild to moderate acne, melanotan 1 treatment reduced symptoms without adverse side effects. [9]
- In patients suffering from polymorphic light eruption (skin rashes due to sun exposure), melanotan 1 treatment reduced skin sensitivity and other related symptoms. [10]
- In patients with skin sensitivity caused by sunlight, afamelanotide enabled patients to spend up to seven times longer in direct sunlight without experiencing pain than patients who received placebo treatment. [11]
- A study reported that afamelanotide can help treat pre-cancerous skin lesions of the head, forearms, and hands in patients who had an organ transplant. [12]
- In patients with sunlight-induced skin sensitivity and itching, melanotan 1 significantly reduced the symptoms and increased the length of exposure to the sun. [13-15]
- In patients, melanotan 1 therapy combined with UV-B light or sunlight was significantly effective for skin tanning in response to light. [16]
- In Caucasian individuals with MC1R variant alleles, melanotan 1 administration effectively increased the melanin content of the skin, which helps protect the epidermis from damage caused by UV light. [17]
B. Prevents Heart Disease
Melanotan 1 may help prevent heart disease by reducing oxidative stress and inflammation, which are key factors in cardiovascular damage. By promoting melanin production, Melanotan 1 can increase antioxidant activity, which helps protect blood vessels from oxidative damage caused by free radicals. Additionally, it has been suggested to modulate inflammatory responses, potentially decreasing chronic inflammation that can lead to atherosclerosis and other heart-related conditions.
- In mice with atherosclerosis (plaque formation within the heart arteries), melanotan 1 prevented the formation of plaques and heart dysfunction. [18]
- A mouse study also found that melanotan 1 induced relaxation of the heart arteries which in turn improved blood circulation. [19]
- A study reported that melanotan 1 could help protect against cardiovascular disease by increasing the levels of nitric oxide, a substance that relaxes the heart arteries to improve blood circulation. [20]
- In rats undergoing a heart attack, Melanotan 1 administration in conjunction with epinephrine during cardiopulmonary resuscitation (CPR) helped restore baseline arterial pressure and heart rate. [21]
C. Fights Inflammation
Melanotan 1 fights inflammation by activating the melanocortin 1 receptor (MC1R) on immune cells, which leads to the suppression of pro-inflammatory cytokines and the promotion of anti-inflammatory signals. This receptor activation reduces the immune system’s inflammatory response, helping to alleviate symptoms in conditions associated with skin inflammation and improving overall skin health.
- A study reported that melanotan 1 induces antioxidant activities, promotes DNA repair, and modulates the inflammatory process. [22]
- In a mouse model of skin inflammation, melanotan 1 inhibited the production of inflammatory substances such as TNF-α and IL-6. [23]
- In a mouse model of nerve pain injury, the lack of melanotan 1 was associated with increased inflammatory pain. [24-25]
- In a rat model of bowel inflammation, melanotan 1 reversed intestinal inflammation. [26-27]
- A study found that melanotan 1 has anti-inflammatory properties that are similar to dexamethasone. [28]
- In mouse models of uveitis, an inflammatory disorder of the eye that can lead to pain and vision loss, systemic and local administration of melanotan 1 was effective in suppressing uveitis with a similar magnitude to that of dexamethasone. [29]
D. Lowers Blood Pressure
Melanotan 1 may help lower blood pressure by stimulating the melanocortin receptors, which can induce vasodilation (the widening of blood vessels). This effect reduces vascular resistance, allowing blood to flow more easily and reducing overall blood pressure. Additionally, the activation of these receptors can influence sympathetic nervous system activity, contributing to further blood pressure regulation.
- In hypertensive mice, melanotan 1 provided protective effects against high blood pressure without affecting other mice with normal blood pressure. [30]
E. Improves Cognitive Function
Melanotan 1 may improve cognitive function by activating melanocortin receptors, which play a role in neuroprotection and brain health. By stimulating these receptors, Melanotan 1 can enhance synaptic plasticity and reduce neuroinflammation, potentially improving memory, focus, and overall cognitive resilience. Additionally, its antioxidant properties may protect brain cells from oxidative stress, further supporting cognitive function.
- In mouse models of Alzheimer’s disease (AD), the administration of a very small dose of melanotan 1 resulted in favorable effects against cognitive decline. [31]
- In mice, once daily administration of nanomolar doses of melanotan 1 for 50 days resulted in a significant reduction of AD-related biomarkers and led to cognitive recovery by boosting neurogenesis (nerve cell formation) through the stimulation of the MC4 receptor. [32]
- In mice, once daily administration of melanotan 1 significantly reduced all AD-related biomarkers which in turn prevented the progression of the disease. [33]
- In rats, melanotan 1 was shown to improve astrocyte functioning, the cells that protect neurons and provide them with nutrition, by increasing levels of brain-derived neurotrophic factor (BDNF). [34]
- In mice and human neurons, melanotan 1 exerted long-lasting neuroprotective effects. [35]
F. Promotes Recovery following a Stroke
Melanotan 1 promotes recovery following a stroke by stimulating melanocortin receptors, which helps reduce inflammation and oxidative stress in the brain. This action minimizes secondary damage to brain tissues, supports neuroprotection, and promotes the survival of neurons in the affected areas, ultimately aiding in functional recovery and healing.
- In gerbil models of stroke, the administration of melatonin 1 and 2 after a stroke reduced brain damage and improved recovery of learning and memory. [36]
G. Promotes Fat Loss
Melanotan 1 may support fat loss indirectly by increasing energy expenditure and influencing fat metabolism through its action on melanocortin receptors. By binding to these receptors, Melanotan 1 can stimulate lipolysis, the breakdown of stored fat, which can contribute to a reduction in body fat over time. This process, combined with its effects on skin pigmentation, provides additional aesthetic benefits for users.
- In mice, melanotan 1 was shown to promote fat burning through the stimulation of MC5R. [37-38]
Melanotan 1 Side Effects
Melanotan 1 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on melanotan 1. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of melanotan 1. Despite this, it was listed as a side effect associated with melanotan 1 even though these associated side effects are very uncommon.
Side effects associated with melanotan 1 may include the following:
- Facial flushing
- Gastrointestinal upset
- Increased moles and freckles
- Loss of appetite
- Nausea
- Tiredness
- Unwanted tanning
- Vomiting
Melanotan 1 Injections
Melanotan 1 injections are a synthetic peptide used to stimulate the production of melanin in the skin, resulting in a tan-like appearance. Administered subcutaneously, typically in the abdomen or thigh, Melanotan 1 works by mimicking the natural hormone alpha-melanocyte stimulating hormone (α-MSH), which activates melanin synthesis. This process helps protect the skin from UV radiation and reduces the risk of sunburn, potentially providing a tan without prolonged sun exposure.
However, the use of Melanotan 1 injections is controversial and not approved for medical use in many countries, including the United States. The peptide’s safety and long-term effects remain a subject of concern among healthcare professionals due to reported side effects such as nausea, increased pigmentation in unintended areas, and the potential for immune system reactions. Users often acquire Melanotan 1 through online suppliers or underground markets, bypassing regulatory oversight and medical guidance.
While some individuals seek Melanotan 1 injections for cosmetic reasons, its use is discouraged by health authorities due to the lack of rigorous clinical testing and potential health risks. The peptide’s ability to induce pigmentation makes it popular among those desiring a tan, but caution is advised regarding its safety, legality, and the potential for adverse reactions.
Melanotan 1 vs 2
Melanotan 1 and Melanotan 2 are synthetic peptides designed to stimulate melanin production in the skin, leading to a tan-like pigmentation. Melanotan 1 primarily focuses on skin pigmentation enhancement with a slower onset but longer-lasting effects compared to Melanotan 2. It requires a longer loading phase for optimal results. In contrast, Melanotan 2 acts more rapidly and also includes potential effects on libido and appetite suppression due to its similarity to alpha-melanocyte-stimulating hormone (α-MSH). Both peptides are administered through subcutaneous injections but vary in dosage and frequency.
Despite their similarities, Melanotan 1 and Melanotan 2 differ in their peptide sequences and mechanisms of action. Melanotan 1’s sequence is Ac-Ser-Tyr-Ser-Nle-Glu-His-D-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2, while Melanotan 2’s sequence includes additional amino acids and differs slightly at the N-terminus. These differences contribute to varying physiological effects and potential side effects, such as nausea, flushing, and increased libido reported more commonly with Melanotan 2.
While both peptides are used primarily for cosmetic purposes to achieve a tan-like appearance, they are not approved for medical use in many countries due to safety concerns and lack of regulatory oversight. Users should exercise caution, especially regarding dosage and source credibility, as the purity and safety of these peptides can vary significantly among suppliers.
Melanotan 1 Dosage
Melanotan 1 dosage varies based on individual tolerance and desired effects. It is typically administered through subcutaneous injections, with initial dosing often starting low to gauge sensitivity. Users commonly begin with a daily dose of around 0.25 to 0.5 milligrams, gradually increasing to assess their body’s response. Once desired pigmentation levels are achieved, maintenance doses may be reduced to once or twice weekly to sustain the tan-like effect.
Due to its potential side effects and individual variability, it’s crucial for users to carefully monitor their reactions and adjust dosage accordingly. Overuse or excessive dosing can lead to unintended effects, including nausea, facial flushing, and darkening of moles or freckles. Consulting with a healthcare provider knowledgeable about peptide therapies can provide guidance on appropriate dosing schedules and help mitigate risks associated with Melanotan 1 use.
In regions where Melanotan 1 is not approved for medical use, obtaining the peptide may involve navigating legal and regulatory considerations. Users should source it from reputable suppliers to ensure product quality and reduce the risk of counterfeit substances that could pose additional health risks. Understanding the recommended dosage guidelines, potential side effects, and legality surrounding its use is essential for safe and responsible administration of Melanotan 1.
What is Afamelanotide?
Afamelanotide is a synthetic analogue of alpha-melanocyte-stimulating hormone (α-MSH), which plays a crucial role in regulating skin pigmentation. Developed by the biopharmaceutical company Clinuvel, afamelanotide has been primarily used to treat erythropoietic protoporphyria (EPP), a rare genetic disorder that causes severe light sensitivity. By stimulating melanin production in the skin, afamelanotide helps increase tolerance to sunlight and reduce the painful reactions EPP patients experience when exposed to light.
Beyond its application in EPP, afamelanotide is being explored for its potential benefits in other conditions related to skin and photoprotection. Its ability to enhance melanin production has made it a candidate for preventing skin damage caused by ultraviolet (UV) radiation, thereby potentially reducing the risk of skin cancer. Additionally, the anti-inflammatory and antioxidant properties of α-MSH suggest that afamelanotide might have therapeutic effects in treating inflammatory skin disorders and certain systemic conditions.
The administration of afamelanotide is typically done through a subcutaneous implant, which releases the drug over a sustained period. This method ensures a gradual increase in melanin levels, providing ongoing protection against UV exposure. The use of afamelanotide represents a significant advancement in dermatological therapies, offering new hope for patients with light sensitivity disorders and possibly extending its benefits to broader dermatological and systemic applications in the future.
Afamelanotide Price
Afamelanotide, a synthetic analogue of the naturally occurring hormone alpha-melanocyte-stimulating hormone (α-MSH), is primarily used to treat erythropoietic protoporphyria (EPP), a rare genetic disorder causing severe light sensitivity. The price of afamelanotide can be substantial, reflecting the costs associated with its development, production, and the rarity of the condition it treats. Given the specialized nature of its use, afamelanotide is often produced in limited quantities, contributing to its high cost.
The pricing of afamelanotide also includes the expenses related to ongoing research and development, regulatory approval processes, and the maintenance of quality and safety standards. Pharmaceutical companies invest significantly in these areas to ensure that the drug is both effective and safe for patients. These factors, combined with the small patient population, lead to higher per-unit costs, which are passed on to the consumer.
Additionally, afamelanotide’s price may vary depending on the healthcare system and country in which it is sold. In countries with strong healthcare insurance coverage or government subsidies, patients might find the cost more manageable. However, in regions without such support, the out-of-pocket expenses can be prohibitively high. Efforts to improve accessibility and affordability of afamelanotide continue to be a critical area of focus for patient advocacy groups and healthcare policymakers.
Melanotan 1 Peptide
Melanotan 1 is a synthetic analog of the naturally occurring alpha-melanocyte-stimulating hormone (α-MSH), which plays a crucial role in the regulation of skin pigmentation. This peptide is designed to mimic the effects of α-MSH, stimulating the production of melanin in the skin. Melanin is the pigment responsible for the coloration of skin, hair, and eyes, and its increased production can lead to a deeper tan. Melanotan 1 is primarily used for its tanning effects and is often marketed as a safer alternative to traditional tanning methods, such as sun exposure or tanning beds, which carry risks of skin damage and cancer.
Beyond its aesthetic applications, Melanotan 1 has been studied for potential therapeutic uses. Research suggests that it may have protective effects against ultraviolet (UV) radiation, reducing the risk of skin cancer by enhancing the natural photoprotective properties of melanin. Additionally, due to its influence on melanocortin receptors, Melanotan 1 may have anti-inflammatory and immune-modulating effects, which could make it beneficial in treating certain skin conditions and autoimmune diseases. However, these potential therapeutic benefits require further clinical investigation to confirm efficacy and safety.
Despite its promising applications, Melanotan 1 is not without risks and controversies. It is not approved by major health authorities like the FDA for tanning or any medical use, and its safety profile is not well-established. Users may experience side effects such as nausea, appetite loss, and changes in skin color, including the appearance of dark spots or freckles. There are also concerns about the long-term effects of using Melanotan 1, given the limited clinical data available. Therefore, individuals considering its use should proceed with caution and consult healthcare professionals to weigh the potential benefits and risks.
Skin Sites Darkened by Melanotan 1 Administration
Melanotan 1, a synthetic analog of the naturally occurring hormone alpha-melanocyte-stimulating hormone (α-MSH), is used to stimulate melanin production in the skin, resulting in a tanned appearance without sun exposure. This peptide works by binding to melanocortin receptors in the skin, promoting the production and distribution of melanin, which can lead to darkened skin sites. The extent and intensity of skin darkening can vary based on individual response, dosage, and duration of administration.
One of the notable effects of Melanotan 1 administration is the uneven darkening of the skin. Certain areas, such as moles, freckles, and existing hyperpigmented spots, may become more pronounced and darker than the surrounding skin. This is due to the higher concentration of melanocytes, the cells responsible for pigment production, in these areas. As a result, users may notice that these pre-existing pigmented regions become more noticeable, creating a contrast with the rest of their skin.
Additionally, prolonged use of Melanotan 1 can lead to a range of pigmentation changes, including the development of new dark spots and an overall increase in skin tone. While many seek the tanning effects for cosmetic purposes, it’s important to monitor skin changes closely, as the increased pigmentation could obscure early signs of skin issues, such as melanoma or other dermatological conditions. Regular skin examinations by a healthcare professional are recommended to ensure any changes are benign and to maintain overall skin health.
Effects of Melanotan 1 on Sun-Exposed Sites
Melanotan 1, a synthetic analog of the melanocyte-stimulating hormone (MSH), primarily stimulates melanin production in the skin, leading to a tanned appearance without significant sun exposure. This compound mimics the natural process of melanogenesis, which is the production of the pigment melanin that gives skin its color and provides some protection against UV radiation. When applied to sun-exposed sites, Melanotan 1 can enhance tanning, making these areas appear darker and potentially reducing the risk of sunburn by increasing the skin’s natural defense against UV damage.
In addition to its tanning effects, Melanotan 1 may have other benefits for sun-exposed sites. The increased melanin production can help reduce the appearance of hyperpigmentation and uneven skin tone caused by sun damage. This can lead to a more even and aesthetically pleasing skin complexion. Furthermore, by promoting melanin synthesis, Melanotan 1 may offer a degree of protection against harmful UV radiation, which is a known risk factor for skin cancers and photoaging. This protective effect could be particularly beneficial for individuals with fair skin who are more susceptible to sunburn and UV-related skin damage.
However, the use of Melanotan 1 is not without potential risks and side effects. Some users have reported issues such as nausea, appetite loss, and increased blood pressure. There are also concerns about the long-term safety and efficacy of the compound, as it has not been extensively studied or approved by many regulatory agencies for cosmetic use. Moreover, the enhanced tanning effect may lead some individuals to engage in riskier sun exposure behaviors, believing they are more protected than they actually are. Therefore, while Melanotan 1 can offer aesthetic and protective benefits for sun-exposed sites, its use should be approached with caution and awareness of potential health risks.
Utilizing Melanotan 1 on More Skin Sites for Enhanced Results
Melanotan 1, a synthetic analog of the alpha-melanocyte-stimulating hormone (α-MSH), is primarily used to induce skin tanning by stimulating melanin production. When applied to more skin sites, the distribution of the peptide can be more uniform, potentially leading to enhanced and more consistent tanning results. By targeting multiple areas, users might achieve a more natural and even skin tone compared to localized application, which could result in uneven pigmentation or patchy tan areas.
Expanding the application to more skin sites can also facilitate better absorption of Melanotan 1. Different parts of the skin have varying absorption rates, and spreading the dosage across multiple sites might maximize the efficacy of the peptide. This approach could potentially reduce the risk of over-concentration in a single area, which might lead to localized side effects such as irritation or dark spots. Ensuring an even application can help in maintaining a balanced and aesthetically pleasing tan.
However, it’s important to consider that increasing the number of application sites also raises the potential for systemic absorption, which could lead to widespread side effects. Users should be cautious and ideally consult with a healthcare professional to tailor the dosage and application strategy to their individual needs and skin type. Proper usage and moderation are key to achieving the desired tanning results while minimizing risks associated with Melanotan 1.
Effects of Melanotan 1 on Irradiated Neck Site
Melanotan 1, a synthetic analog of the alpha-melanocyte-stimulating hormone, has shown potential therapeutic effects on irradiated neck sites. Radiotherapy, often used to treat head and neck cancers, can result in significant skin damage, characterized by erythema, desquamation, and even necrosis in severe cases. Melanotan 1 works by stimulating melanogenesis, which not only leads to tanning but also potentially enhances the skin’s ability to repair and protect itself from further damage.
Studies suggest that Melanotan 1 may promote wound healing and improve skin integrity in irradiated areas. Its melanogenic properties can increase the production of melanin, which acts as a natural barrier against UV radiation and other environmental stressors. Additionally, Melanotan 1 has anti-inflammatory effects, which can help mitigate the inflammatory response typically seen in irradiated skin, thereby reducing pain and discomfort associated with radiation therapy.
However, the application of Melanotan 1 in this context is still under investigation, and its long-term safety profile is not fully established. While initial findings are promising, more comprehensive clinical trials are necessary to determine its efficacy and safety for patients with irradiated neck sites. Healthcare providers must weigh the potential benefits against possible risks, including the unknowns of long-term use, to ensure the best outcomes for patients undergoing radiation therapy.
Efficacy of Melanotan 1 Application on Buttock Site
Melanotan 1, a synthetic analog of the peptide hormone alpha-melanocyte-stimulating hormone (α-MSH), is primarily used to induce skin tanning. The efficacy of its application on the buttock site can be attributed to the subcutaneous fat layer, which provides an ideal depot for slow and sustained release of the peptide into the bloodstream. Studies indicate that subcutaneous injections on the buttock, compared to other sites, may offer more consistent absorption and less localized irritation, making it a preferred site for many users.
Clinical trials and user reports have demonstrated that Melanotan 1 effectively increases melanin production, leading to a deeper and longer-lasting tan. This effect is particularly advantageous for individuals with fair skin who are prone to sunburns. When applied to the buttock site, users have reported uniform tanning without the patchiness that can occur with topical tanning solutions. Additionally, the buttock area is often chosen for its relative lack of aesthetic disruption, making it a discreet option for regular administration.
However, the application of Melanotan 1 on the buttock site is not without potential side effects. Users have reported nausea, flushing, and in some cases, the development of new moles or darkening of existing ones. It is crucial for individuals considering Melanotan 1 to consult healthcare professionals to ensure safe use and to monitor for any adverse reactions. The long-term safety profile of Melanotan 1 remains under investigation, emphasizing the need for cautious and informed use.
FAQ
What is afamelanotide used for?
Afamelanotide is primarily used to treat erythropoietic protoporphyria (EPP), a rare genetic disorder that causes severe photosensitivity and pain upon exposure to sunlight. It helps to increase the production of melanin in the skin, thereby providing protection against sunlight-induced damage from solar UV radiation. By enhancing melanin levels and contributing to the tanning of the skin, afamelanotide reduces the harmful effects of solar UV radiation on the skin. This increased melanin, which aids in the tanning of the skin, not only alleviates the pain associated with EPP but also offers a defense mechanism against the damaging impact of solar UV radiation. The process of tanning of the skin helps in creating a barrier that further protects against sunlight-induced harm.
Who makes afamelanotide?
Afamelanotide is manufactured by the pharmaceutical company Clinuvel Pharmaceuticals. Clinuvel Pharmaceuticals is known for its focus on developing treatments that can address severe skin conditions, including the potential to reduce the risk of skin cancers. By increasing melanin production in the skin, afamelanotide not only helps with conditions like erythropoietic protoporphyria but may also offer some protective benefits against certain types of skin cancers. The ongoing research by Clinuvel Pharmaceuticals includes open label studies to further explore the role of afamelanotide in preventing skin cancers. These open label studies are crucial in understanding how afamelanotide impacts skin cancer risk and contribute to the body of evidence supporting its potential benefits. Clinuvel Pharmaceuticals continues to invest in open label studies to ensure comprehensive evaluation of afamelanotide’s effectiveness and safety.
How do you say afamelanotide?
Afamelanotide is pronounced as “a-fa-mel-an-oh-tide.” It’s important to note that while afamelanotide is different from Melanotan, there have been concerns about melanotan associated melanoma due to increased melanin production, particularly at a higher dose. Understanding these risks is crucial, especially in distinguishing afamelanotide from similar compounds. Being informed about melanotan associated melanoma helps in making safe and educated decisions regarding skin pigmentation treatments, especially when considering a higher dose. Always consult healthcare providers to avoid complications, such as melanotan associated melanoma, which can be exacerbated by a higher dose.
What is the sequence of afamelanotide?
Afamelanotide is a synthetic analog of the natural peptide alpha-melanocyte-stimulating hormone (α-MSH). Its amino acid sequence is Ac-Nle-c[Asp-His-D-Phe-Arg-Trp]-Asp-NH2, where Ac denotes an acetyl group and NH2 represents an amide group. Similar to melanotan II, afamelanotide has been designed to increase melanin production. Both melanotan II and afamelanotide share mechanisms that involve stimulating melanocytes, although their specific applications and regulatory statuses may differ. It’s important to note that while melanotan II is often used for tanning purposes, afamelanotide is primarily used for medical conditions such as erythropoietic protoporphyria, where responses can be highly variable. The effectiveness of these treatments can be highly variable, reflecting individual differences. Additionally, the clinical outcomes associated with afamelanotide can also be highly variable depending on the specific condition being treated.
What is Afamelanotide used for?
Afamelanotide is used to treat erythropoietic protoporphyria (EPP) by increasing melanin production in the skin, which helps to reduce photosensitivity and alleviate associated pain. This treatment can act synergistically with other therapeutic approaches to manage EPP. Similar to melanotan II, this treatment helps those suffering from conditions caused by a lack of melanin. While afamelanotide is specifically targeted for EPP, melanotan II is often discussed in contexts of tanning and skin pigmentation enhancement. Additionally, melanotan II is another synthetic analog of alpha-MSH, which can act synergistically with afamelanotide, highlighting the range of therapeutic applications in the field of dermatology.
How much does Scenesse cost?
The cost of Scenesse (the brand name for afamelanotide) varies depending on the country and healthcare system. In the U.S., a single dose can cost between $10,000 to $15,000, similar to some treatments for erectile dysfunction and pathologic findings. Prices in other countries might differ due to healthcare coverage and pricing regulations, just as they do for erectile dysfunction medications and pathologic findings. When comparing costs internationally, it’s important to consider how erectile dysfunction treatments and pathologic findings are priced in different healthcare systems.
Where can I get Scenesse?
Scenesse is available through specialized pharmacies or medical centers that offer treatments for rare diseases like erythropoietic protoporphyria and erectile dysfunction. It is often prescribed and administered by healthcare professionals who specialize in managing this condition, particularly due to its potential toxic effects. For individuals dealing with rare conditions such as erythropoietic protoporphyria and erectile dysfunction, specialized medical centers provide the necessary treatments and monitor for any toxic effects.
Does Scenesse make you tan?
Yes, Scenesse can induce tanning as a side effect. The increased production of melanin from the use of afamelanotide results in darker skin pigmentation, which can help provide some level of protection against full sunlight and reduce photosensitivity. Although primarily used for conditions like erythropoietic protoporphyria, afamelanotide is not typically associated with treating erectile dysfunction. However, it is important to note that some medications designed for other uses may sometimes have effects on erectile dysfunction. While Scenesse mainly focuses on increasing melanin production, individuals concerned about erectile dysfunction should consult healthcare professionals for appropriate treatments in full sunlight. This is particularly relevant for those who might be exposed to full sunlight regularly.
What are the common side effects of Melanotan 1?
Common side effects of Melanotan 1 include nausea, facial flushing, and appetite loss. Some users also report significantly enhanced tanning, the darkening of existing moles, new mole formation, and eruptive nevi. These side effects may vary in severity and frequency among individuals. Despite the risk of side effects, many users appreciate the significantly enhanced tanning effect. However, it is essential to weigh the potential for significantly enhanced tanning and eruptive nevi against possible adverse reactions and consult a healthcare professional before use.
How is Melanotan 1 administered?
Melanotan 1, often referred to as the “sun tan jab,” is typically administered via subcutaneous injection, meaning it is injected under the skin. The dosage and frequency of administration of the superpotent cyclic melanotropic peptide can vary depending on the desired tanning effect and individual tanning response. It is important to follow proper medical guidance when using this superpotent cyclic melanotropic peptide, commonly known as the sun tan jab, to ensure the best tanning response.
What is the recommended dosage of Melanotan 1?
Some protocols suggest starting with a low dose of melanotan injection and gradually increasing based on tolerance and desired tanning effect. UV B exposure can affect the efficacy of melanotan injection, so consulting a healthcare professional for personalized medicine advice is essential when using melanotan injection. This ensures that the melanotan injection is used safely and effectively to achieve the best results, especially considering UV B sensitivity and the role of medicine in managing potential side effects.
How long does it take for Melanotan 1 to work?
The onset of tanning effects from Melanotan 1 can vary, with some users noticing changes within a few days to a week. Full tanning results may take several weeks of consistent use. Individual response and skin type can influence the time it takes to achieve the desired results, including reaching a minimal erythema dose, which is the amount of UV B exposure required to produce just enough redness for the skin to start producing more melanin. Sunlight only controls how quickly you achieve this minimal erythema dose. Monitoring the minimal erythema dose during Melanotan 1 use, particularly in relation to UV B exposure, can help users gauge their skin’s sensitivity and adjust their dosage accordingly. It’s essential to be mindful that sunlight only controls the effectiveness of UV B exposure to ensure effective and safe tanning. Ultimately, sunlight only controls how the tanning process unfolds and how your skin responds.
Can Melanotan 1 be used for medical purposes?
Melanotan 1 has potential medical applications, particularly in conditions like erythropoietic protoporphyria (EPP), which causes extreme sensitivity to sunlight. It may help patients with EPP tolerate sunlight better by increasing melanin production. Additionally, research suggests that Melanotan 1 might also impact nitric oxide production, which could contribute to its therapeutic effects, potentially affecting spontaneous erections. However, its use for such conditions should be under strict medical supervision, especially considering possible side effects like spontaneous erections.
What is the difference between Melanotan 1 and Melanotan 2?
Melanotan 1 and Melanotan 2 are both analogues of α-MSH but differ in their effects and side effects. Melanotan 1 primarily induces tanning with fewer reported side effects. Melanotan 2, on the other hand, promotes tanning and can have varying effects based on dosage in mg/kg. It also increases libido and can cause more pronounced side effects like nausea and appetite changes, which might be dose-dependent, measured in mg/kg. This differentiation in side effects and effects is important when considering their use, as each has different implications based on the mg/kg dosage used.
Are there any long-term effects of using Melanotan 1?
The long-term effects of Melanotan 1 are not well-documented due to limited research. Potential risks may include prolonged changes in skin pigmentation and the development of new moles, including dysplastic nevi. For assessing safety, it is crucial to evaluate dosage in terms of mg/kg, as well as long-term exposure. Long-term safety and efficacy require more extensive clinical studies, including investigations into the mg/kg dosage to ensure comprehensive understanding of potential risks and benefits, such as the risk of developing dysplastic nevi.
Can Melanotan 1 protect against skin cancer?
While Melanotan 1 increases melanin production, which can offer some protection against UV damage, it should not be considered a substitute for sunscreen or other protective measures. There are concerns that moles linked to increased melanin production may still pose a risk, as it does not eliminate the risk of skin cancer. The main outcome measure is that while it may reduce the risk of some forms of skin damage, regular skin checks and sun protection are still crucial to monitor any moles linked to potential skin issues. The main outcome measure of using Melanotan 1 should always be complemented by traditional protective strategies.
Can Melanotan 1 be used alongside other tanning methods?
Melanotan 1 can be used in conjunction with other tanning methods, such as sun exposure or tanning beds, to enhance the tanning effect. Dermatology clinics often recommend being cautious and avoiding excessive UVB radiation exposure, which can increase the risk of skin damage and cancer. Always use sun protection measures when exposed to UVB radiation. Melanotan 1 may help in achieving a tan, but dermatology clinics emphasize the importance of managing UVB radiation exposure carefully to prevent adverse effects on your skin. For personalized advice, it’s best to consult with dermatology clinics to ensure safe tanning practices.
How should Melanotan 1 be stored?
Melanotan 1 should be stored in a cool, dry place, typically in a refrigerator to maintain its stability and potency. It should be kept away from light and heat, including UV B radiation, to prevent degradation, especially in cases involving eruptive melanocytic naevi. In clinical studies involving human volunteers, proper storage conditions are crucial to ensure the integrity of the product, particularly when studying the effects on eruptive melanocytic naevi. Always follow storage instructions provided with the product to ensure that UV B radiation does not affect its quality, as maintaining these conditions is essential for studies involving human volunteers, including those focused on eruptive melanocytic naevi.
Can Melanotan 1 be used by anyone?
Melanotan 1 can be used in conjunction with other tanning methods, such as sun exposure or tanning beds, to enhance the tanning effect. Dermatology clinics often recommend being cautious and avoiding excessive UVB radiation exposure, which can increase the risk of skin damage and cancer. Always use sun protection measures when exposed to UVB radiation. Melanotan 1 may help in achieving a tan, but dermatology clinics emphasize that the main outcome measure should be managing UVB radiation exposure carefully to prevent adverse effects on your skin. For personalized advice, it’s best to consult with dermatology clinics to ensure safe tanning practices, keeping in mind that the main outcome measure is maintaining skin health. Ultimately, the main outcome measure of any tanning approach should be the balance between achieving the desired tan and protecting your skin.
Are there any contraindications for Melanotan 1?
Melanotan 1 can be used in conjunction with other tanning methods, such as sun exposure or tanning beds, to enhance the tanning effect. Dermatology clinics often recommend being cautious and avoiding excessive UVB radiation exposure, which can increase the risk of skin damage and cancer. Always use sun protection measures when exposed to UVB radiation. Melanotan 1 may help in achieving a tan, but dermatology clinics emphasize the importance of managing UVB radiation exposure carefully to prevent adverse effects on your skin. For personalized advice, it’s best to consult with dermatology clinics to ensure safe tanning practices.
Can Melanotan 1 cause allergic reactions?
Although rare, allergic reactions to Melanotan 1 can occur. Symptoms may include itching, rash, or swelling at the injection site. If you experience any signs of an allergic reaction, discontinue use immediately and seek medical attention. While Melanotan 1 aims to provide an equivalent tanning effect to natural sunlight, it is important to monitor for any adverse reactions. If you experience any symptoms, remember that safety should come first, even if the product offers equivalent tanning results.
What should I do if I miss a dose of Melanotan 1?
If you miss a dose of Melanotan 1, take it as soon as you remember. However, if it is close to the time for your next dose, skip the missed dose and resume your regular dosing schedule. Keep in mind that missing doses of Melanotan 1 may increase the risk of developing atypical moles or other skin irregularities. Do not double up doses to make up for the missed one, as this could potentially exacerbate the risk of atypical moles or other skin issues.
Can Melanotan 1 be used for conditions other than tanning?
Research is ongoing to explore other potential uses of Melanotan 1, including its effects on appetite control and potential benefits for certain skin disorders. The cancer center division is investigating its role in managing skin conditions that might intersect with cancer treatments. However, its primary and most researched use remains skin tanning. Any off-label use should be conducted under medical supervision, with the cancer center division providing guidance for such applications.
How does Melanotan 1 compare to natural tanning?
Melanotan 1 induces tanning by increasing melanin production without the need for UV exposure, while natural tanning relies on UV radiation to stimulate melanin. This process can influence the immune response, potentially offering a safer alternative to natural tanning, which carries a higher risk of skin damage and cancer. However, Melanotan 1’s effectiveness and safety, including its impact on the immune response, require careful consideration and professional guidance.
What are the signs of overuse of Melanotan 1?
Signs of overuse of Melanotan 1 may include excessively darkened skin, new or darkening moles, and persistent nausea. Additionally, users might experience decreased appetite as a side effect, which could lead to unintended weight loss. It is important to use the peptide as directed and consult a healthcare provider if any concerning symptoms arise. Overuse can increase the risk of adverse effects, including decreased appetite, and long-term health issues. To demonstrate safety, users should follow dosage guidelines strictly. It is also crucial to demonstrate safety by seeking professional medical advice when necessary. Regular monitoring and adherence to recommendations can further demonstrate safety and reduce the risk of potential complications.
Can Melanotan 1 cause changes in hair color?
Melanotan 1 primarily affects melanin production in the skin, and its impact on hair color is less pronounced. However, some users may notice slight changes in hair pigmentation over time. These changes are typically minor and vary among individuals. There is no substantial evidence to suggest that Melanotan 1 directly influences sexual desire, although individual experiences may vary. The primary effect of Melanotan 1 remains focused on skin pigmentation rather than alterations in sexual desire.
Is Melanotan 1 effective for all skin types?
Melanotan 1 can be effective for various skin types iii and skin diseases, though individual responses may vary. Those with lighter skin types iii may see more noticeable tanning effects, while individuals with darker skin types iii may experience subtler changes. It is important to start with a low dose and monitor the response to determine effectiveness, especially in individuals with specific skin diseases.
Can I combine Melanotan 1 with other medications?
Combining Melanotan 1 with other medications should be done cautiously and under medical supervision, especially if neck irradiation is part of the treatment plan. Potential interactions with other drugs, including those used in neck irradiation, have not been extensively studied. Always inform your healthcare provider about any other medications you are taking, as well as any recent neck irradiation, before starting Melanotan 1.
What research supports the use of Melanotan 1?
Research on Melanotan 1 includes studies on its effects on melanin production, potential benefits for conditions like EPP, and its safety profile. Melanotan I has been explored for its role in protecting against UV rays, and some studies suggest promising results. While more extensive clinical trials are needed to fully understand its efficacy and safety in reducing UV rays exposure, melanotan I continues to be a subject of interest. Always rely on up-to-date and reputable sources when considering its use, especially concerning melanotan I and its ability to mitigate the harmful effects of UV rays.
How long do the effects of Melanotan 1 last?
The duration of Melanotan 1’s effects can vary, with tanning results typically lasting several weeks to months after discontinuing use. Maintenance doses of peptide hormones may be required to sustain the tan. The longevity of Melanotan I effects depends on individual factors such as skin type and exposure to UV light, as well as the specific peptide hormones involved in the tanning process. Melanotan I’s effects can be influenced by how often the peptide hormones are administered and the individual’s response to Melanotan I.
Can Melanotan 1 be used to treat vitiligo?
Some research suggests that Melanotan 1 may help improve pigmentation in individuals with vitiligo, a condition characterized by loss of skin pigment, through increased eumelanin expression and possibly affecting penile erection. However, its use for vitiligo treatment is not well-established and should be pursued under medical guidance. More research is needed to confirm its effectiveness for this purpose, particularly regarding its role in promoting increased eumelanin expression and its potential impact on penile erection. Furthermore, understanding the relationship between Melanotan 1 and penile erection could provide additional insights into its overall effects on the body.
What should I expect during my first use of Melanotan 1?
During the first use of Melanotan 1, you may experience mild side effects such as nausea or facial flushing. Some users report that it can produce erections as a side effect. To help mitigate these effects, isotonic sodium chloride may be used as a soothing agent. Tanning effects may not be immediately noticeable and can take several days to weeks to develop. It is important to note that Melanotan 1 can produce erections in some individuals. Start with a low dose to assess tolerance and gradually increase as needed, and consider using isotonic sodium chloride to manage any initial discomfort. Additionally, be aware that this peptide can produce erections, which is a common reaction for many users.
Is Melanotan 1 detectable in drug tests?
Melanotan 1 is not typically included in standard drug tests and is unlikely to be detected. However, specialized tests could potentially identify its presence. Some users report that Melanotan 1 can produce penile erections as a side effect, though this is not its primary purpose. If you are subject to drug testing, it is best to disclose any use of Melanotan 1 to the testing authority. Additionally, if you experience any side effects like the ability to produce penile erections, it is important to mention this to your healthcare provider. It is crucial to note that Melanotan 1 may lead to penile erections, so monitoring and reporting any unusual changes to your healthcare provider is advised.
Can Melanotan 1 be used during pregnancy or breastfeeding?
The use of Melanotan 1 during pregnancy or breastfeeding is not recommended due to a lack of safety data. Potential risks to the mother and child are unknown, and it is best to avoid use during these periods. Melanotan 1, which is a highly potent alpha melanotropin, can have effects that are not well understood in these sensitive stages of life. Always consult a healthcare provider for advice on safely combined practices during pregnancy and breastfeeding, especially when dealing with substances like the highly potent alpha melanotropin. The effects of this highly potent alpha melanotropin on fetal development and lactation have not been thoroughly studied, making safely combined caution essential. Ensuring safely combined approaches during these stages is crucial due to the unknown potential risks.
Reference
Lane AM, McKay JT, Bonkovsky HL. Advances in the management of erythropoietic protoporphyria – role of afamelanotide. Appl Clin Genet. 2016;9:179-189. Published 2016 Dec 12. doi:10.2147/TACG.S122030.
Advances in the management of erythropoietic protoporphyria – role of afamelanotide
Erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLPP) are hereditary skin conditions characterized by extreme sensitivity to sunlight and a reduced quality of life due to the excessive production of protoporphyrin (PP). EPP is caused by a genetic defect in the ferrochelatase gene, while XLPP results from a gain-of-function mutation in the gene encoding 5-aminolevulinic acid synthase-2. Current treatments for these conditions are not very effective. Afamelanotide (Scenesse®), a synthetic analog of α-melanocyte-stimulating hormone, increases skin pigmentation by stimulating melanin production, enhancing sunlight tolerance in EPP and XLPP patients. Afamelanotide is approved in the European Union and Switzerland and is under review by the FDA in the United States for EPP/XLPP treatment. This paper reviews the clinical features, existing therapies, pharmacology, and clinical trials of afamelanotide, highlighting its promise as a treatment for these conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5161401/.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-000636-13.
Kim ES, Garnock-Jones KP. Afamelanotide: A Review in Erythropoietic Protoporphyria. Am J Clin Dermatol. 2016 Apr;17(2):179-85. doi: 10.1007/s40257-016-0184-6. PMID: 26979527.
Afamelanotide: A Review in Erythropoietic Protoporphyria
In two multicenter, randomized, double-blind, placebo-controlled trials involving patients with erythropoietic protoporphyria, the safety and efficacy of afamelanotide, an α-melanocyte-stimulating hormone analogue delivered via subcutaneous implants, were assessed. Patients in the European Union (EU) and the United States (US) received regular implants over study periods of 180 days (EU) and 270 days (US). Afamelanotide treatment increased pain-free sun exposure time and improved quality of life in both studies. The US study showed a longer duration of pain-free time at 6 months compared to the placebo group, while the EU study demonstrated similar results at 9 months. Adverse events were mostly mild, with no significant drug-related serious adverse events reported.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4780255/.
Minder EI. Afamelanotide, an agonistic analog of α-melanocyte-stimulating hormone, in dermal phototoxicity of erythropoietic protoporphyria. Expert Opin Investig Drugs. 2010 Dec;19(12):1591-602. doi: 10.1517/13543784.2010.535515. Epub 2010 Nov 13. PMID: 21073357.
Afamelanotide, an agonistic analog of α-melanocyte-stimulating hormone, in dermal phototoxicity of erythropoietic protoporphyria
This review focuses on the significance of afamelanotide, an α-melanocyte stimulating hormone (MSH) agonistic analog, as a potential therapy for protoporphyria (PP), encompassing erythropoietic protoporphyria and X-linked dominant protoporphyria. It discusses the genetic and current treatment aspects of PP, highlights the physiological and pharmacological actions of α-MSH and afamelanotide, including their intracellular signaling and receptor polymorphisms. The review also addresses adverse effects and safety concerns. Readers will gain insight into the clinical severity of PP, the potential efficacy of afamelanotide, and recent trial results, suggesting its promise as a therapeutic option for light-related skin diseases and potentially other medical conditions in the future.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.1517/13543784.2010.535515.
Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider-Yin X, Minder EI. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br J Dermatol. 2015 Jun;172(6):1601-1612. doi: 10.1111/bjd.13598. Epub 2015 Apr 30. PMID: 25494545.
Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria
In patients with erythropoietic protoporphyria (EPP), a condition characterized by painful phototoxic reactions due to protoporphyrin accumulation in the skin, the introduction of afamelanotide has shown significant improvements in light tolerance. Longitudinal observations of 115 EPP patients who received a total of 1023 afamelanotide implants over up to 8 years at two porphyria centers (Rome, Italy, and Zurich, Switzerland) revealed that the treatment has been well-received, with high compliance and a low discontinuation rate of 23%. Quality of life scores improved from 31% to 74% after starting afamelanotide and remained stable. The treatment demonstrated good clinical effectiveness and safety in EPP under long-term routine conditions.
You can read the full article at https://academic.oup.com/bjd/article/172/6/1601/6616051?login=false.
Wensink D, Wagenmakers MAEM, Barman-Aksözen J, Friesema ECH, Wilson JHP, van Rosmalen J, Langendonk JG. Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice. JAMA Dermatol. 2020 May 1;156(5):570-575. doi: 10.1001/jamadermatol.2020.0352. PMID: 32186677; PMCID: PMC7081144.
Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice
In this prospective postauthorization safety and efficacy cohort study, the effectiveness of afamelanotide treatment in patients with erythropoietic protoporphyria (EPP) was evaluated in clinical practice over a longer-term follow-up period. A total of 117 EPP patients were treated with afamelanotide, and nearly all continued treatment (98%) with a median follow-up of 2.0 years. The results showed significant improvements in time spent outside during treatment, disease-specific quality of life, and the severity of phototoxic reactions. The treatment exhibited a good safety profile, with minor self-limiting adverse events. This study highlights the positive clinical outcomes and safety of afamelanotide for EPP patients, suggesting its effectiveness in improving their quality of life and sun tolerance.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7081144/.
Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013 Jan;149(1):68-73. doi: 10.1001/2013.jamadermatol.386. PMID: 23407924.
The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo
In this preliminary report, the use of afamelanotide in combination with narrowband UV-B (NB-UV-B) phototherapy for generalized vitiligo is discussed. Four patients with vitiligo showed promising results with afamelanotide treatment. They received NB-UV-B phototherapy three times a week and a series of monthly implants containing 16 mg of afamelanotide starting in the second month. Afamelanotide led to faster and deeper repigmentation in all cases, with follicular and confluent areas of repigmentation appearing within 2 days to 4 weeks after the initial implant. Additionally, all patients experienced diffuse hyperpigmentation. This suggests that afamelanotide, when combined with NB-UV-B, may be a novel and potentially effective treatment for vitiligo by promoting melanoblast differentiation, proliferation, and eumelanogenesis, but further research is needed to confirm these findings.
You can read the full article at https://jamanetwork.com/journals/jamadermatology/fullarticle/1377949.
Available from https://clinicaltrials.gov/ct2/show/NCT01382589.
Afamelanotide and Narrow-Band Ultraviolet B (NB-UVB) Light in the Treatment of Nonsegmental Vitiligo (NSV)
This study aims to assess the effectiveness of afamelanotide, a synthetic analogue of alpha melanocyte stimulating hormone (alpha-MSH), in combination with narrow-band ultraviolet B (NB-UVB) light for nonsegmental vitiligo patients. Afamelanotide is anticipated to enhance the repigmentation induced by NB-UVB, potentially reducing the need for frequent and high doses of NB-UVB treatment.
You can read the abstract of the article at https://classic.clinicaltrials.gov/ct2/show/NCT01382589.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-018024-15.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-017359-92.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-011018-51.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-007015-89.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2008-002446-39.
Available from https://www.clinicaltrialsregister.eu/ctr-search/search?query=2007-001068-55.
Minder EI, Barman-Aksoezen J, Schneider-Yin X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin Pharmacokinet. 2017 Aug;56(8):815-823. doi: 10.1007/s40262-016-0501-5. PMID: 28063031.
Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders
Afamelanotide, a synthetic analog of α-melanocyte-stimulating hormone (α-MSH), has been extensively studied for its pigmentation-related properties, demonstrating higher activity and stability than natural α-MSH. It binds to the melanocortin-1 receptor (MC1R), promoting melanin synthesis, antioxidant activity, DNA repair, and inflammation modulation. Clinical trials in human volunteers found that subcutaneous injections of afamelanotide resulted in lasting skin pigmentation and were well-tolerated. Despite MC1R variants and fair skin, afamelanotide effectively increased skin pigmentation. It has shown promise in treating conditions like polymorphic light eruption, erythropoietic protoporphyria, solar urticaria, Hailey-Hailey disease, and vitiligo. In 2014, it was approved by the European Medicines Agency for preventing phototoxicity in adult EPP patients, with no reported late effects or significant adverse events in long-term use.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s40262-016-0501-5.
Available from https://jamanetwork.com/journals/jamadermatology/fullarticle/480676.
Fitzgerald LM, Fryer JL, Dwyer T, Humphrey SM. Effect of MELANOTAN, [Nle(4), D-Phe(7)]-alpha-MSH, on melanin synthesis in humans with MC1R variant alleles. Peptides. 2006 Feb;27(2):388-94. doi: 10.1016/j.peptides.2004.12.038. Epub 2005 Nov 15. PMID: 16293341.
Effect of MELANOTAN, [Nle(4), D-Phe(7)]-alpha-MSH, on melanin synthesis in humans with MC1R variant alleles
In this study involving 77 Caucasian individuals, the effects of MELANOTAN (NDP-MSH) were examined in relation to variant MC1R genotypes. MELANOTAN, known for its ability to increase eumelanin in skin cells, was administered to participants. The results revealed a significant (p<0.001) increase in melanin density compared to a placebo in treated individuals. Importantly, MELANOTAN had a more pronounced effect on individuals with variant MC1R alleles, including Val60Leu, Asp84Glu, Val92Met, Arg142His, Arg151Cys, and Arg160Trp, indicating its efficacy in increasing melanin content in those who may benefit from enhanced photoprotection.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0196978105004511?via%3Dihub.
Rinne P, Silvola JM, Hellberg S, Ståhle M, Liljenbäck H, Salomäki H, Koskinen E, Nuutinen S, Saukko P, Knuuti J, Saraste A, Roivainen A, Savontaus E. Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice. Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1346-54. doi: 10.1161/ATVBAHA.113.302963. Epub 2014 May 1. PMID: 24790139.
Pharmacological activation of the melanocortin system limits plaque inflammation and ameliorates vascular dysfunction in atherosclerotic mice
The study aimed to investigate whether melanocortin peptides, known for their anti-inflammatory and vascular endothelial function-promoting properties, could alleviate atherosclerotic plaque inflammation and improve vasoreactivity in atherosclerotic mice. Using low-density lipoprotein receptor-deficient mice on a high-fat diet, they treated the mice with a stable melanocortin analog, melanotan II (MT-II), for four weeks. While MT-II didn’t affect body weight, composition, or cholesterol levels, it reduced inflammation in atherosclerotic plaques, shifted lesional macrophages towards the anti-inflammatory M2 phenotype, and decreased systemic inflammation. Furthermore, MT-II improved aortic vasoreactivity, enhancing endothelium-dependent relaxations and sensitivity to nitric oxide-mediated vasodilation. These findings suggest that melanocortin system activation could be a promising therapeutic approach for atherosclerosis by reducing plaque inflammation and enhancing vascular endothelial function.
You can read the full article at https://www.ahajournals.org/doi/10.1161/ATVBAHA.113.302963?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Rinne P, Nordlund W, Heinonen I, Penttinen AM, Saraste A, Ruohonen ST, Mäkelä S, Vähätalo L, Kaipio K, Cai M, Hruby VJ, Ruohonen S, Savontaus E. α-Melanocyte-stimulating hormone regulates vascular NO availability and protects against endothelial dysfunction. Cardiovasc Res. 2013 Feb 1;97(2):360-8. doi: 10.1093/cvr/cvs335. Epub 2012 Nov 5. PMID: 23131503; PMCID: PMC3543993.
α-Melanocyte-stimulating hormone regulates vascular NO availability and protects against endothelial dysfunction
This study aimed to investigate the role of α-melanocyte-stimulating hormone (α-MSH) in regulating blood vessel tone. The research showed that α-MSH improved endothelium-dependent vasodilation in mouse blood vessels by enhancing endothelial nitric oxide (NO) formation and sensitivity to endothelium-independent relaxation. These effects were mediated by melanocortin 1 (MC1) receptors expressed in the endothelium. Additionally, stable analogues of α-MSH improved endothelial dysfunction in aging and diet-induced obesity in mice, highlighting the potential therapeutic use of α-MSH analogues in conditions characterized by vascular dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543993/.
Weng WT, Wu CS, Wang FS, et al. α-Melanocyte-Stimulating Hormone Attenuates Neovascularization by Inducing Nitric Oxide Deficiency via MC-Rs/PKA/NF-κB Signaling. Int J Mol Sci. 2018;19(12):3823. Published 2018 Nov 30. doi:10.3390/ijms19123823.
α-Melanocyte-Stimulating Hormone Attenuates Neovascularization by Inducing Nitric Oxide Deficiency via MC-Rs/PKA/NF-κB Signaling
This study investigated how α-melanocyte-stimulating hormone (α-MSH) inhibits angiogenesis and its underlying mechanisms. Using human umbilical vein endothelial cells (HUVECs), rat aorta rings, and transgenic zebrafish, the research found that α-MSH reduced nitric oxide (NO) release in a dose-dependent manner. It also suppressed the expression of endothelial and inducible nitric oxide synthase (eNOS/iNOS) and inhibited nuclear factor kappa B (NF-κB) activities. Experiments with NO donor l-arginine reversed α-MSH-induced angiogenesis inhibition. Melanocortin-1 receptor (MC1-R) and melanocortin-2 receptor (MC2-R) were involved in this process, with protein kinase A (PKA) acting as a downstream effector of MC-Rs signaling. Overall, α-MSH exerts its anti-angiogenic effects by modulating NO bioavailability and eNOS/iNOS expression in endothelial cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6321109/.
Ottani A, Neri L, Canalini F, Calevro A, Rossi R, Cappelli G, Ballestri M, Giuliani D, Guarini S. Protective effects of the melanocortin analog NDP-α-MSH in rats undergoing cardiac arrest. Eur J Pharmacol. 2014 Dec 15;745:108-16. doi: 10.1016/j.ejphar.2014.10.022. Epub 2014 Oct 22. PMID: 25446929.
Protective effects of the melanocortin analog NDP-α-MSH in rats undergoing cardiac arrest
In a study using rats subjected to cardiac arrest (CA) followed by cardiopulmonary resuscitation (CPR) and epinephrine treatment, the melanocortin analog [Nle(4), D-Phe(7)]α-melanocyte-stimulating hormone (NDP-α-MSH) was investigated. Rats treated with epinephrine alone showed adverse responses such as low mean arterial pressure (MAP) and heart rate (HR), altered hemogasanalysis parameters, oxidative stress, and inflammation. However, rats treated with epinephrine and NDP-α-MSH during CPR exhibited significant improvements in MAP, HR, metabolic acidosis, and survival rate. This treatment also reduced oxidative stress and inflammation while activating the JAK/STAT signaling pathway, indicating that melanocortins can enhance recovery from CA/CPR-induced complications.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299914007298?via%3Dihub.
Minder EI, Barman-Aksoezen J, Schneider-Yin X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin Pharmacokinet. 2017 Aug;56(8):815-823. doi: 10.1007/s40262-016-0501-5. PMID: 28063031.
Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders
Afamelanotide, a synthetic analogue of α-melanocyte-stimulating hormone (α-MSH), has been extensively studied due to its higher activity and stability compared to the natural hormone. It binds to melanocortin-1 receptor (MC1R), promoting melanin synthesis, antioxidant activity, DNA repair, and anti-inflammatory effects. Clinical trials in human volunteers demonstrated that subcutaneous injection led to long-lasting skin pigmentation and provided insights into its pharmacokinetics. Afamelanotide proved effective in various skin conditions, including polymorphic light eruption, erythropoietic protoporphyria, solar urticaria, Hailey-Hailey disease, and vitiligo. It was approved in 2014 by the European Medicines Agency for preventing phototoxicity in erythropoietic protoporphyria patients, with a favorable safety profile and no reported late effects even after long-term use.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s40262-016-0501-5.
Chen W, Li J, Qu H, Song Z, Yang Z, Huo J, Jiang H, Huang Q, Huo M, Liu B, Zhang Q. The melanocortin 1 receptor (MC1R) inhibits the inflammatory response in Raw 264.7 cells and atopic dermatitis (AD) mouse model. Mol Biol Rep. 2013 Feb;40(2):1987-96. doi: 10.1007/s11033-012-2256-x. Epub 2012 Oct 23. PMID: 23090482.
The melanocortin 1 receptor (MC1R) inhibits the inflammatory response in Raw 264.7 cells and atopic dermatitis (AD) mouse model
The alpha melanocyte stimulating hormone receptor (MC1R), a G-protein coupled receptor in the melanocortin subfamily, is known for regulating fur color in mammals through α-MSH and ACTH agonists. Surprisingly, MC1R was found to be highly expressed in Raw 264.7 cells, key inflammatory cells involved in initiating inflammation. Cyclic AMP, a key molecule in MC1R signaling, was identified not only as part of the MC1R pathway but also as a dampener of innate immune-mediated responses. This led to further studies exploring MC1R’s potential immunoregulatory role. The research investigated MC1R’s immunosuppressive effects on inflammation in LPS-stimulated Raw 264.7 cells and a TNCB-induced atopic dermatitis (AD) model. Results showed that the MC1R antagonist psoralen increased MC1R mRNA levels in Raw 264.7 cells through cumulative feedback regulation, with high psoralen concentrations. Psoralen inhibited LPS-induced TNF-α and IL-6 and increased cyclic AMP protein expression in vitro. In vivo, psoralen promoted histopathologic changes in TNCB-induced AD mice skin tissue. These findings suggest that MC1R can reduce inflammation both in vitro and in vivo and may offer a therapeutic pathway for inflammatory diseases.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s11033-012-2256-x.
Delaney A, Keighren M, Fleetwood-Walker SM, Jackson IJ. Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin. PLoS One. 2010 Sep 13;5(9):e12498. doi: 10.1371/journal.pone.0012498. PMID: 20856883; PMCID: PMC2938350.
Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin
This study investigates the role of the MC1R gene, primarily known for influencing hair color, in pain perception and responses to various pain stimuli. Researchers conducted experiments with mutant mice lacking MC1R or overexpressing an MC1R antagonist and found that female mice without MC1R displayed increased tolerance to noxious heat, reduced responses to inflammatory pain, and decreased aversion to capsaicin, while male mutant mice showed no significant differences. These findings highlight a gender-specific role for MC1R in acute noxious thermal responses and inflammatory pain.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2938350/.
Delaney A, Keighren M, Fleetwood-Walker SM, Jackson IJ. Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin. PLoS One. 2010;5(9):e12498. Published 2010 Sep 13. doi:10.1371/journal.pone.0012498.
Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin
This study explores the genetic influence on pain perception, focusing on the MC1R gene known for its role in hair color. Using mutant mice lacking MC1R or overexpressing an antagonist, researchers assessed their responses to various pain stimuli, including inflammatory and neuropathic pain, as well as aversion to capsaicin. Female mice without MC1R displayed higher tolerance to noxious heat, reduced inflammatory pain responses, and decreased aversion to capsaicin. Male mutant mice showed no significant differences. These findings suggest a gender-specific role for MC1R in acute pain responses and inflammatory pain.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2938350/.
Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J. Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases. Front Pharmacol. 2019 Jan 14;9:1535. doi: 10.3389/fphar.2018.01535. PMID: 30692924; PMCID: PMC6339910.
Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases
In preclinical studies, the melanocortin receptor agonists PL-8177 and PL-8331 were found to exhibit actions akin to α-melanocyte stimulating hormone (α-MSH) in preventing and reversing intestinal and ocular inflammation. These agonists demonstrated effectiveness in reducing inflammation parameters in models of bowel inflammation and experimental autoimmune uveitis similar to α-MSH, and they also showed promise in alleviating dry eye disease. Additionally, PL-8177 and PL-8331 exhibited inhibitory effects on TNF-α similar to adrenocorticotropic hormone (ACTH) and α-MSH in vitro. These findings suggest the potential therapeutic utility of these agonists in addressing inflammatory conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6339910/.
Maaser C, Kannengiesser K, Specht C, Lügering A, Brzoska T, Luger TA, Domschke W, Kucharzik T. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006 Oct;55(10):1415-22. doi: 10.1136/gut.2005.083634. Epub 2006 Mar 16. PMID: 16543288; PMCID: PMC1856418.
Crucial role of the melanocortin receptor MC1R in experimental colitis
The study investigates the impact of a disrupted alpha-Melanocyte stimulating hormone (alpha MSH) and melanocortin1-receptor (MC1R) pathway on colitis. Mice with a mutated MC1R gene experienced severe exacerbation of colitis symptoms, while normal mice showed milder effects. This suggests that MC1R plays a critical role in regulating intestinal inflammation, particularly in non-haematopoietic cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856418/.
Skottner A, Post C, Ocklind A, Seifert E, Liutkevicius E, Meskys R, Pilinkiene A, Biziuleviciene G, Lundstedt T. Anti-inflammatory potential of melanocortin receptor-directed drugs. Ann N Y Acad Sci. 2003 Jun;994:84-9. doi: 10.1111/j.1749-6632.2003.tb03165.x. PMID: 12851301.
Anti-inflammatory potential of melanocortin receptor-directed drugs
The study focuses on developing drugs targeting the melanocortin-1 receptor (MC1R) for treating inflammation. These compounds demonstrate submicromolar binding affinity to MC1R and have varying effects from full agonists to partial agonists and antagonists in vitro. In vivo, these compounds exhibit significant anti-inflammatory effects comparable to dexamethasone. This research suggests that MC1R-based anti-inflammatory drugs may offer advantages over glucocorticosteroids, which are effective but often associated with numerous side effects.
You can read the abstract of the article at https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.2003.tb03165.x?sid=nlm%3Apubmed.
Edling AE, Gomes D, Weeden T, Dzuris J, Stefano J, Pan C, Williams J, Kaplan J, Perricone MA. Immunosuppressive activity of a novel peptide analog of α-melanocyte stimulating hormone (α-MSH) in experimental autoimmune uveitis. J Neuroimmunol. 2011 Jul;236(1-2):1-9. doi: 10.1016/j.jneuroim.2011.04.015. PMID: 21640392.
Immunosuppressive activity of a novel peptide analog of α-melanocyte stimulating hormone (α-MSH) in experimental autoimmune uveitis
Autoimmune uveitis, an eye inflammation disorder with potential vision loss, currently relies on steroids and immunosuppressive drugs with associated toxicities. Seeking safer alternatives, a d-amino acid peptide analog of alpha-melanocyte stimulating hormone (α-MSH), called dRI-α-MSH, was developed, demonstrating improved selectivity for the melanocortin-1 receptor. Systemic or local delivery of dRI-α-MSH effectively suppressed disease progression and preserved retinal structure in experimental autoimmune uveitis (EAU), comparable to the corticosteroid dexamethasone. This suggests the potential therapeutic utility of dRI-α-MSH analogs in uveitis and other autoimmune conditions.
You can read the abstract of the article at https://www.jni-journal.com/article/S0165-5728(11)00136-6/fulltext.
Rinne P, Penttinen AM, Nordlund W, Ahotupa M, Savontaus E. α-MSH analogue attenuates blood pressure elevation in DOCA-salt hypertensive mice. PLoS One. 2013 Aug 16;8(8):e72857. doi: 10.1371/journal.pone.0072857. PMID: 23977363; PMCID: PMC3745458.
α-MSH analogue attenuates blood pressure elevation in DOCA-salt hypertensive mice
Melanocyte-stimulating hormones, including α-MSH, play key roles in regulating physiological functions like energy balance and inflammation. A stable α-MSH analogue called NDP-α-MSH was investigated for its diuretic and natriuretic properties and its potential therapeutic benefits in deoxycorticosterone acetate (DOCA)-salt-induced hypertension in mice. NDP-α-MSH exhibited significant diuretic and natriuretic effects, and when administered chronically, it mitigated blood pressure elevation in hypertensive mice induced by DOCA-salt, without affecting normotensive mice. These findings suggest that α-MSH analogues hold promise for future hypertension treatments.
Read the full article https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0072857
Giuliani D, Galantucci M, Neri L, Canalini F, Calevro A, Bitto A, Ottani A, Vandini E, Sena P, Sandrini M, Squadrito F, Zaffe D, Guarini S. Melanocortins protect against brain damage and counteract cognitive decline in a transgenic mouse model of moderate Alzheimer׳s disease. Eur J Pharmacol. 2014 Oct 5;740:144-50. doi: 10.1016/j.ejphar.2014.06.063. Epub 2014 Jul 15. PMID: 25034807.
Melanocortins protect against brain damage and counteract cognitive decline in a transgenic mouse model of moderate Alzheimer׳s disease
In a study involving 24-week-old APPSwe transgenic mice with moderately severe Alzheimer’s disease (AD), it was found that melanocortins, particularly the melanocortin analog [Nle4,D-Phe7]α-melanocyte-stimulating hormone (NDP-α-MSH), provided neuroprotection and alleviated cognitive decline. Control AD mice exhibited impaired spatial learning and memory, increased β-amyloid deposits, neuronal loss, and reduced synaptic activity-related gene Zif268 in the hippocampus. However, treatment with NDP-α-MSH significantly reduced β-amyloid levels, prevented neuronal loss, increased Zif268 expression, and improved cognitive function in the AD mice. This therapeutic effect was mediated through melanocortin MC4 receptors. These findings suggest that MC4 receptor-stimulating melanocortins could have potential as disease-modifying treatments for AD, particularly in moderate cases.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0014299914005329?via%3Dihub.
Giuliani D, Neri L, Canalini F, Calevro A, Ottani A, Vandini E, Sena P, Zaffe D, Guarini S. NDP-α-MSH induces intense neurogenesis and cognitive recovery in Alzheimer transgenic mice through activation of melanocortin MC4 receptors. Mol Cell Neurosci. 2015 Jul;67:13-21. doi: 10.1016/j.mcn.2015.05.004. Epub 2015 May 21. PMID: 26003413.
NDP-α-MSH induces intense neurogenesis and cognitive recovery in Alzheimer transgenic mice through activation of melanocortin MC4 receptors
In this study involving APPSwe transgenic mice with Alzheimer’s disease (AD), melanocortins, specifically the melanocortin analog [Nle(4),D-Phe(7)]α-melanocyte-stimulating hormone (NDP-α-MSH), were investigated for their potential neurogenic effects. When administered to the mice daily from day 1 to 50, NDP-α-MSH improved brain histology and cognitive function without any signs of toxicity. Immunohistochemical analysis revealed an increased number of proliferating cells that co-localized with mature neurons and functionally integrated neurons in the hippocampus of NDP-α-MSH-treated mice. This neuroprotective and neurogenic effect was mediated by melanocortin MC4 receptors and suggests that MC4 receptor agonists could be a promising and safe approach to counteracting AD progression in humans.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1044743115000858?via%3Dihub.
Giuliani D, Bitto A, Galantucci M, Zaffe D, Ottani A, Irrera N, Neri L, Cavallini GM, Altavilla D, Botticelli AR, Squadrito F, Guarini S. Melanocortins protect against progression of Alzheimer’s disease in triple-transgenic mice by targeting multiple pathophysiological pathways. Neurobiol Aging. 2014 Mar;35(3):537-47. doi: 10.1016/j.neurobiolaging.2013.08.030. Epub 2013 Oct 1. PMID: 24094579.
Melanocortins protect against progression of Alzheimer’s disease in triple-transgenic mice by targeting multiple pathophysiological pathways
In this study using triple-transgenic (3xTg-AD) mice with Alzheimer’s disease (AD), the potential neuroprotective role of melanocortins was investigated. The melanocortin analog [Nle(4),D-Phe(7)]-α-melanocyte-stimulating hormone (NDP-α-MSH) was administered once daily to the mice from 12 to 30 weeks of age. This treatment significantly reduced various AD-related biomarkers, including those involved in amyloid/tau cascade, oxidative/nitrosative stress, inflammation, and apoptosis, in the cerebral cortex and hippocampus. It also decreased neuronal loss, increased the expression of the synaptic activity-dependent gene Zif268, and improved cognitive function. These neuroprotective effects were mediated through melanocortin MC4 receptors, suggesting that MC4 receptor-stimulating melanocortins could be a potential therapeutic approach to counteract AD progression by targeting key pathophysiological mechanisms.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0197458013003758?via%3Dihub.
Ramírez D, Saba J, Carniglia L, Durand D, Lasaga M, Caruso C. Melanocortin 4 receptor activates ERK-cFos pathway to increase brain-derived neurotrophic factor expression in rat astrocytes and hypothalamus. Mol Cell Endocrinol. 2015 Aug 15;411:28-37. doi: 10.1016/j.mce.2015.04.008. Epub 2015 Apr 16. PMID: 25892444.
Melanocortin 4 receptor activates ERK-cFos pathway to increase brain-derived neurotrophic factor expression in rat astrocytes and hypothalamus
Melanocortins, neuropeptides with known anti-inflammatory and anti-apoptotic properties in the brain, exert their effects through melanocortin receptors (MCRs). Among these receptors, MC4R is prominently found in astrocytes within the brain. Prior research demonstrated that MC4R activation by the α-melanocyte stimulating hormone (α-MSH) analog NDP-MSH led to increased expression of brain-derived neurotrophic factor (BDNF) in rat astrocytes via the cAMP-Protein kinase A-cAMP responsive element binding protein pathway. This study explored the involvement of the mitogen-activated protein kinases (MAPK) pathway in MC4R signaling. Treatment of cultured rat astrocytes with NDP-MSH resulted in elevated BDNF expression. Inhibition of extracellular signal-regulated kinase (ERK) and ribosomal p90 S6 kinase (RSK), both components of the ERK pathway, blocked the increase in BDNF induced by NDP-MSH. MC4R activation also upregulated cFos expression, a target of ERK and RSK, with ERK activation involving cAMP, phosphoinositide-3 kinase (PI3K), and the non-receptor tyrosine kinase, Src. Inhibiting PI3K and Src prevented NDP-MSH-induced BDNF expression. Additionally, intraperitoneal injection of α-MSH in male rats was found to induce BDNF expression, MC4R expression, and activate ERK and cFos in the hypothalamus. These findings highlight the involvement of the ERK-RSK-cFos pathway, dependent on PI3K and Src, in MC4R-induced BDNF expression in astrocytes and suggest that melanocortins induce BDNF expression and ERK-cFos activation in the rat hypothalamus.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S030372071500194X?via%3Dihub.
Mykicki N, Herrmann AM, Schwab N, Deenen R, Sparwasser T, Limmer A, Wachsmuth L, Klotz L, Köhrer K, Faber C, Wiendl H, Luger TA, Meuth SG, Loser K. Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci Transl Med. 2016 Oct 26;8(362):362ra146. doi: 10.1126/scitranslmed.aaf8732. PMID: 27797962.
Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease
In neuroinflammatory disorders like multiple sclerosis (MS), immune cell infiltrates lead to demyelination and neuronal damage. Regulatory T cells (Treg) normally control these harmful immune responses, but their function is impaired in MS. A recently approved drug, Nle4-d-Phe7-α-melanocyte-stimulating hormone (NDP-MSH), was found to enhance Treg function, reducing the progression of MS in mice by preventing immune cell infiltration into the central nervous system and maintaining the integrity of the blood-brain barrier. NDP-MSH also exhibited neuroprotective effects, preserving neuron function and preventing cell death in mice and human neurons in vitro. These effects were mediated through melanocortin-1 and orphan nuclear 4 receptors. NDP-MSH shows promise for treating neuroinflammatory diseases like relapsing-remitting MS and related conditions.
You can read the abstract of the article at https://www.science.org/doi/10.1126/scitranslmed.aaf8732?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Giuliani D, Ottani A, Minutoli L, Stefano VD, Galantucci M, Bitto A, Zaffe D, Altavilla D, Botticelli AR, Squadrito F, Guarini S. Functional recovery after delayed treatment of ischemic stroke with melanocortins is associated with overexpression of the activity-dependent gene Zif268. Brain Behav Immun. 2009 Aug;23(6):844-50. doi: 10.1016/j.bbi.2009.03.009. Epub 2009 Apr 5. PMID: 19345727.
Functional recovery after delayed treatment of ischemic stroke with melanocortins is associated with overexpression of the activity-dependent gene Zif268
Melanocortin peptides have demonstrated strong neuroprotective effects and promote functional recovery in experimental ischemic stroke. They are also known for their neurotrophic properties. In this study, the role of the immediate early gene Zif268 in learning and memory recovery following delayed treatment of ischemic stroke with the melanocortin analog [Nle(4), D-Phe(7)]alpha-MSH (NDP-alpha-MSH) was investigated. Gerbils subjected to global cerebral ischemia were treated with NDP-alpha-MSH, starting either 3 or 9 hours after stroke induction. The treatment significantly reduced hippocampal damage, improved learning and memory recovery, and led to Zif268 overexpression, with effects lasting for at least 50 days. These neuroprotective effects were mediated through MC(4) receptors and suggest that melanocortins may trigger repair mechanisms to enhance neuronal function and synaptic plasticity after ischemic stroke, involving the Zif268 gene.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0889159109001020?via%3Dihub.
An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, Jin YJ, Cha BS, Baik JH, Lee WT, Lim SK. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007 Feb 2;282(5):2862-70. doi: 10.1074/jbc.M603454200. Epub 2006 Nov 24. PMID: 17127674.
Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle
To investigate the peripheral effects of melanocortin on fuel homeostasis in skeletal muscle, researchers examined palmitate oxidation and AMP kinase activity in alpha-melanocyte-stimulating hormone (alpha-MSH)-treated muscle cells. The study found that alpha-MSH treatment increased carnitine palmitoyltransferase-1 and fatty acid oxidation (FAO) in a dose-dependent manner. NDP-MSH, a potent melanocortin agonist, also stimulated FAO in muscle cells. However, [Glu6]alpha-MSH-ND, which primarily activates MC4R and MC3R, stimulated FAO only at high concentrations. The study also explored the involvement of MC5R and the cAMP-protein kinase A-AMP-activated protein kinase pathway in alpha-MSH-induced FAO in skeletal muscle.
You can read the full article at https://www.jbc.org/article/S0021-9258(18)38371-6/fulltext.
Møller CL, Raun K, Jacobsen ML, Pedersen TÅ, Holst B, Conde-Frieboes KW, Wulff BS. Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Mol Cell Endocrinol. 2011 Jul 20;341(1-2):9-17. doi: 10.1016/j.mce.2011.03.010. Epub 2011 May 17. PMID: 21616121.
Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved
The melanocortin receptors (MCRs) are a group of G-protein coupled receptors, consisting of five subtypes (MC1R-MC5R). Among them, MC2R and MC5R have been suggested to play a direct role in regulating lipolysis in adipocytes. This study investigated the impact of ACTH, α-melanocyte stimulating hormone (α-MSH), and synthetic MSH analogues on lipolysis in murine 3T3-L1 adipocytes. It revealed that MC2R and MC5R act as mediators of lipolysis in these adipocytes and explored the involvement of various signaling pathways, including cAMP, ERK 1/2, PKB, AMPK, and JNK in MCR-mediated lipolysis. Interestingly, the study found that lipolysis induced by α-MSH, NDP-α-MSH, MT-II, SHU9119, and PG-901 is mediated through MC5R in a cAMP-independent manner. Additionally, it highlighted differences in MCR-mediated lipolysis between 3T3-L1 cells and primary adipocytes.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0303720711001699?via%3Dihub.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








