GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of LL-37
- Key Takeaways
- What is LL-37?
- How LL-37 Works?
- Chemical Structure of LL-37
- Research on LL-37
- LL-37 Side Effects
- LL 37 Sequence
- Cathelicidin LL 37
- LL 37 Peptide Dosage
- What is a Human Antimicrobial Peptide?
- Antimicrobial Peptide LL 37 and other Antimicrobial Peptides
- LL-37 Nasal Spray
- LL 37 Peptide Bodybuilding
- LL 37 Herpes
- LL-37 Peptide Dosage
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of LL-37
- Key Takeaways
- What is LL-37?
- How LL-37 Works?
- Chemical Structure of LL-37
- Research on LL-37
- LL-37 Side Effects
- LL 37 Sequence
- Cathelicidin LL 37
- LL 37 Peptide Dosage
- What is a Human Antimicrobial Peptide?
- Antimicrobial Peptide LL 37 and other Antimicrobial Peptides
- LL-37 Nasal Spray
- LL 37 Peptide Bodybuilding
- LL 37 Herpes
- LL-37 Peptide Dosage
- FAQ
- Reference
Overall Health Benefits of LL-37
LL-37 benefits include promoting wound healing, enhancing immune responses, and possessing antimicrobial properties that help fight off infections. Additionally, LL-37 can reduce inflammation and aid in tissue regeneration.
- Significantly boosts immune function [1-19]
- Fights inflammation [20-29]
- Prevents cancer progression [30-59]
- Accelerates wound healing [60-67]
- Lowers the risk of heart disease [68-70]
- Prevents lung injury [71-72]
- Promotes bone repair [73-77]
Key Takeaways
- Antimicrobial Properties: LL-37 has strong antimicrobial effects, helping to combat bacterial, viral, and fungal infections.
- Immune System Enhancement: It boosts the immune system, aiding in the body’s defense against pathogens.
- Wound Healing: LL-37 promotes wound healing by stimulating tissue regeneration and reducing inflammation.
- Anti-Inflammatory Effects: It reduces inflammation, contributing to overall health and recovery in inflammatory conditions.
- Tissue Regeneration: LL-37 supports tissue regeneration, aiding in the repair and maintenance of healthy tissues.
What is LL-37?
LL-37, also known as Human Cathelicidin Antimicrobial Peptide (CAMP), is touted as a “mammal’s core tool” to fight off various harmful microorganisms in the body. It’s produced by many cell types including natural killer (NK) cells, white blood cells, and skin cells. In addition, different body systems such as the respiratory system, gastrointestinal tract, testes, and ocular surface also produce LL-37. This powerful peptidehas piqued the interest of the research community because its immune-modulating activities have the potential to accelerate tissue recovery and significantly improve the survival rate of patients with chronic debilitating medical conditions.
How LL-37 Works?
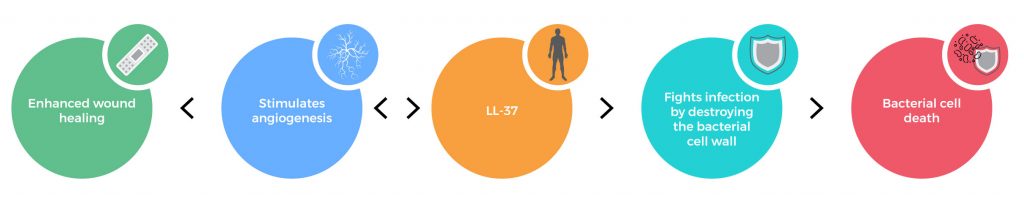
The human cathelicidin LL-37 serves a critical role in the innate immune system by defending against bacterial infections. LL-37 can interact with the molecules of the cell wall and perforate cytoplasmic membranes resulting in bacterial cell death. In addition, LL-37 helps promote wound closure by stimulating the formation of new blood vessels (angiogenesis).
Chemical Structure of LL-37
Research on LL-37
A. Significantly Boosts Immune Function
LL-37 significantly boosts immune function by directly attacking and breaking down bacterial cell walls, leading to rapid bacterial death. It enhances the body’s innate immune defenses by not only acting as an antimicrobial peptide but also by modulating immune responses. LL-37 recruits immune cells to infection sites, reduces inflammation, and promotes wound healing through angiogenesis, thus enhancing the body’s ability to respond to and recover from infections and tissue damage.
- LL-37 boosts immune function by limiting the damage caused by bacterial products and recruiting immune cells to the site of infection. [1]
- A cell study found that LL-37 has the ability to inhibit the formation of bacterial biofilms (densely packed communities of microbial cells). [2]
- A cell study also found that LL-37 may help enhance the immunomodulatory function of human placenta-derived MSCs (pMSCs). [3]
- A study found that LL-37 was deadly against Staphylococcus aureus, the most common cause of upper respiratory tract infections. [4]
- LL-37 demonstrated strong antimicrobial activity against eye pathogens (infectious microorganisms). [5]
- Studies showed that LL-37 destroyed harmful microorganisms by inducing programmed cell death (apoptosis). [6-8]
- A study found that LL-37 can kill a broad spectrum of bacteria. [9-10]
- LL-37 boosts immune response by attracting immune cells including T cells, monocytes, neutrophils, and mast cells to the site of infection. [11-12]
- LL-37 also affects the maturation of dendritic cells (antigen-presenting cells). [13]
- LL-37 stimulates the production of immune cells such as cytokines, chemokines, and their receptors. [14-15]
- Higher blood levels of LL-37 were associated with a lower risk of death from infection in dialysis patients. [16]
- In patients with psoriasis, LL-37 worked synergistically with human beta-defensin 2 (HBD-2) in killing Staphylococcus aureus (S. aureus) bacteria. [17]
- A study showed that LL-37 may be beneficial in treating patients with sepsis. [18]
- A study showed that LL-37 enhanced the effects of lysozyme against S. aureus. [19]
B. Fights Inflammation
LL-37 fights inflammation by modulating the immune response; it does this by binding to specific cell receptors and signaling pathways that regulate inflammation. This peptide reduces pro-inflammatory cytokine release, prevents excessive immune cell activation, and encourages the production of anti-inflammatory molecules. Through these actions, LL-37 helps maintain immune balance, reducing chronic inflammation and promoting healing in inflamed tissues.
- In patients who had surgical removal of the tonsils, those with high levels of LL-37 had lesser inflammation compared to those with low LL-37. [20]
- LL-37 suppresses the translocation of NF-kB to the nucleus, which creates an anti-inflammatory effect. [21]
- In mice, LL-37 administration reduced the risk of inflammatory disease. [22]
- LL-37 reduces inflammation by modulating inflammatory pathways in the body. [23-25]
- In human gum cells, LL-37 strongly reduced the levels of pro-inflammatory cytokines and chemokines. [26]
- LL-37 also modulates the inflammatory and host defense response of human white blood cells. [27]
- In mouse models, LL-37 provided protection against collagen damage which normally occurs in inflammatory arthritis. [28]
- A study showed that LL-37 was effective in regulating inflammation induced by interleukin-32. [29]
C. Prevents Cancer Progression
LL-37 prevents cancer progression by modulating the immune response and affecting tumor cell behavior. It can inhibit tumor cell proliferation, migration, and invasion by interfering with signaling pathways essential for cancer growth. Additionally, LL-37 promotes an anti-tumor immune environment by activating immune cells that target cancer cells, helping to reduce metastasis and tumor expansion.
- A cell study found that LL-37 has the potential to suppress cancer growth. [30]
- In another cell study, LL-37 suppressed the growth of colon cancer by inducing programmed cell death. [31]
- A study found that LL-37 has an anti-cancer effect in colon cancer, gastric cancer, skin cancer, ovarian cancer, lung cancer, breast cancer, prostate cancer, hematologic malignancy, and oral cancer. [32]
- In various human cancer cell lines, LL-37 exhibited anti-cancer effects similar to chemotherapeutic drugs. [33]
- In human lung cancer cell lines, LL-37 suppressed cancer progression by reducing the production of pro-inflammatory cytokines. [34]
- Cell studies have shown that LL-37 can inhibit the migration and invasiveness of prostate cancer cells. [35-40]
- In colon cancer, gastric cancer, and skin cancer cell lines, treatment with LL-37 prevented cancer cell progression.[41-46]
- In colon cancer cell lines, LL-37 destroyed cancer cells by stimulating signaling pathways involved in programmed cell death. [47-51]
- In gastric cancer cell lines, LL-37 inhibited gastric cancer cell proliferation through activation of signaling pathways involved in cell cycle arrest. [52-55]
- In leukemic cell lines, recombinant LL-37 treatment killed malignant cells by activating programmed cell death. [56-57]
- In human oral cancer cell lines, treatment with LL-37 destroyed malignant cells by damaging their DNA. [58-59]
D. Accelerates Wound Healing
LL-37 accelerates wound healing by promoting angiogenesis, which is the formation of new blood vessels essential for tissue repair. It stimulates cells involved in wound closure, such as keratinocytes and fibroblasts, enhancing cell migration and proliferation at the wound site. Additionally, LL-37 has anti-inflammatory and antimicrobial properties that reduce infection risk, creating an optimal environment for healing to proceed efficiently.
- In patients with venous leg ulcers, LL-37 treatment was associated with a faster healing rate (almost six-fold higher) compared to placebo. [60]
- In mice, LL-37 treatment promoted wound healing through the production of new blood vessels. [61]
- A cell study found that LL-37 induces wound healing by stimulating the proliferation and migration of cells necessary for regeneration. [62]
- In mice, LL-37 treatment improved re-epithelialization and granulation tissue formation – both of these processes are necessary for wound healing. [63-64]
- Cell studies found that LL-37 improves wound healing through its antimicrobial activity. [65-66]
- In patients with venous leg ulcers, low-dose LL-37 treatment markedly decreased the mean ulcer area. [67]
E. Lowers the Risk of Heart Disease
LL-37 lowers the risk of heart disease primarily through its anti-inflammatory and antimicrobial effects, which help reduce chronic inflammation and infection-related risks that contribute to heart disease. Additionally, LL-37 promotes the repair and stability of blood vessels by encouraging angiogenesis (new blood vessel formation) and aiding in the prevention of plaque buildup in arteries, thus supporting healthier cardiovascular function.
- A study showed that LL-37 can protect against atherosclerosis (plaque build-up within the heart arteries). [68]
- A study also found that LL-37 inhibited cell death in the heart, suggesting that increasing its level might have therapeutic benefits against heart failure. [69]
- In mice, heart failure was associated with a decrease in LL-37 levels and this deficiency worsened the condition. [70]
F. Prevents Lung Injury
LL-37 helps prevent lung injury by modulating immune responses and reducing inflammation in the lung tissue. It inhibits pro-inflammatory cytokines while promoting anti-inflammatory pathways, limiting excessive immune reactions that can damage lung cells. Additionally, LL-37 enhances the clearance of pathogens, minimizing infections that often lead to lung injury. This protective action supports respiratory health by preventing tissue damage and promoting lung healing processes.
- In mice, LL-37 attenuated the progression of lung injury by controlling inflammation and preventing infection. [71]
- A study reported that LL-37 is an effective inflammatory regulator in various lung diseases. [72]
G. Promotes Bone Repair
LL-37 promotes bone repair by enhancing the recruitment and activity of osteoblasts, the cells responsible for bone formation. It stimulates the production of growth factors and cytokines that aid in bone regeneration, and it also supports angiogenesis, ensuring an adequate blood supply to the damaged bone area. These combined actions help accelerate the healing process and strengthen bone tissue.
- A mice study found that LL-37-treated white blood cells promoted bone formation. [73]
- Cell studies also found that LL-37 can help prevent bone disorders by inhibiting bone breakdown. [74-75]
- In rats with bone defects, LL-37 treatment markedly induced newly formed bones. [76]
- A cell study reported that LL-37 induced bone formation by recruiting stem cells into the site of injury. [77]
LL-37 Side Effects
LL-37 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on LL-37. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of LL-37. Despite this, it was listed as a side effect associated with LL-37 even though these associated side effects are very uncommon.
Side effects associated with LL-37 may include the following:
- Increased inflammation
- Induction of autoimmune disease
- Depression
- Damage to sperm surface membranes
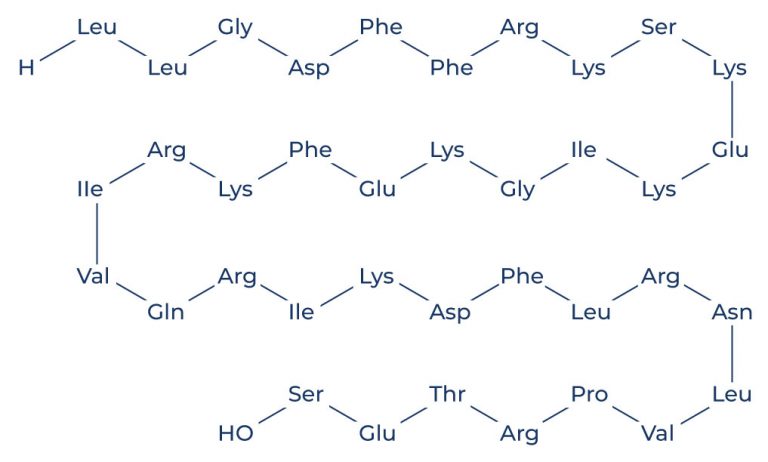
LL 37 Sequence
LL-37, a human antimicrobial peptide, is composed of 37 amino acids, hence the designation “LL-37.” The sequence of LL-37 is LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES, a structure that plays a crucial role in its biological functions. The amphipathic nature of LL-37, with both hydrophilic and hydrophobic regions, allows it to interact with and disrupt microbial membranes, thereby exerting its potent antimicrobial effects.
The sequence of LL-37 is not only essential for its antimicrobial properties but also for its role in modulating the immune system. Specific amino acid residues within the sequence are responsible for binding to and neutralizing lipopolysaccharides (LPS), which are components of the outer membrane of Gram-negative bacteria. By neutralizing LPS, LL-37 helps to prevent excessive inflammatory responses, thus protecting the host from potential damage due to overactive immune reactions.
Furthermore, the sequence of LL-37 is involved in signaling pathways that promote wound healing and tissue regeneration. For instance, certain segments of the peptide can interact with cell surface receptors, triggering pathways that lead to increased cell proliferation and migration. This makes LL-37 a valuable component not only in the innate immune response but also in the processes of healing and tissue repair.
Cathelicidin LL 37
Cathelicidin LL-37 is a crucial component of the innate immune system, known for its broad-spectrum antimicrobial properties. It is a peptide derived from the precursor protein hCAP18, which is produced by various cells, including white blood cells and epithelial cells. LL-37 is effective against a wide range of pathogens, including bacteria, viruses, and fungi, making it a vital first line of defense against infections. Its ability to disrupt microbial membranes and inhibit biofilm formation enhances its role in protecting the body from infectious agents.
Beyond its antimicrobial functions, LL-37 also plays a significant role in modulating the immune response. It acts as a signaling molecule, influencing the activity of immune cells such as macrophages and neutrophils. This peptide can attract immune cells to sites of infection or injury, promoting inflammation to combat pathogens. Additionally, LL-37 helps to regulate the balance between pro-inflammatory and anti-inflammatory responses, ensuring that the immune reaction is effective without causing excessive tissue damage.
LL-37’s contributions extend to wound healing and tissue regeneration. By promoting the migration and proliferation of skin cells, it accelerates the repair of damaged tissues. Its anti-inflammatory properties also help to minimize chronic inflammation, which can impede the healing process. Research has shown that LL-37 can stimulate angiogenesis, the formation of new blood vessels, further supporting tissue repair and regeneration. These multifaceted roles make LL-37 a peptide of great interest in the development of new therapeutic approaches for infections, inflammatory diseases, and wound healing.
LL 37 Peptide Dosage
Determining the appropriate dosage of LL-37 peptide is crucial for maximizing its benefits while minimizing potential side effects. The optimal dosage can vary depending on the intended use, whether it is for wound healing, antimicrobial purposes, or immune system enhancement. Typically, LL-37 is administered through injections, with common dosages ranging from 50 to 100 micrograms per injection. However, the exact dosage and frequency should be tailored to the individual’s needs and health conditions, often under the guidance of a healthcare professional.
The administration of LL-37 requires careful consideration of factors such as the severity of the condition being treated, the patient’s overall health, and any concurrent treatments. For instance, in the case of chronic infections or severe wounds, a higher dosage or more frequent administration might be necessary. Conversely, for general immune support or maintenance, a lower dosage might suffice. It’s important to start with a lower dosage and gradually adjust based on the body’s response and any observed side effects.
Monitoring and adjusting the dosage of LL-37 is an ongoing process. Regular consultations with a healthcare provider are essential to ensure the treatment’s efficacy and safety. Potential side effects, such as irritation at the injection site or systemic inflammatory responses, should be closely monitored. By maintaining a personalized and flexible approach to LL-37 peptide dosage, patients can harness its therapeutic benefits effectively while mitigating risks.
What is a Human Antimicrobial Peptide?
Human antimicrobial peptides (AMPs) are small, naturally occurring molecules found in the human body that play a critical role in the innate immune system. These peptides, which typically consist of 12 to 50 amino acids, possess the ability to destroy a wide range of pathogens, including bacteria, viruses, and fungi. AMPs are produced by various cells and tissues, particularly those that are in constant contact with the external environment, such as the skin, respiratory tract, and gastrointestinal tract. They serve as a first line of defense, rapidly neutralizing potential threats before they can cause infection or disease.
One of the key features of AMPs is their broad-spectrum antimicrobial activity. Unlike traditional antibiotics, which are often specific to certain types of bacteria, AMPs can target a diverse array of microorganisms. This is achieved through mechanisms such as disrupting the microbial cell membrane, thereby causing cell lysis and death. Additionally, AMPs can modulate the host’s immune response, enhancing the ability of immune cells to combat infections. This dual functionality not only helps in directly eliminating pathogens but also in orchestrating a more effective immune response.
Furthermore, AMPs like LL-37 have been found to possess additional biological functions beyond their antimicrobial properties. They can promote wound healing by stimulating cell proliferation and migration, and by reducing inflammation at the site of injury. This makes them not only crucial for fighting infections but also for maintaining overall tissue health and integrity. As research into AMPs continues, their potential therapeutic applications are becoming increasingly evident, offering promising alternatives to conventional antibiotics and treatments for various infectious and inflammatory diseases.
Antimicrobial Peptide LL 37 and other Antimicrobial Peptides
Antimicrobial peptides (AMPs) like LL-37 are a crucial component of the innate immune system, providing a rapid and effective defense against a broad spectrum of pathogens. LL-37, derived from the human cathelicidin antimicrobial peptide, has been extensively studied for its potent antimicrobial properties. It can neutralize bacteria, viruses, and fungi by disrupting their cell membranes, thus preventing infection and promoting healing. LL-37 also has immunomodulatory functions, enhancing the immune system’s response and aiding in the control of inflammation.
Other antimicrobial peptides, such as defensins and magainins, share similar protective roles in various organisms. Defensins, found in humans and many other species, contribute to the immune response by destroying pathogens and recruiting immune cells to infection sites. Magainins, originally discovered in frogs, have shown remarkable effectiveness against a range of microbial infections and are being explored for their potential in developing new therapeutic agents. Each AMP has unique structural features and modes of action, making them versatile tools in the fight against infectious diseases.
The therapeutic potential of AMPs extends beyond their natural roles in immunity. Researchers are investigating their use in treating antibiotic-resistant infections, wound healing, and inflammatory diseases. LL-37, for example, has shown promise in clinical settings for its ability to accelerate tissue repair and reduce inflammation. As antibiotic resistance becomes an increasing global threat, the exploration and development of AMPs offer a promising avenue for new treatments, harnessing the power of these naturally occurring peptides to enhance human health and combat infections.
LL-37 Nasal Spray
LL-37 nasal spray leverages the potent antimicrobial and anti-inflammatory properties of the LL-37 peptide to combat respiratory infections and inflammation. By delivering LL-37 directly to the nasal passages, the spray can target pathogens that cause upper respiratory infections such as colds, sinusitis, and influenza. The localized application helps to maximize the peptide’s effectiveness in eliminating harmful bacteria, viruses, and fungi, providing a powerful tool against common respiratory ailments.
In addition to its antimicrobial benefits, LL-37 nasal spray also plays a significant role in reducing inflammation in the nasal passages and sinuses. This can alleviate symptoms associated with chronic rhinosinusitis, allergic rhinitis, and other inflammatory conditions of the upper respiratory tract. By decreasing inflammation, the spray can improve breathing, reduce nasal congestion, and enhance overall nasal health, providing relief to individuals suffering from these chronic conditions.
Furthermore, LL-37 nasal spray promotes tissue repair and regeneration in the nasal passages. This is particularly beneficial for individuals with damaged or irritated nasal tissues due to frequent infections, allergies, or environmental irritants. The peptide’s ability to stimulate tissue regeneration helps to restore the integrity of the nasal mucosa, leading to improved function and resilience of the lipid bilayers nasal passages. Overall, LL-37 nasal spray offers a multifaceted approach to enhancing respiratory health through its antimicrobial, anti-inflammatory, and tissue-regenerative properties.
LL 37 Peptide Bodybuilding
LL-37 peptide has garnered attention in the bodybuilding community for its potential benefits beyond traditional muscle-building supplements. This naturally occurring peptide, known for its antimicrobial properties, also plays a significant role in enhancing the immune system, which is crucial for bodybuilders who undergo intense training and are at risk of infections. By fortifying the body’s defenses, LL-37 helps athletes maintain their health and continue their rigorous workout routines without interruptions caused by illness.
In addition to immune support, LL-37 contributes to improved recovery times, a critical factor in bodybuilding. Its anti-inflammatory properties aid in reducing muscle soreness and inflammation that often follow strenuous workouts. This allows bodybuilders to train more frequently and with greater intensity, leading to faster gains in muscle mass and strength. The peptide also promotes wound healing and tissue regeneration, which can be particularly beneficial for those recovering from injuries or dealing with minor muscle tears and strains. The role of lipid bilayers is crucial in understanding how LL-37 interacts with cell membranes and exerts its effects.
Furthermore, LL-37’s role in promoting overall health extends to enhancing skin quality and reducing the risk of skin infections, which can be a concern for athletes who sweat heavily and are prone to skin issues. By supporting lipid bilayers skin health, LL-37 ensures that bodybuilders can maintain a clear and healthy complexion, contributing to their overall aesthetic appeal. With its multifaceted benefits, LL-37 peptide emerges as a valuable supplement for bodybuilders aiming to optimize their health, recovery, and performance.
LL 37 Herpes
LL-37, a naturally occurring antimicrobial peptide in the body, shows promise in managing herpes infections. Research suggests that LL-37 can inhibit the replication of herpes simplex viruses (HSV), which cause oral and genital herpes. By disrupting viral entry into cells and interfering with viral replication processes within the outer membrane, LL-37 demonstrates potential as a therapeutic agent against HSV. This peptide’s ability to enhance immune responses further supports its role in controlling herpes outbreaks and reducing the severity of symptoms associated with skin infections the outer membrane.
Studies exploring LL-37’s effectiveness against herpes have highlighted its dual action of directly combating the virus and modulating immune responses in the outer membrane. This dual mechanism not only targets the virus itself but also strengthens the body’s ability to fight off infections involving the outer membrane. By boosting innate immunity and promoting faster healing of herpes lesions, LL-37 offers a multifaceted approach to managing this persistent viral infection, particularly in areas involving the outer membrane.
Despite these promising findings, further research is needed to fully understand the clinical implications of LL-37 in herpes treatment, especially concerning the outer membrane. Challenges such as delivery methods and optimizing therapeutic concentrations in affected tissues, including those in the hydrophobic residues outer membrane, remain to be addressed. Nonetheless, LL-37’s potential as a novel therapeutic option underscores ongoing efforts to harness natural peptides for combating viral infections like herpes effectively, with a particular focus on the outer membrane hydrophobic residues. By targeting the outer membrane, LL-37 could enhance antiviral strategies and improve patient outcomes.
LL-37 Peptide Dosage
The dosage of LL-37 peptide can vary depending on its intended use and the specific condition being treated. Generally, LL-37 is administered in controlled doses to achieve therapeutic effects without adverse reactions. For topical applications, such as wound healing or skin conditions, concentrations typically range from 0.1% to 2%, applied directly to the affected area. This ensures direct exposure to the peptide’s antimicrobial and healing properties.
In cases where LL-37 is used systemically, such as in immune modulation therapies or experimental treatments, dosages are carefully calibrated based on factors like body weight, medical history, and the severity of the condition. Researchers and healthcare providers determine these doses through clinical trials and studies to optimize effectiveness while minimizing potential side effects.
It’s crucial to note that LL-37’s therapeutic use in rheumatoid arthritis is still evolving, with ongoing research exploring its broader applications and optimal dosing strategies. Clinicians and researchers continue to refine dosage guidelines to maximize its benefits in rheumatoid arthritis across various medical contexts, ensuring safe and effective treatment outcomes.
FAQ
What does LL-37 peptide do?
LL-37 peptide, also known as cathelicidin LL-37, functions as an antimicrobial peptide that helps defend against infections by bacteria, viruses, and fungi. As a human host defense peptide, cathelicidin LL-37 also plays roles in wound healing, inflammation modulation, and immune system regulation. This human peptide host defense peptide, cathelicidin LL-37, is crucial in maintaining overall health. By acting as a human host defense peptide, cathelicidin LL-37 ensures a robust response to various pathogens. The understanding of cathelicidin LL-37’s role in disease pathogenesis underscores its significance in biomedical research and therapeutic development.
What are the most effective antimicrobial peptides?
Some of the most effective antimicrobial peptides include LL-37, human beta-defensins (HBDs), and various peptides derived from frogs and other animals known for their antimicrobial properties due to their strong antimicrobial activity. Cathelicidin LL-37 peptides exhibit significant antimicrobial activity, making them potent agents in combating infections. The study of cathelicidin LL-37 antimicrobial activity continues to reveal new insights into their potential therapeutic uses in bacterial surface interactions. These peptides, including cathelicidin LL-37, exhibit significant antimicrobial activity, making them potent agents in combating infections.
What is human cathelicidin?
Human cathelicidin refers to a family of antimicrobial peptides produced in various tissues, primarily by neutrophils, epithelial cells, and amyloid beta, the only human member. LL-37 is the most studied cathelicidin peptide in humans and is known for its strong antimicrobial activity. The antimicrobial activity of LL-37, crucial in the body’s defense mechanisms, includes amyloid beta, the only human member. Studies have shown that amyloid beta and the antimicrobial activity of LL-37 helps protect against a wide range of pathogens.
What does antimicrobial peptide do?
Antimicrobial peptides, like cathelicidin antimicrobial peptide LL-37, serve as natural defense molecules that can kill microbes directly or modulate immune responses to combat infections. They exhibit strong cathelicidin antimicrobial peptide antimicrobial activity, which makes them effective in fighting off various pathogens. They also contribute to cathelicidin antimicrobial peptide wound healing and tissue repair, further highlighting their essential role in the body’s cathelicidin antimicrobial peptide antimicrobial activity. Researchers are continuously exploring the potential of these cathelicidin antimicrobial peptide peptides due to their significant cathelicidin antimicrobial peptide antimicrobial activity and their ability to enhance immune defense mechanisms.
What is LL-37 in saliva?
LL-37 in saliva likely serves a protective role against oral pathogens, contributing to oral health by its antimicrobial actions and potentially influencing immune responses in the bacterial cell envelope in the oral cavity. The presence of LL-37 helps combat bacteria by targeting the bacterial cell envelope, thereby maintaining oral hygiene. Moreover, LL-37’s role in modulating innate and adaptive immunity immune responses further underscores its importance in protecting the bacterial cell envelope in the oral environment. This peptide also interacts with human cells in the oral mucosa, potentially influencing their innate and adaptive immunity immune responses.
What is LL-37 in psoriasis?
In psoriasis, LL-37 is involved in the pathogenesis of the disease. It can form complexes with self-DNA and trigger immune responses that exacerbate inflammation, contributing to the characteristic proteolytic degradation of skin lesions of psoriasis. LL-37’s role in psoriasis has been studied extensively in clinical infectious diseases, highlighting its significant impact on disease progression and immune dysregulation. LL-37 interacts with the cell wall of pathogens and modulates immune responses. The presence of LL-37 on the cell wall affects microbial virulence and survival. Understanding LL-37’s interaction with the cell wall provides insights into host-pathogen interactions. The cell wall composition influences LL-37’s antimicrobial activity. LL-37 binds to the bacterial cell wall and disrupts membrane integrity. The cell wall components affect LL-37’s efficacy against different pathogens. LL-37’s binding to the cell wall enhances bacterial clearance by immune cells. This interaction with the cell wall is crucial for LL-37’s immunomodulatory effects.
What is cathelicidin used for?
Cathelicidin peptides like LL-37 are used for their antimicrobial properties against gram negative bacteria, wound healing capabilities, including promoting epithelial cell migration, and modulation of immune responses against gram negative bacteria. They are being studied for potential therapeutic applications in infections caused by gram negative bacteria, promoting epithelial cell migration in response to gram negative bacteria, and inflammatory conditions. These peptides interact with the gram negative bacteria’s cell wall, aiding in their antimicrobial effects and promoting healing processes.
What does LL-37 do?
LL-37 peptide acts as a multifunctional peptide involved in antimicrobial defense, wound healing, immune modulation, and inflammation regulation, contributing to overall immune health and tissue repair. Its antimicrobial activity is attributed to its ability to induce membrane disruption in gram negative bacteria, pathogens, thereby aiding in immune defense. This membrane disruption capability also facilitates wound healing processes and contributes to the modulation of inflammation, reinforcing its role in maintaining tissue integrity and immune function.
What is the size of LL-37?
LL-37 is a relatively small peptide consisting of 37 amino acids, hence its name. It has a molecular weight of approximately 4.5 kDa. LL-37 is known for its antimicrobial properties, which enable it to interact with gram negative bacteria bacterial cells, disrupting their cytoplasmic membrane and leading to cell death. These interactions make LL-37 a promising candidate for therapeutic applications against various infections caused by gram negative bacteria bacterial cells.
What is antimicrobial peptide used for?
Antimicrobial peptides, including LL-37, are used to combat infections caused by gram negative bacteria, gram negative bacteria, viruses, and gram negative bacteria fungi. They are also studied for their potential in wound healing, inflammation modulation, and immune system support. LL-37, a member of the human cationic antimicrobial protein LL-37 family, is particularly noted for its antimicrobial properties and its role in promoting immune responses and tissue repair. LL-37, a member of the human cationic antimicrobial protein LL-37 family, is particularly noted for its antimicrobial properties and its role in promoting immune responses and tissue repair.
What is the molecular weight of LL-37 in kDa?
LL-37, a human cationic antimicrobial protein, a cationic antimicrobial peptide, has a molecular weight of approximately 4.5 kDa. This human cationic antimicrobial protein is crucial in innate immune responses, serving as a multifunctional molecule involved in antimicrobial defense, wound healing, and inflammation modulation. LL-37’s small size facilitates its rapid diffusion to target sites, enhancing its effectiveness in innate immune responses. LL-37 is also implicated in certain autoimmune diseases, playing a role in autoimmune diseases such as lupus and psoriasis. Its multifunctional nature extends to autoimmune diseases, where LL-37’s involvement influences disease progression and immune responses.
What is LL-37 used for?
LL-37 is used for its antimicrobial properties, wound healing abilities, and its role in modulating innate immune responses, making it potentially valuable in treating infections and inflammatory conditions. LL-37’s antimicrobial properties are particularly effective against invasive bacterial infections, making it a promising candidate for therapeutic applications in combating invasive bacterial infection.
What is the purpose of cathelicidin?
The purpose of cathelicidin peptides like LL-37 is to defend against microbial pathogens, promote tissue repair, and regulate immune responses to maintain overall health and protect against infections. Cathelicidin peptides like LL-37 act by disrupting the integrity of the inner bacterial membrane of pathogens, thereby neutralizing their threat and aiding in the restoration of affected tissues. This dual action supports immune function by assisting in the removal of pathogens and fostering tissue recovery, essential for overall health maintenance and defense against infections. LL-37 also plays a crucial role in modulating the inflammatory response, balancing immune reactions to prevent excessive tissue damage while effectively combating bacterial membranes microbial threats.
What drug is cathelicidin?
Cathelicidin is not a drug itself but refers to a family of antimicrobial peptides naturally produced in the body. LL-37, a member of this human cathelicidin family, is being studied for potential therapeutic applications. LL-37’s antimicrobial properties are particularly noteworthy due to its ability to target a wide range of pathogens by disrupting bacterial cell wall components, thereby triggering a human cathelicidin inflammatory response. This characteristic makes it a promising candidate for treating infections that are resistant to traditional antibiotics, promoting a human cathelicidin inflammatory response.
What is the effect of LL-37 on anti-infective immunity?
LL-37, also known as human cathelicidin, enhances anti-infective immunity by directly killing pathogens and by modulating immune responses, which can help in combating infections effectively. LL-37’s direct antimicrobial action, facilitated by human cathelicidin, disrupts bacterial cell wall components, thereby inhibiting bacterial growth and promoting pathogen clearance. Additionally, LL-37’s ability to modulate immune responses, involving human cathelicidin, enhances the body’s defense mechanisms against infections, further bolstering its efficacy in combating microbial threats. This dual action, mediated by human cathelicidin, involves enhancing the inflammatory response, ensuring a robust defense against pathogens.
What are antimicrobial peptides for UTI?
Antimicrobial peptides, including LL-37, have been studied for their potential use in treating urinary tract infections (UTIs) caused by bacteria. LL-37’s effectiveness lies in its ability to target and disrupt bacterial cell wall components, which are crucial for the survival and virulence of pathogens in the urinary tract. This peptide’s mechanism of action involves directly interacting with human cathelicidin bacterial cell wall components, leading to membrane destabilization and ultimately human cathelicidin bacterial cell death. Such targeted actions make LL-37 a promising candidate for combating UTIs, especially against antibiotic-resistant strains that pose significant challenges in clinical settings. The inflammatory response triggered by LL-37 further enhances its therapeutic potential by promoting immune cell activation and cytokine production, thereby aiding in the resolution of UTIs through multiple pathways.
What does peptide LL-37 do?
LL-37 peptide, also known as human cathelicidin LL-37, acts as an antimicrobial agent, promoting wound healing, modulating inflammation, and regulating immune responses, contributing to overall health and defense against infections. Human cathelicidin LL-37 is particularly crucial in innate immunity, directly combating pathogens and supporting tissue repair processes. Its role in innate immunity extends to modulating inflammation and enhancing immune responses, further bolstering its significance in maintaining health and combating infections.
Where is LL-37 found?
LL-37, also known as human cathelicidin LL-37, is found in various tissues and fluids of the body, including skin, saliva, respiratory tract, and the gastrointestinal tract, where cationic antimicrobial peptides it serves its antimicrobial and immunomodulatory functions. LL-37, cationic antimicrobial peptides LL-37 plays a crucial role in innate immunity by directly combating pathogens and modulating immune responses in these diverse bodily locations.
What are the benefits of LL-37?
The benefits of human cathelicidin LL-37 include its antimicrobial properties against a wide range of pathogens, its role in wound healing, modulation of inflammation, and support for immune system function. Human cathelicidin LL-37, a type of cationic antimicrobial peptides, contributes to innate immunity by directly combating microbes, facilitating tissue repair, and regulating inflammatory responses to enhance overall health and resistance to infections.
What is the function of LL-37?
The function of LL-37, cationic antimicrobial peptides human cathelicidin LL-37, includes antimicrobial defense against bacterial infections, wound healing promotion, inflammation regulation, and immune system modulation, contributing to overall health and disease resistance. Cationic antimicrobial peptides human cathelicidin LL-37 is known for its antimicrobial defense against bacterial infections, promoting wound healing, regulating inflammation, and modulating the immune system, thereby contributing significantly to overall health and disease resistance.
What is the significance of LL-37 on immunomodulation and disease outcome?
LL-37’s role in immunomodulation helps regulate immune responses, influencing disease outcomes in infections, inflammatory conditions, and potentially autoimmune disorders. Studies involving human gingival fibroblasts have shown that LL-37 can significantly affect the immune system’s behavior. The interaction of LL-37 with human gingival fibroblasts may also contribute to its ability to modulate inflammation and promote tissue repair. Thus, understanding LL-37’s effects on human gingival fibroblasts and its interaction with epidermal growth factor receptor is crucial for developing targeted therapies for various diseases.
Is cationic antimicrobial peptide LL-37 effective against both extracellular and intracellular Staphylococcus aureus?
Yes, LL-37 has demonstrated effectiveness against both extracellular and intracellular forms of Staphylococcus aureus, highlighting its broad antimicrobial capabilities. LL-37 also interacts with dendritic cells, enhancing their ability to initiate immune responses. Additionally, the presence of epidermal growth factor receptor LL-37 in various tissues, including its impact on dendritic cells, underscores its multifunctional role in the immune system. This interaction with dendritic cells further illustrates LL-37’s significance in modulating immune defenses and combating infections.
How is cathelicidin produced?
Cathelicidin peptides like LL-37 are produced in various tissues, including human dendritic cells, by proteolytic cleavage of larger precursor proteins, typically in response to infection or inflammation. Human dendritic cells play a key role in initiating immune responses, and the production of LL-37 by these cells enhances their antimicrobial capabilities. Additionally, human dendritic cells contribute to the modulation of immune responses through the activity of cathelicidin peptides like LL-37.
What is a high range for herpes?
A high range for herpes typically refers to a significant increase in viral load or antibody titers, indicating active infection or recent exposure. Dendritic cells play a crucial role in recognizing and responding to herpes virus infections, particularly in how they interact with the bacterial membrane. These dendritic cells are essential in initiating immune responses and managing the viral load. Understanding how dendritic cells interact with the herpes virus and the bacterial membrane can help in developing targeted therapies for active infections or recent exposures. By studying the interaction between dendritic cells, the herpes virus, and the bacterial membrane, researchers can gain valuable insights into more effective treatments.
What is a positive herpes score?
A positive herpes score generally indicates the presence of antibodies against herpes simplex virus (HSV), suggesting either current infection or past exposure. In cases involving human bronchial epithelial cells, detecting these antibodies can be crucial for understanding the spread of HSV in respiratory tissues, especially in individuals with systemic lupus erythematosus. This is particularly important since human bronchial epithelial cells can also be a site of viral infection. Therefore, a positive herpes score in the context of human bronchial epithelial cells highlights the need for further investigation and potential treatment, especially in the presence of systemic lupus erythematosus. Additionally, systemic lupus erythematosus patients may require more careful monitoring due to their potentially compromised immune systems.
What is herpes virus type 7 (HH7)?
Herpesvirus type 7 (HHV-7) is a virus belonging to the Herpesviridae family, closely related to HHV-6. The amino acid sequence of HHV-7 can cause roseola, a common childhood illness, and has been associated with other diseases involving reactive oxygen species. The involvement of reactive oxygen species in the pathogenesis of HHV-7-related conditions highlights the importance of oxidative stress in viral infections. Understanding the role of reactive oxygen species and the amino acid sequence can provide insights into potential therapeutic approaches for managing HHV-7 and its associated diseases. Further research into the amino acid sequence of HHV-7 may reveal more about its mechanisms and potential treatments.
What is L-lysine and herpes?
Human antimicrobial peptide L-lysine is an amino acid supplement often used to manage herpes simplex virus infections. It is believed to reduce viral replication and frequency of outbreaks, although scientific evidence is mixed. By understanding how human antimicrobial peptide works in conjunction with L-lysine and amino acid sequence, researchers hope to uncover new therapeutic approaches. Human antimicrobial peptide is an area of active research due to its potential in reducing viral loads. Using human antimicrobial peptide with L-lysine and amino acid sequence might enhance the efficacy against herpes simplex virus.
Reference
Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169(7):3883-91.
The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses
The study by Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE, titled “The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses,” published in the Journal of Immunology in 2002, discusses the diverse roles of LL-37, a human antimicrobial peptide, in modulating innate immune responses. LL-37, part of the cathelicidin family, is known for its direct antimicrobial activity against a broad spectrum of pathogens, including bacteria, viruses, and fungi. This study extends the understanding of LL-37 by demonstrating its multifunctional role in the innate immune system beyond its antimicrobial activity.
The research highlights how LL-37 influences various aspects of the immune response, including chemotaxis (the attraction of immune cells to infection sites), modulation of inflammatory responses, and promotion of wound healing. LL-37 acts as a bridge between innate and adaptive immunity, enhancing the immune system’s ability to fight infections while also regulating inflammatory processes to prevent excessive damage to host tissues.
Full study on https://journals.aai.org/jimmunol/article/169/7/3883/35412
Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76(9):4176-82.
Human host defense peptide LL-37 prevents bacterial biofilm formation
The study by Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE, titled “Human host defense peptide LL-37 prevents bacterial biofilm formation,” published in Infection and Immunity in 2008, investigates the role of the human antimicrobial peptide LL-37 in preventing the formation of bacterial biofilms. Biofilms are complex communities of bacteria that adhere to surfaces and are encased in a protective matrix, making them significantly more resistant to antibiotics and immune system actions than free-floating bacterial cells.
The study demonstrates that LL-37, known for its broad-spectrum antimicrobial activity and modulation of immune responses, also possesses the ability to inhibit the formation of bacterial biofilms. This is a critical finding, as biofilms are implicated in a wide range of chronic infections and are notoriously difficult to eradicate with conventional antibiotic treatments.
Full study on https://journals.asm.org/doi/abs/10.1128/iai.00318-08
Oliveira-Bravo M, Sangiorgi BB, Schiavinato JL, et al. LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells. Stem Cell Res Ther. 2016;7(1):189. Published 2016 Dec 30. doi:10.1186/s13287-016-0448-3.
LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells
The study by Oliveira-Bravo M, Sangiorgi BB, Schiavinato JL, et al., titled “LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells,” published in Stem Cell Research & Therapy in December 2016, explores the interaction between the human antimicrobial peptide LL-37 and placenta-derived mesenchymal stromal cells (MSCs), focusing on the immunomodulatory effects of this interaction. MSCs are known for their potential therapeutic uses, including their ability to modulate immune responses, making them promising candidates for treating various inflammatory and autoimmune diseases.
This study demonstrates that LL-37 enhances the immunosuppressive capabilities of placenta-derived MSCs. Specifically, LL-37 treatment led to an increase in the MSCs’ ability to suppress T-cell proliferation, which is a crucial aspect of the immune response. This effect suggests that LL-37 could play a significant role in modulating the immune system by enhancing the immunosuppressive functions of MSCs, potentially leading to more effective treatments for conditions characterized by excessive or inappropriate immune responses.
Full study on https://stemcellres.biomedcentral.com/articles/10.1186/s13287-016-0448-3
Aboualaiwa MH, Reznikov LR, Gansemer ND, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. ProcNatlAcadSci USA. 2014;111(52):18703-8.
pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37
The study by Aboualaiwa MH, Reznikov LR, Gansemer ND, et al., titled “pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37,” published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) in 2014, investigates how pH levels influence the antimicrobial effectiveness of β-defensin-3 and LL-37 in the airway surface liquid (ASL), which is crucial for lung health and defense against respiratory pathogens.
The ASL is a thin layer of fluid covering the airways, playing a vital role in the lung’s defense mechanism by trapping and clearing inhaled pathogens and particles. The study highlights that both β-defensin-3 and LL-37, which are key components of the innate immune system present in the ASL, exhibit their antimicrobial activities against common respiratory pathogens more effectively at certain pH levels. Importantly, it was found that their activity is enhanced in a more acidic environment, which is contrary to the neutral to slightly alkaline pH typically observed in healthy airway surface liquid.
Full study on https://www.pnas.org/doi/abs/10.1073/pnas.1422091112
Huang LC, Petkova TD, Reins RY, Proske RJ, Mcdermott AM. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci. 2006;47(6):2369-80.
Multifunctional roles of human cathelicidin (LL-37) at the ocular surface
The study by Huang LC, Petkova TD, Reins RY, Proske RJ, McDermott AM, titled “Multifunctional roles of human cathelicidin (LL-37) at the ocular surface,” published in Investigative Ophthalmology & Visual Science in 2006, explores the diverse functions of the antimicrobial peptide LL-37 at the ocular surface. LL-37, a member of the cathelicidin family, is known for its broad-spectrum antimicrobial activity and its role in modulating immune responses. This study extends our understanding of LL-37 by highlighting its multifaceted roles in eye health and disease.
The research demonstrates that LL-37 is not only involved in providing antimicrobial protection against various pathogens at the ocular surface but also plays a significant role in wound healing, inflammation modulation, and potentially in protecting against ocular surface diseases. The study found that LL-37 can stimulate epithelial cell migration, which is crucial for wound healing, and can modulate the inflammatory response, reducing the risk of excessive inflammation that can lead to tissue damage.
Full study on https://iovs.arvojournals.org/article.aspx?articleid=2126385
De yang, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069-74.
LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells
The study by De Yang, Chen Q, Schmidt AP, et al., titled “LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells,” published in the Journal of Experimental Medicine in 2000, focuses on the chemotactic properties of LL-37 and its interaction with the formyl peptide receptor-like 1 (FPRL1) on immune cells.
LL-37, a human cathelicidin antimicrobial peptide, is known for its broad-spectrum antimicrobial activity. This study expands its known functions by demonstrating LL-37’s role in immune regulation, specifically its ability to attract (chemoattract) various types of immune cells, including neutrophils, monocytes, and T cells, to sites of infection or inflammation. The study identifies FPRL1, a G protein-coupled receptor (GPCR), as the receptor through which LL-37 mediates this chemotactic activity.
Full study on https://rupress.org/jem/article-abstract/192/7/1069/8245
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27-55.
Mechanisms of antimicrobial peptide action and resistance
The review article by Yeaman MR, Yount NY, titled “Mechanisms of antimicrobial peptide action and resistance,” published in Pharmacological Reviews in 2003, provides a comprehensive overview of the actions of antimicrobial peptides (AMPs) and the mechanisms by which microbes develop resistance against them. AMPs are a diverse group of molecules that play a crucial role in the innate immune system across a wide range of organisms, providing a first line of defense against microbial pathogens.
The article discusses the various mechanisms through which AMPs exert their antimicrobial effects. These mechanisms include disrupting microbial membrane integrity, interfering with intracellular targets, and modulating the host immune response. The review highlights that AMPs target microbial cells through mechanisms that are distinct from those of conventional antibiotics, which often target specific biochemical pathways. This broad mode of action reduces the likelihood of resistance development but does not eliminate it.
Full study on https://pharmrev.aspetjournals.org/content/55/1/27.short
Cassagnes LE, Hervé V, Nepveu F, Hureau C, Faller P, Collin F. The catalytically active copper-amyloid-Beta state: coordination site responsible for reactive oxygen species production. AngewChemInt Ed Engl. 2013;52(42):11110-3.
The catalytically active copper‐amyloid‐beta state: coordination site responsible for reactive oxygen species production
The study by Cassagnes LE, Hervé V, Nepveu F, Hureau C, Faller P, Collin F, titled “The catalytically active copper-amyloid-Beta state: coordination site responsible for reactive oxygen species production,” published in Angewandte Chemie International Edition in 2013, focuses on the interaction between copper ions and amyloid-beta (Aβ) peptides, which are critically involved in Alzheimer’s disease (AD) pathology. The study aims to elucidate the molecular mechanisms underlying the generation of reactive oxygen species (ROS) in the presence of copper-bound Aβ peptides, a process that contributes to oxidative stress and neuronal damage in AD.
Amyloid-beta peptides can bind metal ions, including copper, leading to the formation of a complex that has been shown to catalyze the production of ROS. These highly reactive molecules can damage cellular components, contributing to the neurodegeneration observed in Alzheimer’s disease. Understanding the specific coordination site of copper in the Aβ peptide that is responsible for ROS production is crucial for elucidating the role of metal ions in AD pathology and for developing potential therapeutic strategies targeting metal-peptide interactions.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.201305372
Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. BiochimBiophysActa. 2006 Sep;1758:1408–1425.
LL-37, the only human member of the cathelicidin family of antimicrobial peptides
The review article by Dürr UHN, Sudheendra US, Ramamoorthy A, titled “LL-37, the only human member of the cathelicidin family of antimicrobial peptides,” published in Biochimica et Biophysica Acta (BBA) – Biomembranes in September 2006, provides an extensive overview of LL-37, the sole human cathelicidin antimicrobial peptide. Cathelicidins are a family of peptides known for their broad-spectrum antimicrobial activities and are found in various species. LL-37 plays a crucial role in the human innate immune system, providing a first line of defense against a wide range of pathogens, including bacteria, viruses, fungi, and parasites.
The article discusses the structure, mechanism of action, and the diverse biological functions of LL-37 beyond its antimicrobial activity. LL-37 is produced as a precursor protein, hCAP-18, which is cleaved to release the active LL-37 peptide. This peptide is unique in its ability to interact with microbial membranes, leading to membrane disruption and pathogen death. Moreover, LL-37 is involved in modulating the immune response, including inducing chemokine production, influencing cell proliferation and migration, and playing roles in wound healing and inflammation regulation.
Full study on https://www.sciencedirect.com/science/article/pii/S000527360600126X
Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013;4:143.
The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds
The article by Duplantier AJ, van Hoek ML, titled “The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds,” published in Frontiers in Immunology in 2013, discusses the therapeutic potential of LL-37, the only human cathelicidin antimicrobial peptide, in treating wounds infected with multiple types of pathogens. Polymicrobial infections, which involve more than one microbial species, are particularly challenging to treat due to the complex interactions between different pathogens and the host’s immune response. Such infections are common in chronic wounds, including diabetic ulcers, venous leg ulcers, and pressure ulcers, and they significantly hinder the healing process.
The review highlights LL-37’s broad-spectrum antimicrobial activity against bacteria, viruses, fungi, and parasites, making it a promising agent for treating polymicrobial wound infections. LL-37 not only directly kills pathogens but also modulates the immune response to promote healing. It can enhance the recruitment of immune cells to the site of infection, stimulate angiogenesis (the formation of new blood vessels), and promote re-epithelialization (the restoration of the epidermis).
Full study on https://www.frontiersin.org/articles/10.3389/fimmu.2013.00143/full
De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000 Oct 2;192:1069–1074
LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells
The study by De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al., titled “LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells,” was published in the Journal of Experimental Medicine on October 2, 2000. This research investigates the role of LL-37, a human cathelicidin antimicrobial peptide, in chemotaxis, specifically its ability to attract various immune cells from peripheral blood.
LL-37 is known for its antimicrobial properties, but this study delves into its additional function as a chemoattractant for immune cells. The research demonstrates that LL-37 has the capacity to chemoattract peripheral blood neutrophils, monocytes, and T cells, which are essential components of the immune system involved in defense against infections and immune responses.
Full study on https://rupress.org/jem/article-abstract/192/7/1069/8245
Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002 May;106:20–26.
A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis
The study by Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al., titled “A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis,” was published in Immunology in May 2002. This research investigates the effects of LL-37, a member of the cathelicidin family of human antibacterial peptides, on mast cells, which are immune cells involved in inflammatory and allergic responses.
The study reveals that LL-37 has the capacity to induce chemotaxis, the directed migration of cells, in mast cells. Chemotaxis is an essential process in the immune response, as it allows immune cells to move toward sites of infection or inflammation. LL-37’s ability to attract mast cells suggests its involvement in regulating immune responses, particularly those associated with inflammation and allergy.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2567.2002.01398.x
Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J ImmunolBaltimMd 1950. 2004 Jan 15;172:1146–1156.
The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization
The study by Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, et al., titled “The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization,” was published in the Journal of Immunology (Baltimore, Md. : 1950) on January 15, 2004. This research investigates the impact of LL-37, a cationic antimicrobial peptide, on the differentiation of dendritic cells and the subsequent polarization of T cells.
Dendritic cells are critical antigen-presenting cells that play a central role in initiating and shaping immune responses. This study demonstrates that LL-37 has a significant influence on dendritic cell differentiation, leading to the development of dendritic cells with altered characteristics. LL-37-treated dendritic cells exhibit changes in surface markers and cytokine production, suggesting a modification in their maturation and function.
Full study on https://journals.aai.org/jimmunol/article/172/2/1146/71732
Pistolic J, Cosseau C, Li Y, Yu JJ, Filewod NCJ, Gellatly S, et al. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun. 2009;1:254–267.
Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-κB signaling pathway
The study by Pistolic J, Cosseau C, Li Y, Yu JJ, Filewod NCJ, Gellatly S, et al., titled “Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway,” was published in the Journal of Innate Immunity in 2009. This research investigates the interaction between LL-37, a host defense peptide, and human bronchial epithelial cells, with a focus on its effect on the expression of interleukin-6 (IL-6) and the involvement of the NF-kappaB signaling pathway.
The study demonstrates that LL-37 has the capacity to induce the expression of IL-6 in human bronchial epithelial cells. IL-6 is a pro-inflammatory cytokine that plays a crucial role in the immune response, particularly in inflammation and infection. LL-37’s ability to stimulate IL-6 production suggests its role in initiating and amplifying the local immune response in the respiratory tract.
Full study on https://karger.com/jin/article-abstract/1/3/254/179822
Montreekachon P, Chotjumlong P, Bolscher JGM, Nazmi K, Reutrakul V, Krisanaprakornkit S. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011 Jun;46:327–337.
Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts
The study by Montreekachon P, Chotjumlong P, Bolscher JGM, Nazmi K, Reutrakul V, Krisanaprakornkit S, titled “Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts,” was published in the Journal of Periodontal Research in June 2011. This research investigates the mechanisms through which LL-37, a host defense peptide, up-regulates interleukin-8 (IL-8) in human gingival fibroblasts, focusing on the involvement of the P2X(7) purinergic receptor and the MEK1/2 signaling pathway.
The study reveals that LL-37 has the ability to up-regulate the expression of IL-8, a pro-inflammatory chemokine, in human gingival fibroblasts. IL-8 plays a crucial role in recruiting immune cells to sites of inflammation, and its up-regulation suggests LL-37’s involvement in the local immune response within gingival tissues.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0765.2011.01346.x
Gombart AF, Bhan I, Borregaard N, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48(4):418-24
Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis
The study by Gombart AF, Bhan I, Borregaard N, et al., titled “Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis,” was published in Clinical Infectious Diseases in 2009. This research investigates the relationship between the plasma levels of cathelicidin antimicrobial peptide (hCAP18) and infectious disease mortality in patients undergoing hemodialysis.
Hemodialysis is a medical procedure used to treat patients with kidney failure, and these patients are known to have an increased susceptibility to infections. Cathelicidin antimicrobial peptide (hCAP18) is a host defense peptide with antimicrobial properties, and it plays a crucial role in the innate immune system’s defense against infections.
The study reveals that patients undergoing hemodialysis with low plasma levels of hCAP18 are at a higher risk of infectious disease-related mortality. In other words, individuals with lower levels of this antimicrobial peptide are more vulnerable to severe infections and have a higher likelihood of succumbing to infectious diseases.
Full study on https://academic.oup.com/cid/article-abstract/48/4/418/283660
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002 Oct 10;347(15):1151-60. doi: 10.1056/NEJMoa021481. PMID: 12374875.
Endogenous antimicrobial peptides and skin infections in atopic dermatitis
The study by Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY, titled “Endogenous antimicrobial peptides and skin infections in atopic dermatitis,” was published in the New England Journal of Medicine on October 10, 2002. This research explores the role of endogenous antimicrobial peptides in the context of skin infections in individuals with atopic dermatitis, a chronic inflammatory skin condition.
Atopic dermatitis is known for its association with recurrent skin infections, and this study investigates the mechanisms underlying the susceptibility of individuals with atopic dermatitis to skin infections. One aspect of the study focuses on endogenous antimicrobial peptides, which are naturally occurring molecules that play a crucial role in the skin’s defense against infections.
Full study on https://www.nejm.org/doi/full/10.1056/NEJMoa021481
Ciornei CD, Sigurdardóttir T, Schmidtchen A, Bodelsson M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005 Jul;49(7):2845-50. doi: 10.1128/AAC.49.7.2845-2850.2005. PMID: 15980359; PMCID: PMC1168709.
Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37
The study by Ciornei CD, Sigurdardóttir T, Schmidtchen A, Bodelsson M, titled “Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37,” was published in Antimicrobial Agents and Chemotherapy in July 2005. This research explores various properties and activities of analogs of the human cathelicidin LL-37, including their antimicrobial activity, chemoattractant activity, lipopolysaccharide (LPS) neutralization, cytotoxicity, and susceptibility to inhibition by serum.
Cathelicidin LL-37 is an antimicrobial peptide known for its role in host defense against infections. The study investigates analogs of LL-37, which are similar molecules with potential therapeutic applications.
Full study on https://journals.asm.org/doi/abs/10.1128/AAC.49.7.2845-2850.2005
Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005 Nov;40(2):123-32. doi: 10.1016/j.jdermsci.2005.03.014. Epub 2005 Jun 15. PMID: 15963694.
Synergistic effect of antibacterial agents human β-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli
In a study published in the Journal of Dermatological Science in November 2005, researchers investigated the synergistic effects of natural antibacterial agents, including human beta-defensins, cathelicidin LL-37, and lysozyme, against common bacterial pathogens Staphylococcus aureus and Escherichia coli. Their findings revealed that the combination of these antibacterial agents had a synergistic effect, resulting in enhanced antibacterial activity against both pathogens compared to individual agents. This research sheds light on the intricate mechanisms employed by the innate immune system to combat bacterial infections and suggests potential applications for developing more effective antibacterial treatments by harnessing the synergy between these natural antimicrobial molecules.
Full study on https://www.sciencedirect.com/science/article/pii/S0923181105001374
Elenius V, Palomares O, Waris M, et al. The relationship of serum vitamins A, D, E and LL-37 levels with allergic status, tonsillar virus detection and immune response. PLoS ONE. 2017;12(2):e0172350.
The relationship of serum vitamins A, D, E and LL-37 levels with allergic status, tonsillar virus detection and immune response
In a study published in PLOS ONE in 2017, researchers investigated the interplay between serum levels of vitamins A, D, E, and the antimicrobial peptide LL-37 in relation to allergic status, tonsillar virus detection, and immune responses. This comprehensive analysis aimed to uncover associations between these serum components and various aspects of the immune system. The findings provided insights into the potential roles of these nutrients and LL-37 in influencing immune function, allergic conditions, and the presence of viral infections in the tonsils, contributing to our understanding of their relevance in immune responses and health outcomes.
Full study on https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0172350
Mookherjee, N. et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol 176, 2455–2464 (2006).
Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37
In a study published in the Journal of Immunology in 2006, Mookherjee and colleagues investigate the impact of the endogenous human host defense peptide LL-37 on the Toll-like receptor (TLR)-mediated inflammatory response, a critical component of the innate immune system’s defense against pathogens. LL-37, known for its antimicrobial properties, is revealed to have a regulatory role in modulating the inflammatory response triggered by TLR activation. Depending on the specific TLRs involved and the context of the immune response, LL-37 can either enhance or suppress the production of inflammatory cytokines. These findings illuminate LL-37’s multifaceted role in immune regulation, providing insights into its potential applications for modulating immune responses to microbial challenges and contributing to our understanding of innate immune system dynamics.
Full study on https://journals.aai.org/jimmunol/article/176/4/2455/73544
Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. Immunity. 2015 Aug 18;43(2):304-17.
Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota
In a study published in Immunity in 2015, Sun et al. investigate the pivotal role of pancreatic β-cells and an immunoregulatory antimicrobial peptide influenced by the gut microbiota in restraining autoimmune diabetes. Focusing on the autoimmune attack on pancreatic β-cells seen in conditions like type 1 diabetes, the research unveils the expression of an immunoregulatory antimicrobial peptide that appears to play a critical role in immune regulation. Notably, the study highlights the influence of the gut microbiota on the production of this peptide, suggesting a complex interplay between pancreatic β-cells, gut microorganisms, and immune modulation. These findings contribute to our understanding of the mechanisms underlying autoimmune diabetes and offer potential insights into therapeutic strategies for its management.
Full study on https://www.cell.com/immunity/pdf/S1074-7613(15)00302-7.pdf
Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191(10):4895-901.
Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease
In their 2013 review published in the Journal of Immunology, Kahlenberg and Kaplan examine the pivotal role of the antimicrobial peptide LL-37 in inflammation and autoimmune diseases. LL-37, produced by various cell types, emerges as a multifaceted player in immune regulation. The review explores LL-37’s dual nature, capable of both promoting and dampening inflammatory responses, depending on the context. Moreover, it delves into LL-37’s involvement in autoimmune diseases like systemic lupus erythematosus (SLE) and psoriasis, shedding light on its contributions to disease pathogenesis. This comprehensive overview underscores LL-37’s intricate interactions within the immune system and highlights its potential as a target for innovative therapeutic strategies in autoimmune and inflammatory disorders.
Full study on https://journals.aai.org/jimmunol/article/191/10/4895/86783
Yu X, Quan J, Long W, et al. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 2018;372(2):178-187.
LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway
In a study published in Experimental Cell Research in 2018, Yu et al. investigate the versatile role of the antimicrobial peptide LL-37. They find that LL-37 demonstrates dual functionality by inhibiting lipopolysaccharide (LPS)-induced inflammation and promoting the osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). The study reveals that LL-37 exerts its anti-inflammatory effects through the P2X7 receptor, a cell membrane receptor, and engages the MAPK signaling pathway, a crucial intracellular signaling pathway. This research sheds light on LL-37’s potential as a therapeutic agent in conditions involving inflammation and bone health, offering insights into its mechanisms of action and signaling pathways involved.
Full study on https://www.sciencedirect.com/science/article/pii/S001448271830572X
Tjabringa GS, Rabe KF, Hiemstra PS. The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung. PulmPharmacolTher. 2005;18(5):321-7.
The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung
In their 2005 review published in Pulmonary Pharmacology & Therapeutics, Tjabringa, Rabe, and Hiemstra provide a comprehensive exploration of the multifunctional role of the human cathelicidin LL-37 in infection and inflammation within the lung. Focusing on this versatile antimicrobial peptide, the review underscores LL-37’s significance in combating lung infections by targeting and neutralizing pathogens. Furthermore, it illuminates LL-37’s capacity for immunomodulation, shaping inflammatory responses in the lung. The article also delves into LL-37’s relevance in lung diseases such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis, where inflammation and infections are prominent features. By elucidating the therapeutic potential of LL-37 and related peptides in managing lung infections and inflammation, this review contributes to a deeper understanding of LL-37’s multifaceted roles in maintaining respiratory health.
Full study on https://www.sciencedirect.com/science/article/pii/S1094553905000155
Jönsson D, Nilsson BO. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J Periodont Res. 2012;47(3):330-5.
The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells
In their 2012 study published in the Journal of Periodontal Research, Jönsson and Nilsson investigate the impact of the antimicrobial peptide LL-37 on human periodontal ligament cells, a critical component of the structures supporting teeth. The research uncovers that LL-37 possesses dual functionality, demonstrating anti-inflammatory properties by attenuating inflammatory responses within periodontal ligament cells. Additionally, LL-37 exhibits proapoptotic effects, suggesting its potential involvement in programmed cell death, which can be significant for tissue homeostasis and repair. These findings contribute to a deeper understanding of LL-37’s role in modulating inflammation and apoptosis within periodontal tissues, offering insights into its implications for periodontal health.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0765.2011.01436.x
Alalwani SM, Sierigk J, Herr C, et al. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol. 2010;40(4):1118–1126. doi:10.1002/eji.200939275.
The antimicrobial peptide LL‐37 modulates the inflammatory and host defense response of human neutrophils
In their 2010 study published in the European Journal of Immunology, Alalwani et al. delve into the multifaceted effects of the antimicrobial peptide LL-37 on human neutrophils, pivotal immune cells crucial for defending against infections. The research uncovers that LL-37 plays a dynamic role in modulating inflammatory responses within neutrophils, capable of both enhancing and suppressing inflammatory signaling pathways, contingent on the specific context. Moreover, LL-37 emerges as a promoter of host defense mechanisms within neutrophils, enhancing their abilities in processes such as phagocytosis and microbial killing. These findings shed light on LL-37’s intricate involvement in the immune system, illuminating its potential as a therapeutic agent in infectious and inflammatory conditions.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/eji.200939275
Chow LN, Choi KY, Piyadasa H, Bossert M, Uzonna J, Klonisch T, Mookherjee N. Human cathelicidin LL-37-derived peptide IG-19 confers protection in a murine model of collagen-induced arthritis. Mol Immunol. 2014 Feb;57(2):86-92. doi: 10.1016/j.molimm.2013.08.011. Epub 2013 Oct 1. PMID: 24091294.
Human cathelicidin LL-37-derived peptide IG-19 confers protection in a murine model of collagen-induced arthritis
In their 2014 study published in Molecular Immunology, Chow et al. investigate the therapeutic potential of the human cathelicidin LL-37-derived peptide IG-19 in a murine model of collagen-induced arthritis, a representative model for rheumatoid arthritis. Focusing on IG-19, a peptide derived from the immune-modulating antimicrobial peptide LL-37, the research unveils that IG-19 exerts protective effects in this arthritis model by ameliorating disease severity. These findings highlight IG-19’s promising role as a therapeutic agent for rheumatoid arthritis and autoimmune disorders, shedding light on its immunomodulatory properties that contribute to its beneficial impact.
Full study on https://www.sciencedirect.com/science/article/pii/S0161589013004938
Choi KY, Napper S, Mookherjee N. Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation. Immunology. 2014 Sep;143(1):68-80. doi: 10.1111/imm.12291. PMID: 24666281; PMCID: PMC4137957.
Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation
In their 2014 study published in Immunology, Choi et al. investigate the regulatory effects of the human cathelicidin LL-37 and its derivative IG-19 on interleukin-32-induced inflammation. Interleukin-32 (IL-32) is known to play a role in promoting inflammatory responses. The research reveals that LL-37 and IG-19 exert anti-inflammatory actions by inhibiting IL-32-induced inflammation. These peptides effectively suppress IL-32-induced cytokine production and immune cell activation. This study provides valuable insights into the immunomodulatory properties of LL-37 and its derivative IG-19, suggesting their potential as therapeutic agents for conditions involving excessive inflammation.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1111/imm.12291
Hayashi M, Kuroda K, Ihara K, Iwaya T, Isogai E. Suppressive effect of an analog of the antimicrobial peptide of LL‑37 on colon cancer cells via exosome‑encapsulated miRNAs. Int J Mol Med. 2018;42(6):3009-3016.
Suppressive effect of an analog of the antimicrobial peptide of LL‑37 on colon cancer cells via exosome‑encapsulated miRNAs
In their 2018 study published in the International Journal of Molecular Medicine, Hayashi et al. investigate the potential anti-cancer properties of an analog of the antimicrobial peptide LL-37 in the context of colon cancer cells. This research reveals that the LL-37 analog exerts suppressive effects on colon cancer cell growth and proliferation. Furthermore, the study uncovers a novel mechanism involving exosomes, small vesicles released by cells, and their encapsulated miRNAs, which mediate the anticancer actions of the LL-37 analog. These findings offer insights into the potential therapeutic applications of LL-37 analogs in combatting colon cancer and shed light on the role of exosome-mediated mechanisms in regulating cancer cell behavior.
Full study on https://www.spandidos-publications.com/ijmm/42/6/3009
Ren SX, Cheng AS, To KF, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72(24):6512-23.
Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer
In their 2012 study published in Cancer Research, Ren et al. investigate the role of the host immune defense peptide LL-37 in combatting colon cancer. This research uncovers that LL-37 possesses notable anticancer properties by inducing apoptosis, a form of cell death, in colon cancer cells through a caspase-independent mechanism. These findings highlight LL-37’s potential as a promising agent for suppressing colon cancer growth and offer insights into novel pathways for apoptosis induction in cancer cells, providing valuable contributions to cancer therapeutics.
Full study on https://aacrjournals.org/cancerres/article-abstract/72/24/6512/576212
Chen X, Zou X, Qi G, et al. Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell PhysiolBiochem. 2018;47(3):1060-1073.
Roles and mechanisms of human cathelicidin LL-37 in cancer
In their 2018 study published in Cell Physiology and Biochemistry, Chen et al. conduct a comprehensive investigation into the diverse roles and underlying mechanisms of the human cathelicidin LL-37 in the context of cancer. The research explores LL-37’s multifaceted impact on cancer, including its effects on cancer cell growth, proliferation, apoptosis, and metastasis. Moreover, the study highlights LL-37’s immunomodulatory properties within the tumor microenvironment, shedding light on its influence on the interplay between cancer cells and immune cells. This research suggests that LL-37 may hold promise as a potential therapeutic target in cancer treatment and underscores its significance in the broader landscape of cancer biology and therapy.
Full study on https://karger.com/cpb/article/47/3/1060/75103
Kuroda K, Okumura K, Isogai H, Isogai E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front Oncol. 2015;5:144.
The human cathelicidin antimicrobial peptide LL-37 and mimics are potential anticancer drugs
In their 2015 study published in Frontiers in Oncology, Kuroda et al. investigate the potential of the human cathelicidin antimicrobial peptide LL-37 and LL-37 mimics as candidates for anticancer drugs. This research delves into the role of LL-37 in cancer therapy, highlighting its capacity to inhibit cancer cell growth and induce apoptosis. Additionally, the study explores LL-37 mimics, synthetic compounds designed to replicate LL-37’s properties, as promising agents for cancer treatment. The findings suggest that LL-37 and its mimics hold significant potential as novel anticancer drugs, offering a new avenue for cancer therapy development and emphasizing their relevance in oncology research.
Full study on https://www.frontiersin.org/articles/10.3389/fonc.2015.00144/full
Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, Rabe KF, Hiemstra PS: The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 2003; 171: 6690-6696.
The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor
In their 2003 study published in the Journal of Immunology, Tjabringa et al. investigate the role of the antimicrobial peptide LL-37 in activating innate immunity at the airway epithelial surface. The research reveals that LL-37 achieves this by transactivating the epidermal growth factor receptor (EGFR), a key molecular signaling pathway. This transactivation of EGFR leads to the initiation of innate immune responses in the airway epithelium, contributing to the host’s defense against infections and highlighting LL-37’s multifunctional properties in immune regulation and host defense.
Full study on https://journals.aai.org/jimmunol/article/171/12/6690/72072
Piktel E, Niemirowicz K, Wnorowska U, Wątek M, Wollny T, Głuszek K, Góźdź S, Levental I, Bucki R: The role of cathelicidin LL-37 in cancer development. Arch ImmunolTherExp (Warsz) 2016; 64: 33-46.
The role of cathelicidin LL-37 in cancer development
In their 2016 review published in the Archives of Immunology and Experimental Therapy, Piktel et al. explore the role of the cathelicidin LL-37 in cancer development. The review provides an overview of LL-37’s multifaceted functions, including its antimicrobial properties and involvement in immune responses. It also delves into LL-37’s potential impact on cancer development and progression. The authors discuss how LL-37 may influence various aspects of cancer biology, such as tumor cell proliferation, angiogenesis, and metastasis, and its potential as a target for cancer therapy. This comprehensive review sheds light on the complex relationship between LL-37 and cancer and highlights the need for further research to elucidate its precise role in cancer development.
Full study on https://link.springer.com/article/10.1007/s00005-015-0359-5
Choi KY, Napper S, Mookherjee N: Human cathelicidin LL-37 and its derivative IG-19 regulate interleukin-32-induced inflammation. Immunology 2014; 143: 68-80.
Human cathelicidin LL‐37 and its derivative IG‐19 regulate interleukin‐32‐induced inflammation
In their 2014 study published in Immunology, Choi et al. investigate the regulatory role of the human cathelicidin LL-37 and its derivative IG-19 in interleukin-32-induced inflammation. The research explores how LL-37 and IG-19 modulate the inflammatory responses triggered by interleukin-32. The study reveals that both LL-37 and IG-19 have the capacity to regulate and dampen interleukin-32-induced inflammation, highlighting their potential as anti-inflammatory agents. This research contributes to a better understanding of the intricate mechanisms involved in immune regulation and inflammation control, particularly in the context of interleukin-32-mediated responses.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1111/imm.12291
Di Virgilio F, Falzoni S, Giuliani AL, Adinolfi E: P2 receptors in cancer progression and metastatic spreading. CurrOpinPharmacol 2016; 29: 17-25.
P2 receptors in cancer progression and metastatic spreading
In their 2016 review published in Current Opinion in Pharmacology, Di Virgilio et al. provide insights into the role of P2 receptors in cancer progression and metastatic spreading. The review explores the significance of P2 receptors in the context of cancer biology and how they contribute to the various stages of cancer development, including tumor growth, invasion, and metastasis. The authors discuss the potential of P2 receptors as therapeutic targets in cancer treatment and shed light on the complex interplay between purinergic signaling and cancer progression. This review offers valuable perspectives on the role of P2 receptors in cancer and their potential implications for the development of novel anticancer strategies.
Full study on https://www.sciencedirect.com/science/article/pii/S1471489216300455
Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG: P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One 2014; 9:e114371.
P2X7 mediates ATP-driven invasiveness in prostate cancer cells
In their 2014 study published in PLoS One, Qiu et al. investigate the role of the P2X7 receptor in mediating ATP-driven invasiveness in prostate cancer cells. The research focuses on how ATP, acting through the P2X7 receptor, influences the invasiveness of prostate cancer cells. The study reveals that activation of P2X7 by ATP promotes the invasiveness of these cancer cells, suggesting a potential mechanism by which purinergic signaling contributes to cancer progression and metastasis in the context of prostate cancer. This study provides insights into the molecular pathways involved in cancer cell invasiveness and highlights the importance of P2X7 as a potential therapeutic target in prostate cancer research and treatment.
Full study on https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0114371
Cha HR, Lee JH, Hensel JA, Sawant AB, Davis BH, Lee CM, Deshane JS, Ponnazhagan S: Prostate cancer-derived cathelicidin-related antimicrobial peptide facilitates macrophage differentiation and polarization of immature myeloid progenitors to protumorigenic macrophages. Prostate 2016; 76: 624-636.
Prostate cancer-derived cathelicidin-related antimicrobial peptide facilitates macrophage differentiation and polarization of immature myeloid progenitors to protumorigenic macrophages
In their 2016 study published in Prostate, Cha et al. investigate the role of a prostate cancer-derived cathelicidin-related antimicrobial peptide (CRAMP) in promoting macrophage differentiation and polarization of immature myeloid progenitors towards protumorigenic macrophages. The research explores how this CRAMP, produced by prostate cancer cells, contributes to shaping the tumor microenvironment. The study reveals that CRAMP facilitates the differentiation of immature myeloid progenitors into tumor-promoting macrophages, suggesting a mechanism by which prostate cancer cells can manipulate the immune response in their favor. These findings provide insights into the complex interactions within the tumor microenvironment and highlight the potential impact of CRAMP on immune cell polarization in the context of prostate cancer.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/pros.23155
Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang LH: Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. BiochimBiophysActa 2015; 1848: 2584-2602.
Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives
In their 2015 review published in Biochimica et Biophysica Acta, Roger et al. provide a comprehensive overview of the roles of the P2X7 receptor in solid tumor progression and discuss potential therapeutic perspectives. The review delves into the multifaceted functions of the P2X7 receptor in the context of various solid tumors, including its involvement in cancer cell proliferation, migration, angiogenesis, and immune evasion. The authors also explore the potential therapeutic strategies targeting the P2X7 receptor as a means to impede cancer progression. This review offers valuable insights into the complex relationship between purinergic signaling via the P2X7 receptor and solid tumor development, highlighting its significance in cancer research and therapy.
Full study on https://www.sciencedirect.com/science/article/pii/S0005273614003642
Piktel E, Niemirowicz K, Wnorowska U, Wątek M, Wollny T, Głuszek K, Góźdź S, Levental I, Bucki R: The role of cathelicidin LL-37 in cancer development. Arch ImmunolTherExp (Warsz) 2016; 64: 33-46.
The role of cathelicidin LL-37 in cancer development
In their 2016 study published in Archives of Immunology and Experimental Therapy, Piktel et al. investigate the role of the cathelicidin LL-37 in cancer development. This comprehensive review explores the multifaceted functions of LL-37, including its antimicrobial properties and its potential impact on cancer biology. The authors discuss the intricate relationship between LL-37 and various aspects of cancer, such as tumor cell proliferation, angiogenesis, and metastasis. Additionally, the review highlights the immunomodulatory properties of LL-37 within the tumor microenvironment and its potential implications for cancer progression. This study provides valuable insights into the complex interplay between LL-37 and cancer development, emphasizing the need for further research to elucidate its precise role in oncology.
Full study on https://link.springer.com/article/10.1007/s00005-015-0359-5
Tuomela JM, Sandholm JA, Kaakinen M, Hayden KL, Haapasaari KM, Jukkola-Vuorinen A, Kauppila JH, Lehenkari PP, Harris KW, Graves DE, Selander KS: Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro. Breast Cancer Res Treat 2016; 155: 261-271.
Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro
In their 2016 study published in Breast Cancer Research and Treatment, Tuomela et al. investigate the impact of telomeric G-quadruplex-forming DNA fragments on breast cancer cells. The research focuses on how these DNA fragments induce invasion in breast cancer cells in vitro, particularly through Toll-like receptor 9 (TLR9)-mediated mechanisms. The study also explores the regulatory role of LL-37, a cathelicidin peptide, in this process. The findings suggest that telomeric DNA fragments can promote invasion in breast cancer cells, and LL-37 plays a role in regulating this invasive behavior. This research contributes to our understanding of the complex interactions between DNA fragments, innate immune receptors, and LL-37 in the context of breast cancer cell invasion.
Full study on https://link.springer.com/article/10.1007/s10549-016-3683-5
Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D, Wu K, Yu J, Sung JJ, Cho CH: Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer 2010; 127: 1741-1747.
Emerging roles of the host defense peptide LL‐37 in human cancer and its potential therapeutic applications
In their 2010 study published in the International Journal of Cancer, Wu et al. explore the emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. The research investigates the multifunctional properties of LL-37 in the context of cancer, including its effects on cancer cell proliferation, angiogenesis, and immune responses. The study also discusses the potential therapeutic applications of LL-37 in cancer treatment, highlighting its role as a novel candidate for cancer therapy. This research sheds light on the evolving understanding of LL-37’s involvement in cancer biology and its potential as a therapeutic target in the fight against cancer.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.25489
Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH: The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol 2010; 223: 178-186.
The host defense peptide LL‐37 activates the tumor‐suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells
In their 2010 study published in the Journal of Cellular Physiology, Wu et al. investigate the impact of the host defense peptide LL-37 on bone morphogenetic protein (BMP) signaling in gastric cancer cells. The research focuses on how LL-37 activates the tumor-suppressing BMP signaling pathway by inhibiting the proteasome in these cancer cells. The study reveals a novel mechanism by which LL-37 influences intracellular signaling, leading to the suppression of gastric cancer cell growth. This research provides valuable insights into the intricate interactions between LL-37 and cellular pathways that contribute to its potential role in cancer therapy, particularly in the context of gastric cancer.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/jcp.22026
An LL, Ma XT, Yang YH, Lin YM, Song YH, Wu KF: Marked reduction of LL-37/hCAP-18, an antimicrobial peptide, in patients with acute myeloid leukemia. Int J Hematol 2005; 81: 45-7.
Marked reduction of LL-37/hCAP-18, an antimicrobial peptide, in patients with acute myeloid leukemia
In their 2005 study published in the International Journal of Hematology, An et al. report a significant reduction in LL-37/hCAP-18, an antimicrobial peptide, in patients with acute myeloid leukemia (AML). The research highlights the marked decrease in LL-37 levels in AML patients, suggesting a potential association between LL-37 and this hematological malignancy. This finding raises questions about the role of LL-37 in immune responses and its potential implications in AML. Further investigation into LL-37’s involvement in AML may provide insights into the disease’s pathogenesis and potential therapeutic avenues.
Full study on https://link.springer.com/article/10.1532/IJH97.A10407
Chen X, Qi G, Qin M, Zou Y, Zhong K, Tang Y, Guo Y, Jiang X, Liang L, Zou X: DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity. Oncotarget 2017; 8: 27943-27952.
DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity
In their 2017 study published in Oncotarget, Chen et al. explore the regulation of the human cathelicidin antimicrobial peptide gene (CAMP) promoter activity by DNA methylation. The research investigates the direct downregulation of the CAMP promoter activity through DNA methylation. The study highlights the epigenetic mechanisms that can affect the expression of cathelicidin antimicrobial peptides, such as LL-37. Understanding how DNA methylation influences CAMP expression has implications for various physiological and pathological processes, including host defense and cancer, as it may impact the antimicrobial and immunomodulatory functions of LL-37. This research sheds light on the intricate regulatory mechanisms governing LL-37 expression in different contexts.
Full study on https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438620/
Piktel E, Niemirowicz K, Wnorowska U, Wątek M, Wollny T, Głuszek K, Góźdź S, Levental I, Bucki R: The role of cathelicidin LL-37 in cancer development. Arch ImmunolTherExp (Warsz) 2016; 64: 33-46.
The role of cathelicidin LL-37 in cancer development
In their 2016 review published in the Archives of Immunology and Experimental Therapy, Piktel et al. explore the multifaceted role of cathelicidin LL-37 in cancer development. The review delves into the various functions of LL-37, including its antimicrobial properties and its potential impact on cancer biology. The authors discuss the complex interplay between LL-37 and cancer, encompassing aspects such as tumor cell proliferation, angiogenesis, and metastasis. Additionally, the review addresses the immunomodulatory properties of LL-37 within the tumor microenvironment and its potential implications for cancer progression. This comprehensive study provides valuable insights into the intricate relationship between LL-37 and cancer development, emphasizing the need for further research to elucidate its precise role in oncology.
Full study on https://link.springer.com/article/10.1007/s00005-015-0359-5
Tuomela JM, Sandholm JA, Kaakinen M, Hayden KL, Haapasaari KM, Jukkola-Vuorinen A, Kauppila JH, Lehenkari PP, Harris KW, Graves DE, Selander KS: Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro. Breast Cancer Res Treat 2016; 155: 261-271.
Telomeric G-quadruplex-forming DNA fragments induce TLR9-mediated and LL-37-regulated invasion in breast cancer cells in vitro
In their 2016 study published in Breast Cancer Research and Treatment, Tuomela et al. investigate the role of telomeric G-quadruplex-forming DNA fragments in inducing invasion in breast cancer cells in vitro. The research focuses on the activation of Toll-like receptor 9 (TLR9) and the regulation of invasion by the host defense peptide LL-37. The study highlights the intricate interactions between these elements, where telomeric DNA fragments stimulate TLR9-mediated responses and LL-37 regulates the invasive behavior of breast cancer cells. These findings provide valuable insights into the complex mechanisms involved in breast cancer invasion and suggest a potential role for LL-37 in modulating tumor cell behavior in response to specific DNA structures.
Full study on https://link.springer.com/article/10.1007/s10549-016-3683-5
Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, Ng SS, Chiu LC, Marquez VE, Gallo RL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH: Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res 2012; 72: 6512-6523.
Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer
In their 2012 study published in Cancer Research, Ren et al. investigate the impact of the host immune defense peptide LL-37 on colon cancer. The research reveals that LL-37 activates caspase-independent apoptosis, a form of programmed cell death, and suppresses colon cancer cell growth. This suggests a potential role for LL-37 in inhibiting the progression of colon cancer. These findings provide insights into LL-37’s anti-cancer properties and its ability to modulate key cellular processes in colon cancer cells, highlighting its potential as a therapeutic target for colon cancer treatment.
Full study on https://aacrjournals.org/cancerres/article-abstract/72/24/6512/576212
Niemirowicz K, Prokop I, Wilczewska AZ, Wnorowska U, Piktel E, Wątek M, Savage PB, Bucki R: Magnetic nanoparticles enhance the anti-cancer activity of cathelicidin LL-37 peptide against colon cancer cells. Int J Nanomedicine 2015; 10: 3843-3853.
Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells
In their 2015 study published in the International Journal of Nanomedicine, Niemirowicz et al. explore the enhanced anti-cancer activity of the cathelicidin LL-37 peptide against colon cancer cells when combined with magnetic nanoparticles. The research demonstrates that magnetic nanoparticles can augment the effectiveness of LL-37 in inhibiting colon cancer cell growth. This study offers a novel approach to enhancing the therapeutic potential of LL-37 in the context of colon cancer treatment. By combining LL-37 with magnetic nanoparticles, the researchers open up new possibilities for improving the targeted delivery and anti-cancer properties of this host defense peptide in cancer therapy.
Full study on https://www.tandfonline.com/doi/abs/10.2147/IJN.S76104
Niemirowicz K, Durnaś B, Tokajuk G, Piktel E, Michalak G, Gu X, Kułakowska A, Savage PB, Bucki R: Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci Rep 2017; 7: 4610.
Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells
In their 2017 study published in Scientific Reports, Niemirowicz et al. investigate the formulation and candidacidal (fungicidal against Candida species) activity of magnetic nanoparticles coated with two antimicrobial agents, cathelicidin LL-37 and ceragenin CSA-13. The research focuses on developing an innovative approach to combat Candida infections, a significant medical concern. By coating magnetic nanoparticles with LL-37 and CSA-13, the study demonstrates enhanced candidacidal activity, potentially offering a promising therapeutic strategy against Candida-related diseases. This research highlights the potential of combining LL-37 with nanotechnology to improve antimicrobial treatments for fungal infections.
Full study on https://www.tandfonline.com/doi/abs/10.2147/IJN.S76104
Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH: The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol 2010; 223: 178-186.
The host defense peptide LL‐37 activates the tumor‐suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells
In their 2010 study published in the Journal of Cellular Physiology, Wu et al. explore the impact of the host defense peptide LL-37 on gastric cancer cells. The research demonstrates that LL-37 activates tumor-suppressing bone morphogenetic protein (BMP) signaling in gastric cancer cells by inhibiting proteasome activity. These findings suggest a potential role for LL-37 in suppressing the progression of gastric cancer by modulating BMP signaling and inhibiting proteasome-mediated degradation of tumor-suppressing proteins. This study provides valuable insights into the mechanisms through which LL-37 can exert its anti-cancer effects in the context of gastric cancer, offering potential implications for the development of novel therapeutic strategies.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/jcp.22026
Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J, Sung JJ: Proteasome inhibition: a new therapeutic strategy to cancer treatment. Cancer Lett 2010; 293: 15-22.
Proteasome inhibition: a new therapeutic strategy to cancer treatment
In their 2010 review published in Cancer Letters, Wu et al. discuss proteasome inhibition as a promising therapeutic strategy for cancer treatment. The review highlights the importance of the proteasome in regulating protein degradation and cell cycle control, making it a potential target for cancer therapy. By inhibiting the proteasome, researchers can disrupt the degradation of specific proteins involved in cancer cell growth and survival, ultimately leading to cancer cell death. This review provides valuable insights into the potential of proteasome inhibitors as a novel approach to cancer treatment, shedding light on the development of targeted therapies for various types of cancer.
Full study on https://www.sciencedirect.com/science/article/pii/S0304383509006958
Prevete N, Liotti F, Visciano C, Marone G, Melillo RM, de Paulis A: The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 2015; 34: 3826-3838.
The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis
In their 2015 study published in Oncogene, Prevete et al. investigate the role of the formyl peptide receptor 1 (FPR1) in human gastric cancer. The research reveals that FPR1 functions as a tumor suppressor in gastric cancer by inhibiting angiogenesis, the process of new blood vessel formation that is crucial for tumor growth and metastasis. FPR1 activation leads to the suppression of angiogenic factors and pathways, ultimately hindering the development of blood vessels within tumors. These findings provide insights into the potential therapeutic implications of targeting FPR1 to inhibit angiogenesis in gastric cancer, suggesting a novel approach to cancer treatment by disrupting the tumor’s blood supply.
Full study on https://www.nature.com/articles/onc2014309
Li L, Chen K, Xiang Y, Yoshimura T, Su S, Zhu J, Bian XW, Wang JM: New development in studies of formyl-peptide receptors: critical roles in host defense. J LeukocBiol 2016; 99: 425-435.
New development in studies of formyl-peptide receptors: critical roles in host defense
In their 2016 review article published in the Journal of Leukocyte Biology, Li et al. provide an overview of recent developments in the study of formyl-peptide receptors (FPRs) and their critical roles in host defense. The review highlights the importance of FPRs in the immune response, including their involvement in host defense against pathogens. FPRs are known for their ability to recognize formyl peptides, which are released by bacteria and mitochondria during infection or cellular stress. Activation of FPRs plays a crucial role in immune cell recruitment, chemotaxis, and inflammation regulation. The authors discuss the diverse functions of FPRs in various immune cells and their potential as therapeutic targets for immune-related disorders. This review provides valuable insights into the significance of FPRs in host defense mechanisms.
Full study on https://academic.oup.com/jleukbio/article-abstract/99/3/425/6935941
Mader JS, Mookherjee N, Hancock RE, Bleackley RC: The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res 2009; 7: 689-702.
The human host defense peptide LL-37 induces apoptosis in a calpain-and apoptosis-inducing factor–dependent manner involving bax activity
In their 2009 study published in Molecular Cancer Research, Mader et al. investigated the mechanisms by which the human host defense peptide LL-37 induces apoptosis. They found that LL-37 triggers apoptosis in a manner that involves calpain activation and the release of apoptosis-inducing factor (AIF). Additionally, Bax activity was implicated in this process. This study provides important insights into the molecular pathways through which LL-37, an antimicrobial peptide, can induce cell death, shedding light on its potential roles beyond antimicrobial defense, particularly in the context of cancer and apoptosis.
Full study on https://aacrjournals.org/mcr/article-abstract/7/5/689/90438
Yang D, Chertov O, Oppenheim JJ: Participation of mammalian defensins and cathelicidins in antimicrobial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J LeukocBiol 2001; 69: 691-697.
Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37)
The 2001 review by Yang, Chertov, and Oppenheim, titled “Participation of mammalian defensins and cathelicidins in antimicrobial immunity: receptors and activities of human defensins and cathelicidin (LL-37),” provides a comprehensive overview of the roles of human defensins and the cathelicidin LL-37 in antimicrobial immunity. It discusses the receptors and activities of these antimicrobial peptides, shedding light on their functions in host defense against microbial pathogens. This review is a valuable resource for understanding the immune responses mediated by these peptides in the context of antimicrobial defense.
Full study on https://academic.oup.com/jleukbio/article-abstract/69/5/691/6926932
Okumura K, Itoh A, Isogai E, Hirose K, Hosokawa Y, Abiko Y, Shibata T, Hirata M, Isogai H: C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett 2004; 212: 185-194.
C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells
In the study titled “C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells,” conducted by Okumura et al. in 2004, the researchers investigated the effects of the C-terminal domain of the human CAP18 antimicrobial peptide on oral squamous cell carcinoma SAS-H1 cells. Their findings revealed that this peptide induces apoptosis, or programmed cell death, in these cancer cells. This study suggests a potential therapeutic application for antimicrobial peptides in the context of cancer treatment, specifically in inducing apoptosis in oral squamous cell carcinoma cells.
Full study on https://www.sciencedirect.com/science/article/pii/S0304383504002794
Chen X, Qi G, Qin M, Zou Y, Zhong K, Tang Y, Guo Y, Jiang X, Liang L, Zou X: DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity. Oncotarget 2017; 8: 27943-27952.
DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity
In the study by Chen et al. published in 2017 titled “DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity,” the authors investigated the regulation of the CAMP gene, which encodes the human cathelicidin antimicrobial peptide LL-37, through DNA methylation. They found that DNA methylation of the CAMP promoter region directly leads to the downregulation of CAMP gene expression. This study highlights the epigenetic control of LL-37 production and its potential implications in various physiological and pathological conditions where LL-37 plays a role in host defense and immune responses. Understanding the regulation of LL-37 expression at the DNA methylation level may have implications for therapeutic interventions.
Full study on https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438620/
Available from https://www.promorepharma.com/en/ll-37-healing-of-chronic-wounds/.
Ramos R, Silva JP, Rodrigues AC, et al. Wound healing activity of the human antimicrobial peptide LL37. Peptides. 2011;32(7):1469-76.
Wound healing activity of the human antimicrobial peptide LL37
The study titled “Wound healing activity of the human antimicrobial peptide LL37” by Ramos et al. investigated the potential wound healing properties of the human antimicrobial peptide LL37. The researchers conducted experiments to evaluate the effects of LL37 on wound healing in a laboratory setting. They found that LL37 exhibited wound healing activity, which suggests that this antimicrobial peptide may have therapeutic potential in promoting the healing of wounds.
Full study on https://www.sciencedirect.com/science/article/pii/S0196978111002294
Shaykhiev R, Beisswenger C, Kändler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L842-8.
Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure
The study titled “Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure” by Shaykhiev et al. investigated the effects of the human endogenous antibiotic LL-37 on airway epithelial cells. The researchers found that LL-37 had a stimulating effect on airway epithelial cell proliferation and wound closure. This suggests that LL-37 may play a role in promoting the repair and healing of damaged airway epithelial cells.
Full study on https://journals.physiology.org/doi/abs/10.1152/ajplung.00286.2004
Carretero M, Escámez MJ, García M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128(1):223-36.
In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37
The study titled “In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37” by Carretero et al. investigated the wound healing properties of the human cathelicidin LL-37 both in vitro and in vivo. The researchers found that LL-37 exhibited wound healing-promoting activities, contributing to the repair and closure of wounds. This suggests that LL-37 has potential applications in promoting wound healing and tissue repair.
Full study on https://www.sciencedirect.com/science/article/pii/S0022202X15336071
Heilborn JD, Nilsson MF, Kratz G, et al. J Invest Dermatol. 2003;120:379–389.
The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium
The study by Heilborn et al. published in the Journal of Investigative Dermatology in 2003, titled “The Cathelicidin Antimicrobial Peptide LL-37 is Involved in Re-Epithelialization of Human Skin Wounds,” investigated the role of the cathelicidin antimicrobial peptide LL-37 in the re-epithelialization of human skin wounds. The research found that LL-37 plays a role in the wound healing process, particularly in the re-epithelialization phase, where it contributes to the closure of skin wounds.
Full study on https://www.sciencedirect.com/science/article/pii/S0022202X15301913
Saporito P, Vangmouritzen M, Løbner-olesen A, Jenssen H. LL-37 fragments have antimicrobial activity against Staphylococcus epidermidis biofilms and wound healing potential in HaCaT cell line. J Pept Sci. 2018;24(7):e3080.
LL‐37 fragments have antimicrobial activity against Staphylococcus epidermidis biofilms and wound healing potential in HaCaT cell line
The study conducted by Saporito et al., published in the Journal of Peptide Science in 2018, titled “LL-37 Fragments have Antimicrobial Activity Against Staphylococcus epidermidis Biofilms and Wound Healing Potential in HaCaT Cell Line,” explored the antimicrobial activity of LL-37 fragments against Staphylococcus epidermidis biofilms and their potential for promoting wound healing in HaCaT cell line. The research found that LL-37 fragments exhibited antimicrobial activity against biofilms formed by Staphylococcus epidermidis, a common pathogen associated with biofilm-related infections. Additionally, these LL-37 fragments showed potential in promoting wound healing in HaCaT cell lines.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/psc.3080
Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013;4:143. Published 2013 Jul 3. doi:10.3389/fimmu.2013.00143.
The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds
The research article titled “The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds,” authored by Duplantier AJ and van Hoek ML, was published in Frontiers in Immunology in 2013. This study investigated the potential use of the human cathelicidin antimicrobial peptide LL-37 as a treatment for polymicrobial infected wounds. The research explored the antimicrobial properties of LL-37 and its ability to combat infections caused by a mixture of different microorganisms commonly found in wound infections. The findings suggested that LL-37 could be a promising candidate for the treatment of polymicrobial infected wounds.
Full study on https://www.frontiersin.org/articles/10.3389/fimmu.2013.00143/full
Grönberg A, Mahlapuu M, Ståhle M, Whately-smith C, Rollman O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014;22(5):613-21.
Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial
The study titled “Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial” was conducted by Grönberg A, Mahlapuu M, Ståhle M, Whately-Smith C, and Rollman O. It was published in the journal Wound Repair and Regeneration in 2014. This randomized clinical trial aimed to assess the safety and effectiveness of LL-37 in promoting the healing of hard-to-heal venous leg ulcers. The results indicated that treatment with LL-37 was both safe and effective in enhancing the healing process of these challenging wounds.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1111/wrr.12211
Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, Xu Q, Yan ZQ. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006 Jul;26(7):1551-7. doi: 10.1161/01.ATV.0000223901.08459.57. Epub 2006 Apr 27. PMID: 16645154..
Involvement of the antimicrobial peptide LL-37 in human atherosclerosis
This reference is for a scientific article published in the journal Arteriosclerosis, Thrombosis, and Vascular Biology in July 2006. The article, authored by Edfeldt K, Agerberth B, Rottenberg ME, Gudmundsson GH, Wang XB, Mandal K, Xu Q, Yan ZQ, explores the role of the antimicrobial peptide LL-37 in the development of human atherosclerosis. LL-37 is part of the human body’s innate immune system, known for its broad-spectrum antimicrobial activity. This study suggests a link between LL-37 and atherosclerosis, a disease characterized by the buildup of fats, cholesterol, and other substances in and on artery walls (plaque), which can restrict blood flow.
Full study on https://www.ahajournals.org/doi/abs/10.1161/01.ATV.0000223901.08459.57
Bei Y, Pan LL, Zhou Q, Zhao C, Xie Y, Wu C, Meng X, Gu H, Xu J, Zhou L, Sluijter JPG, Das S, Agerberth B, Sun J, Xiao J. Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Med. 2019 Feb 20;17(1):42. doi: 10.1186/s12916-019-1268-y. PMID: 30782145; PMCID: PMC6381635.
Cathelicidin-related antimicrobial peptide protects against myocardial ischemia/reperfusion injury
The citation you’ve provided refers to a research article published in BMC Medicine in February 2019. The study, conducted by Bei Y, Pan LL, Zhou Q, Zhao C, Xie Y, Wu C, Meng X, Gu H, Xu J, Zhou L, Sluijter JPG, Das S, Agerberth B, Sun J, Xiao J, investigates the protective effects of cathelicidin-related antimicrobial peptide (CRAMP) against myocardial ischemia/reperfusion (I/R) injury.
Cathelicidin-related antimicrobial peptides, similar to LL-37 in humans, are a part of the innate immune system, known for their antimicrobial properties as well as their role in modulating inflammation. Myocardial ischemia/reperfusion injury is a significant complication of acute myocardial infarction, characterized by tissue damage and inflammation when blood supply returns to the tissue after a period of ischemia or lack of oxygen.
Full study on https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1268-y
Zhou Q, Pan LL, Xue R, et al. The anti-microbial peptide LL-37/CRAMP levels are associated with acute heart failure and can attenuate cardiac dysfunction in multiple preclinical models of heart failure. Theranostics. 2020;10(14):6167-6181. Published 2020 May 15. doi:10.7150/thno.46225.
The anti-microbial peptide LL-37/CRAMP levels are associated with acute heart failure and can attenuate cardiac dysfunction in multiple preclinical models of heart failure.
This reference points to a study published in Theranostics in 2020, authored by Zhou Q, Pan LL, Xue R, et al., focusing on the antimicrobial peptide LL-37 (human) / CRAMP (mouse) in the context of acute heart failure. The study highlights that the levels of LL-37/CRAMP are associated with acute heart failure and presents evidence that this peptide can attenuate cardiac dysfunction in various preclinical models of heart failure.
The antimicrobial peptides LL-37 in humans and its murine equivalent CRAMP are recognized for their role in the innate immune system, providing a first line of defense against pathogens through their antimicrobial properties. Beyond their antimicrobial functions, these peptides have been found to possess immunomodulatory activities, which can influence inflammation, wound healing, and immune cell recruitment.
Full study on https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7255020/
Qin X, Zhu G, Huang L, Zhang W, Huang Y, Xi X. LL-37 and its analog FF/CAP18 attenuate neutrophil migration in sepsis-induced acute lung injury. J Cell Biochem. 2019 Apr;120(4):4863-4871. doi: 10.1002/jcb.27641. Epub 2018 Dec 9. PMID: 30537236.
LL‐37 and its analog FF/CAP18 attenuate neutrophil migration in sepsis‐induced acute lung injury
This citation refers to a research article published in Journal of Cellular Biochemistry in April 2019, authored by Qin X, Zhu G, Huang L, Zhang W, Huang Y, Xi X. The study focuses on the antimicrobial peptide LL-37 and its analog FF/CAP18, investigating their effects on neutrophil migration in the context of sepsis-induced acute lung injury (ALI).
Sepsis-induced ALI is a critical condition characterized by widespread inflammation in the lungs, leading to severe respiratory distress. It is often exacerbated by the excessive recruitment of neutrophils, a type of white blood cell that plays a crucial role in the body’s immune response to infection. However, when neutrophils are overly activated or recruited in large numbers, they can contribute to tissue damage and increased inflammation, worsening the condition.
Full study on https://onlinelibrary.wiley.com/doi/abs/10.1002/jcb.27641
Available at https://www.jacionline.org/article/S0091-6749(06)00727-5/abstract#articleInformation.
Zhang Z, Shively JE. Generation of novel bone forming cells (monoosteophils) from the cathelicidin-derived peptide LL-37 treated monocytes. PLoS One. 2010 Nov 15;5(11):e13985. doi: 10.1371/journal.pone.0013985. PMID: 21085494; PMCID: PMC2981577.
Generation of novel bone forming cells (monoosteophils) from the cathelicidin-derived peptide LL-37 treated monocytes
The reference is for a research article published in PLoS ONE in November 2010 by Zhang Z and Shively JE. This study investigates the effects of treating monocytes with the cathelicidin-derived peptide LL-37, leading to the generation of novel bone-forming cells termed “monoosteophils.”
LL-37 is a human cathelicidin antimicrobial peptide known for its roles in the innate immune response, including direct antimicrobial activity and modulation of inflammation. Beyond these functions, research like this study explores the broader biological activities of LL-37, including its potential in regenerative medicine and tissue repair.
Full study on https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013985
Yu X, Quan J, Long W, Chen H, Wang R, Guo J, Lin X, Mai S. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 2018 Nov 15;372(2):178-187. doi: 10.1016/j.yexcr.2018.09.024. Epub 2018 Oct 1. PMID: 30287143.
LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway
The study published in *Experimental Cell Research* on November 15, 2018, by Yu X, Quan J, Long W, and colleagues, investigates the antimicrobial peptide LL-37 and its role in inhibiting lipopolysaccharide (LPS)-induced inflammation and promoting osteogenic differentiation of bone marrow stromal cells (BMSCs) through the P2X7 receptor and MAPK signaling pathway. This research highlights LL-37’s potential beyond its known antimicrobial functions, suggesting it could mitigate inflammatory responses and enhance bone regeneration. By elucidating the mechanisms of LL-37’s action, specifically its interaction with the P2X7 receptor and activation of the MAPK pathway, the study opens up new avenues for therapeutic applications in treating inflammatory diseases and bone-related disorders, showcasing the peptide’s versatility in modulating inflammation and tissue repair processes. The findings, detailed under DOI 10.1016/j.yexcr.2018.09.024, provide valuable insights into leveraging LL-37 for medical treatments, emphasizing its significance in immune modulation and regenerative medicine.
Full study on https://www.sciencedirect.com/science/article/pii/S001448271830572X
Supanchart C, Thawanaphong S, Makeudom A, Bolscher JG, Nazmi K, Kornak U, Krisanaprakornkit S. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. 2012 Nov;91(11):1071-7. doi: 10.1177/0022034512460402. Epub 2012 Sep 13. PMID: 22983411.
The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis
The study by Supanchart C, Thawanaphong S, Makeudom A, and colleagues, published in the *Journal of Dental Research* in November 2012, focuses on the antimicrobial peptide LL-37 and its effects on osteoclastogenesis, the process by which osteoclasts, the cells responsible for bone resorption, are formed. The research demonstrates that LL-37 inhibits osteoclastogenesis in vitro, suggesting a potential role for this peptide in regulating bone metabolism and preventing bone loss. This finding is significant as it indicates that LL-37, known primarily for its antimicrobial and immunomodulatory functions, may also play a role in bone health by impacting the balance between bone formation and resorption. The inhibition of osteoclastogenesis by LL-37 could have therapeutic implications for conditions characterized by excessive bone loss, such as osteoporosis. The study, available under DOI 10.1177/0022034512460402, adds to the growing body of evidence on the multifunctional nature of LL-37, highlighting its potential as a target for the development of new treatments for bone-related diseases.
Full study on https://journals.sagepub.com/doi/abs/10.1177/0022034512460402
Kittaka M, Shiba H, Kajiya M, Fujita T, Iwata T, Rathvisal K, Ouhara K, Takeda K, Fujita T, Komatsuzawa H, Kurihara H. The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect. Peptides. 2013 Aug;46:136-42. doi: 10.1016/j.peptides.2013.06.001. Epub 2013 Jun 12. PMID: 23770151.
The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect
The research conducted by Kittaka M, Shiba H, Kajiya M, and their team, published in *Peptides* in August 2013, explores the role of the antimicrobial peptide LL37 in bone regeneration. Their study, using a rat calvarial bone defect model, demonstrates that LL37 significantly promotes bone healing and regeneration. This finding is particularly notable as it underscores LL37’s potential beyond its established antimicrobial and immunomodulatory functions, highlighting its capability to enhance bone repair processes. The study suggests that LL37 could be a valuable therapeutic agent for bone regeneration, offering promising implications for the treatment of bone defects and injuries. The effectiveness of LL37 in promoting bone regeneration in this preclinical model opens avenues for further research into its mechanisms of action and potential applications in regenerative medicine and dentistry. The publication, accessible via DOI 10.1016/j.peptides.2013.06.001, provides critical insights into the multifunctional role of LL37 in tissue healing and regeneration, positioning it as a candidate for developing new strategies for enhancing bone repair.
Full study on https://www.sciencedirect.com/science/article/pii/S0196978113002180
He Y, Mu C, Shen X, Yuan Z, Liu J, Chen W, Lin C, Tao B, Liu B, Cai K. Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment. Acta Biomater. 2018 Oct 15;80:412-424. doi: 10.1016/j.actbio.2018.09.036. Epub 2018 Sep 25. PMID: 30266635.
Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment
The study by He Y, Mu C, Shen X, and colleagues, published in *Acta Biomaterialia* on October 15, 2018, investigates the use of the antimicrobial peptide LL-37 as a coating on micro-structured titanium implants to enhance bone formation in vivo. The research focuses on LL-37’s ability to facilitate bone regeneration by recruiting mesenchymal stem cells (MSCs) to the implant site. This approach leverages LL-37’s properties not just for its antimicrobial capabilities but also for its role in cell signaling and tissue regeneration. By coating titanium implants with LL-37, the study demonstrates a novel strategy to improve osseointegration, which is critical for the success of dental and orthopedic implants. The findings suggest that LL-37 coated implants could promote better bone healing and integration by attracting MSCs, which are key players in bone regeneration. This research opens up new avenues for enhancing the performance of bone implants through bioactive coatings that support tissue healing and integration, offering promising implications for the development of improved implantable devices for bone repair and regeneration. The publication, available via DOI 10.1016/j.actbio.2018.09.036, provides valuable insights into the potential of utilizing bioactive peptides like LL-37 to improve the outcomes of surgical implants in orthopedics and dentistry.
Full study on https://www.sciencedirect.com/science/article/pii/S1742706118305658
Other Peptides
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

