Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Liraglutide
- Key Takeaways
- What is Liraglutide?
- How Liraglutide Works
- Chemical Structure of Liraglutide
- Research on Liraglutide
- Liraglutide Side Effects
- Liraglutide Brand Name
- Victoza Drug
- Victoza Medication
- Liraglutide Saxenda
- Liraglutide Medication
- Liraglutide Injection
- Victoza Liraglutida/Victoza Liraglutide
- Victoza and Weight Loss
- Saxenda Dose
- Victoza Pen
- Saxenda vs Victoza
- Is Liraglutide Once a Week Injection for Type 2 Diabetes Good?
- Victoza Dosage for Weight Loss
- Liraglutide Cost
- Liraglutide Generic
- Liraglutide for Weight Loss in Non Diabetics
- Liraglutide and Thyroid Cancer
- Liraglutide and Low Blood Sugar
- Blog
- FAQ
- Reference
Table of Contents
- Overall Health Benefits of Liraglutide
- Key Takeaways
- What is Liraglutide?
- How Liraglutide Works
- Chemical Structure of Liraglutide
- Research on Liraglutide
- Liraglutide Side Effects
- Liraglutide Brand Name
- Victoza Drug
- Victoza Medication
- Liraglutide Saxenda
- Liraglutide Medication
- Liraglutide Injection
- Victoza Liraglutida/Victoza Liraglutide
- Victoza and Weight Loss
- Saxenda Dose
- Victoza Pen
- Saxenda vs Victoza
- Is Liraglutide Once a Week Injection for Type 2 Diabetes Good?
- Victoza Dosage for Weight Loss
- Liraglutide Cost
- Liraglutide Generic
- Liraglutide for Weight Loss in Non Diabetics
- Liraglutide and Thyroid Cancer
- Liraglutide and Low Blood Sugar
- Blog
- FAQ
- Reference
Overall Health Benefits of Liraglutide
Liraglutide benefits include weight loss, improved blood sugar control, and reduced risk of cardiovascular events in patients with type 2 diabetes. This medication also aids in appetite regulation and has shown positive effects on overall metabolic health.
- Treats diabetes and improves blood sugar levels [1-9]
- Lowers the risk of heart disease [10-18]
- Lowers blood pressure [19-24]
- Helps lose weight [25-33]
- Accelerates wound healing [34-38]
- Prevents cancer [39-42]
- Boosts brain power [43-57]
Key Takeaways
- Weight Loss: Liraglutide is effective in promoting significant weight loss, making it beneficial for individuals with obesity or those needing to manage their weight.
- Blood Sugar Control: It improves blood sugar levels in patients with type 2 diabetes, helping to stabilize glucose levels and reduce HbA1c.
- Cardiovascular Health: Liraglutide has been shown to lower the risk of major cardiovascular events, such as heart attacks and strokes, in people with type 2 diabetes.
- Appetite Regulation: The medication helps regulate appetite, leading to reduced food intake and assisting in long-term weight management.
- Metabolic Health: Liraglutide contributes to overall metabolic health by improving insulin sensitivity and promoting better glycemic control.
What is Liraglutide?
Liraglutide is mainly used in the treatment of type 2 diabetes mellitus and obesity. It stimulates the release of a hormone known as insulin, which brings down the levels of blood sugar. The more insulin that the pancreas secretes, the lower the chance that blood sugars will spike up. There’s increasing evidence suggesting that this medication can offer other health benefits.
How Liraglutide Works
IMG
Liraglutide works by helping the pancreas to release the right amount of insulin when blood sugar levels are elevated. Insulin allows the blood sugar to move from the blood into other body tissues where it is converted into energy. In addition, liraglutide also promotes weight loss by slowing the emptying of the stomach to decrease appetite.
Chemical Structure of Liraglutide
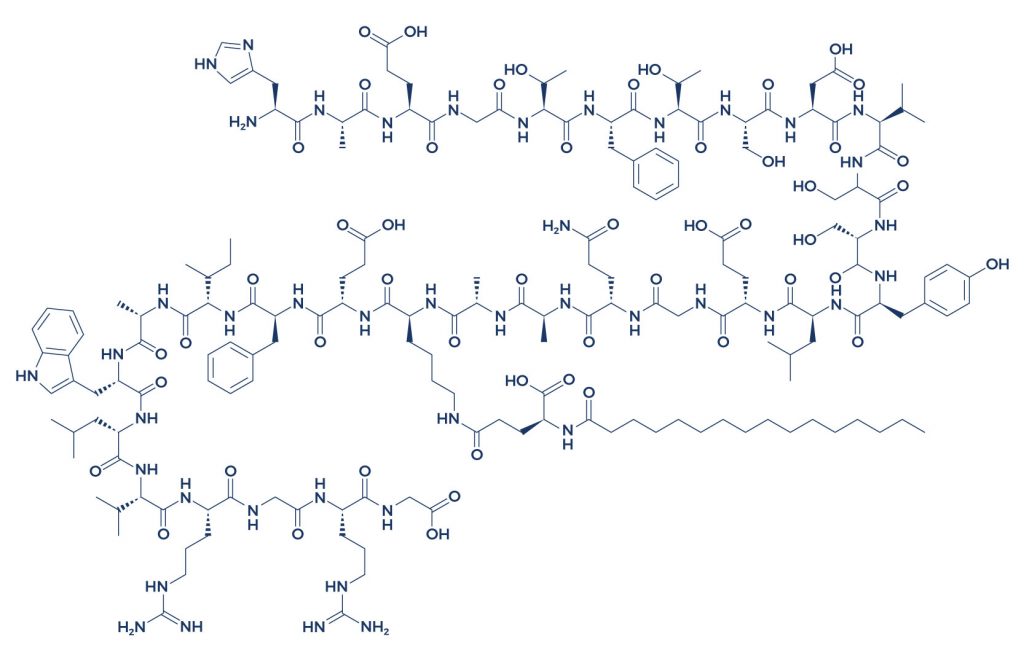
Research on Liraglutide
A. Treats Diabetes and Improves Blood Sugar Levels
Liraglutide treats diabetes and improves blood sugar levels by mimicking the action of the incretin hormone GLP-1, which enhances insulin secretion in response to meals. It also slows gastric emptying, reducing the speed at which glucose enters the bloodstream, and decreases glucagon release, which helps lower blood sugar levels. Additionally, liraglutide promotes weight loss, which further contributes to improved glycemic control, making it an effective treatment for patients with type 2 diabetes.
- In diabetic patients, liraglutide significantly improved blood sugar control with a low risk of hypoglycemia (abnormally low blood sugar). [1]
- In children and adolescents with type 2 diabetes mellitus (T2D), a daily dose of liraglutide produced beneficial effects in controlling blood sugar levels. [2]
- In patients with type 1 diabetes mellitus (T1D), liraglutide plus insulin treatment was effective at lowering blood sugar levels. [3]
- In patients with T2D, liraglutide monotherapy or in combination with oral antidiabetic drugs was found to be efficacious and well-tolerated. [4]
- In patients with T2D, liraglutide treatment was effective in improving the symptoms associated with diabetes. [5]
- In overweight diabetic patients, liraglutide treatment resulted in a reduced risk of complications. [6]
- In diabetic patients undergoing intensive insulin therapy, liraglutide treatment improved blood sugar control. [7]
- In patients with T2D, liraglutide as an add-on treatment for 3 months was effective in decreasing glucose variability and ameliorating hyperglycemia without increasing the incidence of hypoglycemia. [8]
- In Indian patients with T2D, administration of liraglutide in addition to anti-diabetic drugs resulted in significant improvements in glycemic parameters. [9]
B. Lowers the Risk of Heart Disease

Liraglutide lowers the risk of heart disease by improving cardiovascular health through multiple mechanisms. It enhances glycemic control and promotes weight loss, both of which are critical in reducing heart disease risk factors like high blood sugar and obesity. Additionally, liraglutide reduces inflammation and oxidative stress, which play a role in atherosclerosis development. Clinical studies have shown that liraglutide can decrease the likelihood of major cardiovascular events, such as heart attack and stroke, particularly in people with type 2 diabetes or other risk factors for heart disease.
- In patients with advanced type 2 diabetes, liraglutide therapy significantly lowered the mortality rate from cardiovascular causes. [10]
- In diabetic patients, treatment with liraglutide was associated with reduced cases of cardiovascular-related deaths. [11]
- In patients with type 2 diabetes, liraglutide was shown to have beneficial effects on the function of the heart’s left ventricle. [12]
- A review of studies reported that liraglutide treatment lowered the risk of heart problems in diabetic patients. [13]
- In diabetic patients with high cardiovascular risk, liraglutide treatment lowered the cardiovascular outcomes. [14]
- A study showed that the use of liraglutide was associated with a lower risk of major heart diseases. [15]
- Liraglutide treatment was shown to be beneficial in patients with or without a history of heart failure. [16]
- In diabetic patients, liraglutide administration greatly lowered the risk of major adverse cardiovascular events. [17]
- In diabetic patients with coronary artery disease, monotherapy of liraglutide improved cardiovascular function. [18]
C. Lowers Blood Pressure
Liraglutide, a GLP-1 receptor agonist, lowers blood pressure by improving endothelial function and promoting vasodilation. It enhances nitric oxide production, which relaxes blood vessels, reducing vascular resistance and improving blood flow. Additionally, liraglutide helps with weight loss and glycemic control, both of which contribute to lowering blood pressure. By addressing these metabolic factors, liraglutide can reduce systolic and diastolic blood pressure in individuals with obesity, type 2 diabetes, or cardiovascular risk factors.
- A review of studies showed that liraglutide could significantly reduce blood pressure (BP). [19]
- In diabetic patients, treatment with liraglutide successfully reduced BP and provided extra cardiovascular benefits. [20]
- In diabetic patients, liraglutide produced systolic blood pressure (SBP) and weight reduction effects. [21]
- In diabetic patients with or without antihypertensive medication, liraglutide treatment greatly improved their SBP. [22]
- In Asian diabetic patients, daily administration of liraglutide resulted in significantly lowered SBP. [23]
- In patients with type 2 diabetes, liraglutide treatment lowered SBP. [24]
D. Helps Lose Weight

Liraglutide helps with weight loss by mimicking a natural hormone in the body called GLP-1, which regulates appetite and food intake. It works by slowing down the emptying of the stomach, making you feel full for longer, and reducing hunger. Liraglutide also interacts with areas of the brain that control appetite, leading to a decrease in calorie consumption, which ultimately promotes weight loss when combined with a healthy diet and exercise.
- In patients with heart failure, liraglutide treatment was shown to be effective as a weight-loss agent. [25]
- In Asian patients, liraglutide produced weight-reducing effects. [26]
- When combined with a healthy diet and mild exercise, liraglutide was shown to produce significant weight reduction. [27]
- In obese Chinese patients with type 2 diabetes, daily injection of liraglutide greatly reduced their weight and waist circumference. [28]
- In obese patients with type 2 diabetes, prolonged liraglutide treatment reduced body weight. [29]
- In overweight adults with bodyweight-related disease, the addition of liraglutide to a healthy diet and exercise resulted in weight reduction. [30]
- In adolescents with obesity, the administration of liraglutide (3.0 mg) in addition to lifestyle therapy produced a significant reduction in body mass index (BMI) than placebo plus lifestyle therapy. [31]
- A review of randomized, placebo-controlled trials of liraglutide for weight management found that the treatment can help induce and sustain weight loss in patients with obesity. [32]
- In overweight and obese participants with type 2 diabetes, the administration of subcutaneous liraglutide at 3.0 mg daily, compared with placebo, produced significant weight loss over 56 weeks. [33
E. Accelerates Wound Healing
Liraglutide accelerates wound healing by enhancing tissue repair processes, primarily through its anti-inflammatory and pro-angiogenic effects. It promotes the formation of new blood vessels (angiogenesis) and improves blood flow to the wound site, which facilitates oxygen and nutrient delivery essential for healing. Additionally, liraglutide reduces inflammation and oxidative stress, helping to create a more favorable environment for cell regeneration and tissue repair.
- In diabetic mice with skin ulcers, the application of liraglutide-containing ointment improved wound healing. [34]
- A study suggested that liraglutide can help treat diabetic foot ulcers possibly by reducing blood sugar levels. [35]
- In patients with diabetic wounds, the application of liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats accelerated the wound repair process. [36]
- In mice, liraglutide improved wound healing and showed its potential in treating diabetic skin ulcers. [37]
- In patients with type 2 diabetes, liraglutide treatment was associated with a reduced risk of diabetes-related foot ulcer amputations. [38]
F. Prevents Cancer
Liraglutide, a GLP-1 receptor agonist, may help prevent cancer by reducing insulin resistance and lowering blood sugar levels, which are linked to cancer growth. It also promotes weight loss, reducing obesity-related cancer risks. Additionally, liraglutide may inhibit cancer cell proliferation and induce apoptosis (cell death) in certain cancer cells, contributing to its potential protective effects. These mechanisms make it a promising candidate for cancer prevention in metabolic-related cancers, such as colorectal and pancreatic cancer.
- The combined treatment of liraglutide and docetaxel (anti-cancer drug) worked synergistically in reducing the prostate cancer cells’ viability, causing cell cycle arrest, and inducing apoptosis (programmed cell death). [39]
- A review of studies showed that liraglutide treatment resulted in reduced multiplication of breast cancer cells. [40]
- A study reported that liraglutide treatment together with metformin produced anti-tumor effects against pancreatic cancer cells. [41]
- In pancreatic cancer cells, liraglutide inhibited cell proliferation, migration, and invasion. [42]
G. Boosts Brain Power

Liraglutide may boost brain power by enhancing cognitive function through its neuroprotective effects. It works by activating GLP-1 receptors, which improve glucose metabolism in the brain and reduce inflammation, oxidative stress, and the accumulation of amyloid-beta plaques linked to neurodegenerative conditions like Alzheimer’s disease. This can lead to improved memory, learning, and overall brain health.
- In mice, liraglutide administration produced neuroprotective effects. [43]
- A study showed that liraglutide has beneficial effects on objective measures of cognitive function. [44]
- In mice, liraglutide prevented the loss of brain receptors and synapses and reversed memory impairment induced by Alzheimer’s disease (AD). [45]
- In medication-induced diabetic mice that exhibited impaired learning and memory, liraglutide treatment attenuated these effects. [46]
- A study showed that liraglutide can help treat AD by targeting the root cause of the disease. [47]
- In rats with ovarian cysts, liraglutide treatment reduced memory impairment. [48]
- In type 2 diabetic rats, liraglutide administration exerted a protective effect against brain cell damage. [49]
- In diabetic rats with cognitive impairment, liraglutide improved learning and memory. [50]
- In the mouse model of epilepsy, liraglutide reduced seizure susceptibility and cognitive dysfunction and exerted neuroprotective effects. [51]
- A study showed that liraglutide protected against diabetes-induced cognitive impairment. [52]
- In diabetic mice, liraglutide administration improved cognitive function. [53]
- In mice fed with a high-fat diet, liraglutide treatment improved metabolic parameters and had a beneficial effect on cognitive function. [54]
- In a mouse model of Alzheimer’s disease, liraglutide administration significantly increased memory retention and the total number of brain neurons. [55]
- In aged mice, liraglutide reversed memory impairment and synaptic loss and reduced plaque load. [56]
- In diabetic patients, liraglutide slowed down memory function decline. [57]
Liraglutide Side Effects

Liraglutide side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on liraglutide. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of liraglutide. Despite this, it was listed as a side effect associated with liraglutide even though these associated side effects are very uncommon.
Side effects associated with liraglutide may include the following:
- Constipation
- Cough
- Headache
- Heartburn
- Pain or burning sensation when urinating
- Runny nose
- Sneezing
- Tiredness
Liraglutide Brand Name
Liraglutide is marketed under the brand name Victoza, primarily prescribed for the management of type 2 diabetes. Victoza works by mimicking the action of a natural hormone called GLP-1 (glucagon-like peptide-1), which helps regulate blood sugar levels. By stimulating insulin release and inhibiting glucagon secretion, it effectively reduces blood sugar levels, making it an essential medication for individuals struggling to control their diabetes through diet and exercise alone.
In addition to its use in diabetes management, Liraglutide is also sold under the brand name Saxenda for the treatment of obesity. Saxenda is administered at higher doses than Victoza and is specifically designed to aid in weight loss for adults and certain adolescents with obesity-related health issues. By promoting a feeling of fullness and reducing appetite, Saxenda helps patients achieve significant weight reduction, which can lead to improved overall health and a lower risk of obesity-related conditions.
Both Victoza and Saxenda have been thoroughly studied and proven effective in their respective uses, making Liraglutide a versatile medication with broad applications. The availability of Liraglutide under different brand names allows healthcare providers to tailor treatment plans to meet the specific needs of their patients, whether they require blood sugar management, weight loss, or both. This adaptability makes Liraglutide a valuable tool in addressing some of the most prevalent and challenging health issues today.
Victoza Drug
Victoza, a brand name for the drug liraglutide, is a medication primarily used to treat type 2 diabetes. It works by mimicking the action of the hormone GLP-1 (glucagon-like peptide-1), which increases insulin secretion in response to high blood sugar levels, thereby aiding in blood glucose control. By enhancing insulin production and lowering the amount of glucose produced by the liver, Victoza helps patients achieve better glycemic management, reducing the risk of long-term complications associated with diabetes.
In addition to its role in blood sugar regulation, Victoza has shown significant benefits in promoting weight loss. Many patients with type 2 diabetes struggle with obesity, which exacerbates their condition. Victoza addresses this by slowing gastric emptying and increasing feelings of fullness, leading to reduced calorie intake. Clinical trials have demonstrated that patients using Victoza not only experience improved glycemic control but also achieve meaningful weight loss, enhancing their overall health and well-being.
Beyond diabetes and weight management, Victoza has been proven to lower the risk of cardiovascular events in patients with type 2 diabetes and established cardiovascular disease. Studies have indicated that it can reduce the occurrence of heart attacks, strokes, and other cardiovascular complications. This dual benefit of managing both blood sugar and cardiovascular health makes Victoza a valuable therapeutic option for patients seeking comprehensive care for their diabetes and associated health risks.
Victoza Medication
Victoza, a brand name for the medication liraglutide, is an injectable prescription drug primarily used to manage type 2 diabetes. It belongs to a class of drugs called GLP-1 receptor agonists, which work by mimicking the incretin hormones that the body usually produces naturally to stimulate insulin release in response to meals. By enhancing insulin secretion, Victoza helps lower blood sugar levels and offers better control over diabetes, making it an essential treatment option for many patients struggling with this chronic condition.
One of the significant benefits of Victoza is its ability to promote weight loss. Many people with type 2 diabetes are overweight or obese, and Victoza’s appetite-suppressing properties can lead to significant weight reduction. This weight loss is beneficial not only for blood sugar control but also for reducing the risk of cardiovascular complications often associated with diabetes. Clinical trials have demonstrated that patients taking Victoza often experience improved metabolic health, which contributes to better overall management of diabetes and its related risks.
In addition to managing blood sugar and aiding in weight loss, Victoza has been shown to reduce the risk of major cardiovascular events, such as heart attacks and strokes. This cardiovascular benefit makes it a particularly valuable medication for patients with type 2 diabetes who are at higher risk for heart disease. By addressing multiple aspects of diabetes management—blood sugar control, weight reduction, and cardiovascular risk—Victoza offers a comprehensive approach to improving the health and quality of life for individuals with type 2 diabetes.
Liraglutide Saxenda
Liraglutide Saxenda is a medication primarily used for weight management in adults with obesity or overweight conditions. It is a glucagon-like peptide-1 (GLP-1) receptor agonist, which mimics the action of the hormone GLP-1. By stimulating insulin secretion and reducing appetite, Saxenda helps patients achieve and maintain a healthier weight. Clinical studies have demonstrated that Saxenda, when combined with a reduced-calorie diet and increased physical activity, leads to significant weight loss in many users.
Beyond its primary function of weight management, Liraglutide Saxenda also offers additional health benefits. It has been shown to improve metabolic health by enhancing insulin sensitivity and lowering blood glucose levels. This makes it particularly beneficial for individuals with prediabetes or metabolic syndrome, as it helps in managing and potentially preventing the progression to type 2 diabetes. Moreover, the weight loss achieved through Saxenda can lead to reductions in blood pressure, cholesterol levels, and overall cardiovascular risk, contributing to improved overall health and well-being.
Despite its benefits, Liraglutide Saxenda is not without potential side effects. Common side effects include nausea, vomiting, diarrhea, and constipation, which often subside as the body adjusts to the medication. More serious but less common side effects can include pancreatitis and gallbladder problems. Therefore, it is important for patients to be closely monitored by their healthcare provider while using Saxenda. Overall, Liraglutide Saxenda represents a valuable tool in the fight against obesity, offering significant benefits for weight loss and metabolic health when used appropriately.
Liraglutide Medication
Liraglutide is a medication primarily used for the treatment of type 2 diabetes and chronic weight management. As a glucagon-like peptide-1 (GLP-1) receptor agonist, liraglutide works by stimulating insulin secretion in response to meals, thereby improving blood sugar control. This medication also slows gastric emptying and promotes satiety, which helps reduce food intake and supports weight loss efforts. For individuals struggling with obesity or type 2 diabetes, liraglutide offers a dual benefit of glycemic control and significant weight reduction.
In clinical trials, liraglutide has demonstrated its efficacy in reducing HbA1c levels, a key marker of long-term blood sugar control, and in achieving meaningful weight loss. Patients using liraglutide have reported improved overall metabolic health, including better insulin sensitivity and lower cardiovascular risk. The medication’s ability to lower the risk of major cardiovascular events such as heart attacks and strokes adds an essential layer of protection for those with type 2 diabetes, who are often at higher risk for these complications.
Beyond its benefits in managing diabetes and obesity, liraglutide also addresses some of the challenges patients face with appetite control. By enhancing feelings of fullness and reducing hunger, liraglutide makes it easier for patients to adhere to dietary changes and maintain a healthier lifestyle. This medication is typically administered via a daily injection, and while it is generally well-tolerated, some patients may experience side effects such as nausea or gastrointestinal discomfort. Despite these potential drawbacks, liraglutide remains a valuable tool in the management of type 2 diabetes and obesity, offering significant benefits for long-term health and quality of life.
Liraglutide Injection
Liraglutide injection is a medication primarily used to manage type 2 diabetes and obesity. Administered once daily, it mimics the action of the glucagon-like peptide-1 (GLP-1) hormone, which plays a crucial role in regulating blood sugar levels and appetite. By enhancing insulin secretion in response to meals and inhibiting the release of glucagon, liraglutide helps stabilize blood glucose levels and reduces the risk of hyperglycemia.
Beyond its blood sugar regulation benefits, liraglutide injection is also effective in promoting weight loss. It works by slowing gastric emptying and increasing feelings of fullness, which helps individuals reduce their food intake. Clinical trials have demonstrated significant weight reduction in patients using liraglutide, making it a valuable option for those struggling with obesity or those who need to manage their weight due to associated health conditions.
Moreover, liraglutide has shown positive effects on cardiovascular health. Studies indicate that it can lower the risk of major cardiovascular events, such as heart attacks and strokes, in patients with type 2 diabetes. This added benefit makes liraglutide an important therapeutic option not only for glycemic control and weight management but also for enhancing overall cardiovascular health in individuals with diabetes.
Victoza Liraglutida/Victoza Liraglutide
Victoza, known generically as Liraglutide, is a medication primarily used for managing type 2 diabetes mellitus. It belongs to the class of glucagon-like peptide-1 receptor agonists (GLP-1 receptor agonists), which work by mimicking the effects of a hormone called GLP-1. This hormone helps regulate blood sugar levels by stimulating insulin secretion and inhibiting glucagon release from the pancreas after meals. By enhancing these processes, Victoza helps to lower blood sugar levels effectively, thereby improving glycemic control in diabetic patients.
In addition to its role in diabetes management, Victoza has garnered attention for its benefits beyond glucose control. It is also approved for use in weight management in adults with obesity or who are overweight and have at least one weight-related comorbidity. Studies have shown that Victoza can lead to significant weight loss when combined with lifestyle modifications, making it a valuable tool in addressing both diabetes and obesity—a common dual challenge in many patients.
Victoza is typically administered once daily via subcutaneous injection. Its convenience, coupled with its dual benefits of improving blood sugar levels and aiding weight loss, has made it a preferred choice for many healthcare providers and patients alike in managing type 2 diabetes and related conditions. However, like all medications, it is essential for individuals to discuss with their healthcare providers to determine if Victoza is suitable for their specific health needs and to manage any potential side effects effectively.
Victoza and Weight Loss
Victoza, known generically as Liraglutide, has gained recognition for its role in weight loss beyond its primary use in managing type 2 diabetes. This medication, when used at higher doses than typically prescribed for diabetes treatment, has been found to promote significant weight loss in clinical trials. Studies have shown that patients using Victoza experience greater reductions in body weight compared to those using a placebo, highlighting its potential as an effective tool for obesity management.
The mechanism behind Victoza’s weight loss benefits involves its action as a GLP-1 receptor agonist. By mimicking the effects of glucagon-like peptide-1 (GLP-1), Victoza enhances feelings of fullness, reduces appetite, and slows gastric emptying, which collectively contribute to reduced food intake and subsequent weight loss. This dual benefit of improving blood sugar control while aiding weight loss makes Victoza a valuable option for patients with type 2 diabetes who struggle with obesity or overweight conditions.
Beyond its pharmacological effects, Victoza’s use in weight management is supported by its safety profile and tolerability. Clinical trials have generally reported mild to moderate side effects, such as nausea and gastrointestinal discomfort, which tend to diminish over time. This combination of efficacy and manageable side effects underscores Victoza’s role not only in diabetes management but also in addressing the complex challenges of obesity and associated metabolic disorders.
Saxenda Dose
Saxenda, a brand name for liraglutide, is prescribed for weight management in adults who have obesity or overweight conditions with at least one weight-related health issue. The dosing regimen for Saxenda is crucial for achieving optimal results. Initially, patients typically start with a low dose to minimize potential side effects, such as nausea, which is common during the initial weeks of treatment. The dosage is gradually increased over several weeks to reach the maintenance dose, which is usually determined based on individual response and tolerance.
The recommended starting dose of Saxenda is typically 0.6 mg once daily. Over the course of the first five weeks, the dose is increased weekly by increments of 0.6 mg until the maintenance dose of 3.0 mg is reached. This gradual titration helps the body adjust to the medication and reduces the likelihood of side effects. It’s important for patients to follow their healthcare provider’s instructions closely regarding the dosing schedule and any adjustments that may be necessary based on their response to the treatment.
Monitoring and adherence to the prescribed Saxenda dose are essential for maximizing its effectiveness in weight management. Patients are advised to administer the injection at the same time each day, preferably around the same mealtime, to maintain consistency. Regular follow-up with healthcare providers ensures that any necessary adjustments to the Saxenda dose can be made based on individual progress and health considerations.
Victoza Pen
The Victoza pen is a user-friendly medical device designed for the administration of liraglutide, a medication used primarily for managing type 2 diabetes and promoting weight loss. This pen-like injector offers convenience and ease of use, allowing patients to self-administer their medication with minimal training. It features a pre-filled cartridge of Victoza (liraglutide) and a fine needle that delivers the precise dosage subcutaneously.
Patients using the Victoza pen appreciate its compact design and intuitive mechanism, which simplifies the injection process. The device typically includes dose adjustment settings to accommodate individual treatment needs, ensuring accurate delivery of the medication. This functionality not only enhances patient compliance but also supports consistent therapeutic outcomes.
Moreover, the Victoza pen incorporates safety features to prevent accidental needle sticks and misuse, prioritizing patient comfort and adherence. Its portable nature allows for discreet use in various settings, promoting flexibility in managing diabetes and supporting lifestyle integration. Overall, the Victoza pen exemplifies modern advancements in medical technology aimed at improving patient experience and treatment efficacy in diabetes management.
Saxenda vs Victoza
Saxenda and Victoza are both medications developed by Novo Nordisk, but they serve different purposes despite sharing the same active ingredient, liraglutide. Victoza, primarily used for managing type 2 diabetes, helps lower blood sugar levels by stimulating insulin release and inhibiting glucagon secretion. It is administered once daily via injection and is effective in improving glycemic control and reducing the risk of cardiovascular events in diabetic patients. In contrast, Saxenda is specifically approved for weight management in individuals with obesity or overweight conditions, regardless of whether they have diabetes. It operates by mimicking a natural hormone that regulates appetite and food intake, helping users achieve and maintain weight loss goals. Saxenda requires a higher dose than Victoza and is also administered via daily injection, but its focus is on managing obesity rather than diabetes control.
Despite their differences, both Saxenda and Victoza share a commonality in their active ingredient, liraglutide, which enhances the body’s response to insulin, improves glucose control, and offers potential cardiovascular benefits. This shared foundation underscores their efficacy in different therapeutic contexts: Victoza as a cornerstone for diabetes management and Saxenda as a tool for weight loss management. Understanding these distinctions is crucial for healthcare providers and patients alike in determining the most suitable treatment based on individual health needs and goals.
In terms of side effects, both medications may cause gastrointestinal issues such as nausea, diarrhea, and constipation, though these symptoms often diminish over time as the body adjusts to treatment. Additionally, Saxenda users may experience injection site reactions and potential thyroid changes, requiring monitoring during therapy. Conversely, Victoza may carry a risk of pancreatitis and should be used cautiously in patients with a history of this condition. Overall, while both Saxenda and Victoza offer significant therapeutic benefits, their distinct applications and potential side effects should be carefully considered in consultation with healthcare providers to optimize treatment outcomes.
Is Liraglutide Once a Week Injection for Type 2 Diabetes Good?
Liraglutide, administered once weekly, offers significant advantages for managing type 2 diabetes. This formulation provides convenience and improved adherence compared to daily medications, reducing the burden of frequent dosing for patients. By maintaining consistent levels of the medication throughout the week, it helps stabilize blood sugar levels effectively. This can lead to better glycemic control over time, lowering the risk of diabetes-related complications such as cardiovascular disease and kidney damage.
Moreover, the once-weekly injection of Liraglutide has demonstrated efficacy in promoting weight loss, which is particularly beneficial for individuals with type 2 diabetes who often struggle with obesity. This dual benefit of glucose control and weight management makes it a valuable treatment option, potentially enhancing overall quality of life for patients. Clinically, Liraglutide has shown to be well-tolerated with manageable side effects, further supporting its role as a favorable choice in diabetes management.
In conclusion, Liraglutide’s once-weekly injection offers a compelling option for individuals with type 2 diabetes seeking effective and convenient treatment. Its ability to improve both glycemic control and aid in weight loss underscores its significance in comprehensive diabetes care strategies, potentially leading to better long-term health outcomes for patients.
Victoza Dosage for Weight Loss
Victoza, known generically as liraglutide, is prescribed off-label at a higher dosage for weight loss compared to its standard use in managing type 2 diabetes. The typical starting dose for weight loss is 0.6 mg daily, gradually increasing to 3 mg daily over a period of weeks. This gradual titration helps patients adjust to the medication’s effects and minimize gastrointestinal side effects, such as nausea and diarrhea, which can occur during initial use.
The dosage escalation of Victoza for weight loss aims to maximize its efficacy in reducing appetite and promoting weight loss. Clinical studies have shown that patients often experience significant reductions in body weight when using liraglutide at higher doses, alongside improvements in metabolic parameters like blood sugar levels and cholesterol profiles.
Patients considering Victoza for weight loss should consult with their healthcare provider to determine the appropriate dosage and monitor for any potential side effects. Regular follow-up appointments are essential to assess progress and make adjustments to dosage or treatment plans as needed to achieve optimal weight management goals.
Liraglutide Cost
Liraglutide, marketed under brand names like Victoza and Saxenda, can be costly due to its status as a specialty medication. The price of Liraglutide varies depending on the dosage strength and whether it’s prescribed for diabetes management or weight loss. Patients often encounter higher costs for Liraglutide compared to traditional diabetes medications due to its unique mechanisms and additional benefits, such as weight loss support. Insurance coverage and pharmacy discounts may help mitigate some of these expenses, but out-of-pocket costs can still be significant for many individuals.
Despite its higher cost, Liraglutide’s effectiveness in managing both type 2 diabetes and aiding weight loss can justify its expense for those who benefit from its therapeutic effects. Healthcare providers may consider Liraglutide’s comprehensive benefits when determining its affordability and suitability for patients. In some cases, alternative medications or therapeutic approaches may be explored to balance clinical efficacy with financial considerations, ensuring that patients receive optimal care without undue financial strain.
Liraglutide Generic
Liraglutide, originally marketed under the brand name Victoza, is now also available as a generic medication. Generic Liraglutide offers a more affordable option for individuals managing type 2 diabetes and obesity, as it typically costs less than the brand-name version. This availability broadens access to effective treatment options, ensuring that more patients can benefit from its therapeutic effects without financial strain.
Generic medications undergo rigorous testing to ensure they are bioequivalent to their brand-name counterparts, meaning they have the same active ingredients and produce similar therapeutic outcomes. For patients using Liraglutide, switching to the generic version is generally safe and effective, provided they consult their healthcare provider to ensure continuity of care and monitoring. This transition to generic Liraglutide supports healthcare cost-efficiency initiatives and enhances affordability for both patients and healthcare systems.
Healthcare providers may recommend generic Liraglutide as part of a comprehensive treatment plan for managing type 2 diabetes and obesity. Its availability reinforces the importance of affordable access to essential medications, empowering patients to maintain their health through consistent and cost-effective treatment options. As with any medication change, patient education and adherence to prescribed regimens remain crucial to achieving optimal health outcomes with generic Liraglutide.
Liraglutide for Weight Loss in Non Diabetics
Liraglutide, originally developed as a treatment for type 2 diabetes, has also shown promising results as a weight loss medication for non-diabetic individuals. Its mechanism of action involves mimicking the effects of GLP-1, a hormone that regulates appetite and food intake. By binding to GLP-1 receptors in the brain, Liraglutide helps to reduce feelings of hunger and increase feelings of fullness, making it easier for non-diabetic users to adhere to reduced-calorie diets.
Clinical trials have demonstrated significant weight loss benefits with Liraglutide in non-diabetic populations. Studies indicate that patients using Liraglutide often achieve greater weight loss compared to those using placebo or other weight loss medications. This effectiveness has led to its approval for weight management in non-diabetic adults with a body mass index (BMI) above a certain threshold, typically 27 kg/m² or higher with comorbidities, or 30 kg/m² or higher without comorbidities.
Moreover, Liraglutide’s benefits extend beyond weight reduction alone. Users often experience improvements in metabolic health markers such as reduced waist circumference and improved blood pressure levels. These metabolic improvements underscore Liraglutide’s potential as a comprehensive treatment option for non-diabetic individuals seeking effective and sustainable weight loss solutions.
Liraglutide and Thyroid Cancer
Victoza liraglutide, a medication commonly used to treat type 2 diabetes and obesity, has been the subject of some scrutiny regarding its potential association with thyroid cancer. Several studies have explored this concern, particularly focusing on whether there is an increased risk of thyroid tumors or cancer development among users of victoza liraglutide. While initial animal studies suggested a possible link with victoza liraglutide, subsequent clinical trials and observational studies in humans have provided mixed results. Some studies have indicated a potential increased risk with victoza liraglutide, particularly in rodent models at high doses, but evidence in humans remains inconclusive and often conflicting.
In clinical practice, healthcare providers are cautious when prescribing victoza liraglutide, particularly in patients with a history of thyroid cancer or those at high risk. Regular monitoring of thyroid function and careful evaluation of risk factors are recommended to mitigate any potential concerns associated with victoza liraglutide. Despite these considerations, many healthcare professionals emphasize that the benefits of victoza liraglutide in managing diabetes and obesity generally outweigh the perceived risks associated with thyroid cancer, especially when victoza liraglutide is used judiciously and alongside appropriate medical supervision.
Overall, while the association between victoza liraglutide and thyroid cancer warrants ongoing research and vigilance, current evidence does not conclusively establish a causal relationship. Continued investigation and long-term studies are essential to further clarify the potential risks and benefits of victoza liraglutide, ensuring that healthcare decisions are informed by the most up-to-date scientific evidence available.
Liraglutide and Low Blood Sugar
Liraglutide, a medication commonly used to treat type 2 diabetes and obesity, has been the subject of some scrutiny regarding its potential association with thyroid cancer. Several studies have explored this concern, particularly focusing on whether there is an increased risk of thyroid tumors or cancer development among users. While initial animal studies suggested a possible link, subsequent clinical trials and observational studies in humans have provided mixed results. Some studies have indicated a potential increased risk, particularly in rodent models at high doses, but evidence in humans remains inconclusive and often conflicting.
In clinical practice, healthcare providers are cautious when prescribing victoza liraglutide, particularly in patients with a history of thyroid cancer or those at high risk. Regular monitoring of thyroid function and careful evaluation of risk factors are recommended to mitigate any potential concerns. Despite these considerations, many healthcare professionals emphasize that the benefits of victoza liraglutide in managing diabetes and obesity generally outweigh the perceived risks associated with thyroid cancer, especially when used judiciously and alongside appropriate medical supervision.
Overall, while the association between victoza liraglutide and thyroid cancer warrants ongoing research and vigilance, current evidence does not conclusively establish a causal relationship. Continued investigation and long-term studies are essential to further clarify the potential risks and benefits, ensuring that healthcare decisions are informed by the most up-to-date scientific evidence available.
FAQ
Is liraglutide the same as Ozempic?
No, liraglutide is not the same as Ozempic. Liraglutide is the active ingredient in Victoza and Saxenda, while Ozempic contains semaglutide. Both medications can affect blood sugar levels, and low blood sugar can be a concern. It’s important to monitor for signs of low blood sugar when using these medications. While liraglutide and Ozempic are used to manage different conditions, both can potentially lead to low blood sugar, especially if combined with other treatments.
Is liraglutide effective for weight loss?
Yes, liraglutide (sold under the brand Saxenda for weight loss) has been shown to be effective for weight loss in clinical studies. However, it is important to be aware of the potential for an allergic reaction. Allergic reactions can vary in severity, so monitoring for any signs of an allergic reaction is crucial when using liraglutide.
Why was Victoza taken off the market?
Victoza has not been taken off the market. It is still available and prescribed for diabetes management. However, it is important to note that some individuals may experience a serious allergic reaction when using this medication. If you have any concerns about a serious allergic reaction, consult your healthcare provider. Monitoring for any signs of a serious allergic reaction is crucial while taking Victoza.
What are the cons of liraglutide?
Some potential cons of liraglutide on a reduced calorie diet include gastrointestinal side effects such as nausea, vomiting, and diarrhea, as well as the need for daily injections (except for Saxenda, which is weekly). Additionally, individuals on a reduced calorie diet may experience these side effects more intensely. It’s important to monitor any adverse reactions while following a reduced calorie diet and using liraglutide.
What brand of liraglutide is used for weight loss?
Saxenda is the brand of liraglutide used for weight loss, and it’s important to be aware of serious allergic reactions that may occur with its use. Saxenda, containing liraglutide, is prescribed for weight management, but serious allergic reactions can potentially occur. It’s crucial to monitor for signs of serious allergic reactions when using Saxenda for weight loss.
Is Trulicity the same as liraglutide?
No, Trulicity contains dulaglutide, which is a different medication from liraglutide. If you missed dose, it’s important to consult your healthcare provider for guidance on when to take the next dose and how to manage any potential effects of missed dose.
What is the drug Victoza used for?
Victoza is used to treat type 2 diabetes by helping to lower blood sugar levels. It works by mimicking the action of a hormone called GLP-1 (glucagon-like peptide 1) that helps regulate blood sugar. Victoza is injected under the skin, typically in the abdomen, thigh, or upper arm area, once a day at any time, with or without food. It should not be injected into a vein or muscle.
Is Victoza an Ozempic?
No, Victoza is not Ozempic. They are different medications. It’s important to consult your doctor before switching between them, as the side effects and efficacy can vary significantly. Patients have reported experiencing severe pain when transitioning from Victoza to Ozempic due to the differences in formulation and dosage. It’s crucial to manage any severe pain with appropriate medical guidance.
Is Victoza a weight loss injection?
While Victoza is primarily used for diabetes management, it has also been prescribed off-label for weight loss. Some patients may experience trouble breathing when using Victoza, so it’s important to consult a healthcare provider if you encounter trouble breathing. In clinical trials, trouble breathing was reported in a small percentage of patients using Victoza for weight loss.
Is Victoza the same as Ozempic?
No, Victoza and Ozempic are different medications. Victoza, which contains liraglutide, and Ozempic, which contains semaglutide, have distinct formulations and uses. It’s essential to understand the side effects of Victoza and Ozempic before starting either medication.
How much weight do you lose on Victoza?
Weight loss with Victoza can vary, but clinical studies have shown significant weight loss in some patients. It’s important to be aware of the side effects of Victoza, as they can impact individual experiences with the medication. Victoza side effects may include gastrointestinal issues, such as nausea and diarrhea. Despite potential side effects of Victoza, many patients have found it effective in managing weight loss.
How much weight can I lose in a month with Saxenda?
On average, patients using Saxenda can lose around 5-10% of their body weight over 6 months to a year, which typically translates to gradual weight loss over several months. It’s important to discuss Saxenda with your healthcare provider to understand its potential benefits and risks. Working closely with your healthcare provider can help you achieve and maintain your weight loss goals effectively.
Which is better, Saxenda or Ozempic?
The choice between Saxenda (liraglutide) and Ozempic (semaglutide) depends on individual patient factors, including the specific health goals and medical history. Both can be effective but have different dosing schedules and potential side effects. It’s important to discuss these options with a healthcare provider to determine the most suitable treatment.
Is Saxenda a good weight loss drug?
Saxenda has been shown to be effective for weight loss in clinical trials and is approved for chronic weight management. Before starting Saxenda, consult your healthcare provider to discuss if it’s right for you. Your healthcare provider will assess your medical history and current health status to determine if Saxenda is suitable. Follow the guidance of your healthcare provider closely while using Saxenda.
What does liraglutide do to the body?
Liraglutide (and similar medications like semaglutide) mimics a hormone called GLP-1, which helps regulate blood sugar levels, insulin release, and can also help reduce appetite and promote weight loss. These medications are particularly beneficial for individuals with a personal or family history of diabetes or obesity-related conditions.
Is liraglutide good for weight loss?
Yes, liraglutide (Saxenda) is considered effective for weight loss in individuals who are overweight or obese, especially when there is a personal or family history of weight management challenges.
Is liraglutide the same as metformin?
No, liraglutide and metformin are different medications with different mechanisms of action. Metformin primarily works to lower blood sugar levels by decreasing glucose production in the liver and improving insulin sensitivity. Trouble swallowing liraglutide and metformin are different medications with different mechanisms of action. Metformin primarily works to lower blood sugar levels by decreasing glucose production in the liver and improving insulin sensitivity.
What kind of drug is liraglutide?
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. It mimics the action of GLP-1, a hormone that regulates insulin secretion and blood sugar levels. Some patients using liraglutide may experience trouble swallowing due to its effects on gastrointestinal function. This trouble swallowing typically resolves with continued use or dose adjustment. If you encounter trouble swallowing while taking liraglutide, consult your healthcare provider for guidance.
How much weight can you lose with liraglutide?
Weight loss with liraglutide can vary among individuals but clinical studies have shown significant weight loss in many patients. Medullary thyroid carcinoma can affect the body’s metabolism and sometimes lead to unintended weight changes. Medullary thyroid carcinoma patients may experience weight fluctuations due to the nature of the disease. However, liraglutide has shown promising results in promoting weight loss even in cases involving medullary thyroid carcinoma.
What does liraglutide do for weight loss?
Liraglutide helps with weight loss by reducing appetite, increasing feelings of fullness, and slowing digestion, which collectively leads to reduced calorie intake and weight loss. Liraglutide should not be administered in double or extra doses to avoid potential adverse effects. Double or extra doses of liraglutide can increase the risk of side effects and complications. Therefore, it is important to follow the prescribed dosage carefully and not exceed the recommended amount.
Where do you inject liraglutide?
Liraglutide (Saxenda) is injected subcutaneously (under the skin), typically in the abdomen, thigh, or upper arm. It is important to discuss with your healthcare provider if you have any history of kidney problems before starting this medication. Liraglutide has been associated with an increased risk of kidney problems in some patients, so monitoring kidney function regularly is recommended, especially if you have pre-existing kidney problems. Your healthcare provider will assess whether Saxenda is appropriate for you based on your individual health history, including any kidney problems.
What are the disadvantages of liraglutide?
Disadvantages may include gastrointestinal side effects (like nausea and diarrhea), the need for over the counter medicines injections, and cost considerations.
What is the most common side effect of liraglutide?
“Nausea is one of the most common side effects of liraglutide, particularly at the injection site. Some patients may experience tenderness or redness at the injection site after using liraglutide. It’s important to monitor for any signs of irritation at the injection site and consult a healthcare provider if symptoms persist.”
What are the risks of taking liraglutide?
Risks may include pancreatitis, thyroid tumors, and potential allergic reactions, although these weight related medical problems are rare.
How fast is weight loss on liraglutide?
Weight loss on liraglutide (Saxenda) varies, but noticeable weight loss typically occurs over several weeks to months of treatment. It’s important to monitor patients closely for any signs of end stage renal disease such as changes in urine output or swelling in the legs. Patients with end stage renal disease may experience slower weight loss due to impaired kidney function, so adjustments to treatment may be necessary in these cases.
Why is Victoza being recalled?
There is no indication that Victoza is being recalled due to weight related medical problems. It remains an approved medication for weight related medical problems and diabetes management. If you experience difficulty breathing while using Victoza, consult your healthcare provider immediately.
How quickly do you lose weight on Victoza?
Weight loss with Victoza can occur gradually over several months, with individual results varying. It’s important to note that Victoza should be used under medical supervision, as it may cause side effects such as nausea or heart attack weight loss with Victoza can occur gradually over several months, with individual results varying.
Do you lose more weight on Ozempic or Victoza?
Both medications can promote weight loss, but individual responses can vary. Clinical trials have shown both to be effective in heart attack weight management. However, it’s important to consult with your doctor to determine which option is best for you and to monitor any potential side effects.
Can non-diabetics take Victoza for weight loss?
Some healthcare providers may prescribe Victoza off-label for weight loss in pediatric patients, non-diabetic patients, but this use should be carefully monitored.
Can a doctor prescribe Victoza for weight loss?
Yes, doctors can prescribe Victoza off-label for weight loss, though it’s primarily indicated for diabetes management. Victoza is available in various dosage forms, and its off-label use for weight loss has been observed in clinical practice.
Is Victoza as good as Ozempic for weight loss?
Both Victoza and Ozempic have shown efficacy for weight loss, but the choice between them depends on individual patient factors and preferences. The availability of different dosage forms can also influence the decision.
Can my doctor prescribe Victoza for weight loss?
Yes, your doctor can prescribe Victoza off-label for weight loss if they deem it appropriate for your situation. Victoza has shown effectiveness in helping patients lose weight. It works by reducing appetite and helping control blood sugar levels. If you’re struggling to lose weight, Victoza may be an option to consider under medical supervision.
What is the Victoza pen used for?
The Victoza pen is used to administer liraglutide (Victoza) subcutaneously for the treatment of type 2 diabetes. Victoza has also been prescribed off-label to help patients lose weight by reducing appetite and slowing digestion.
How much weight did you lose on Victoza?
Individual weight loss with Victoza can vary widely. Results depend on factors such as diet, exercise, and overall health. It’s important to note that Victoza is not a guaranteed solution for weight loss. The effectiveness of Victoza in helping individuals lose weight can be influenced by various lifestyle choices and medical conditions. Therefore, consulting with a healthcare provider is crucial to determine if Victoza is suitable for losing weight and achieving your health goals.
What is better than Saxenda for weight loss?
Different weight loss medications may be more suitable depending on individual health conditions and responses. Consultation with a healthcare provider is recommended to determine the most effective approach to lose weight. It’s important to consider the right medication that fits your needs for losing weight, and consulting with a healthcare provider can provide valuable guidance in this process
Which is better Saxenda or Ozempic?
The choice between Saxenda (liraglutide) and Ozempic (semaglutide) depends on individual patient factors, including dosing preferences, side effect profiles, and other health considerations. Both medications are effective in helping to improve glycemic control. Saxenda and Ozempic are often chosen based on their respective dosing schedules and tolerability profiles, which play crucial roles in managing diabetes
Is Victoza as strong as Ozempic?
Both medications have similar mechanisms of action in adult patients, but individual responses can vary. Consult with a healthcare provider in adult patients to determine the most suitable option for adult patients.
How much weight can you lose on Saxenda in a month?
Weight loss with Saxenda, a prescription drug, varies, but gradual weight loss over several months is typical. Individual results can differ. Saxenda is a prescription drug that can help with weight loss by promoting a feeling of fullness and reducing appetite. It’s important to follow your healthcare provider’s guidance when using prescription drugs like Saxenda for weight management.
What is the best once a week injection for type 2 diabetes?
Ozempic (semaglutide) is a once-weekly injection that is highly effective for type 2 diabetes management. Individuals experiencing gallbladder problems may find Ozempic beneficial due to its weekly dosing schedule, which can simplify treatment adherence.
What is the new injection for type 2 diabetes?
Trulicity (dulaglutide) and Ozempic (semaglutide) are relatively newer once-weekly injections for type 2 diabetes. These medications often require dosage adjustment to ensure effective management of blood sugar levels over time.
What injection is given once a week for diabetic?
Ozempic (semaglutide) and Trulicity (dulaglutide) are once-weekly injections commonly used for type 2 diabetes, along with several other drugs. These medications are typically administered in the upper stomach area to ensure effective absorption and consistent release throughout the week. It’s important to monitor for potential kidney damage, especially in patients with pre-existing conditions.
Is there a diabetic shot once a week for weight loss?
Yes, medications like Ozempic (semaglutide) and possibly other drugs, prescribed off-label, are used for both diabetes management and weight loss. It’s important to consult a medication guide to understand their proper use and potential side effects. These medications, often accompanied by a medication guide, can provide significant benefits in managing diabetes and supporting weight loss goals. Always follow the recommendations in the medication guide provided by your healthcare provider or pharmacist to ensure safe and effective use.
How much does liraglutide cost without insurance?
The cost of liraglutide (Saxenda) without insurance can vary, but it is generally expensive. Prices may differ depending on pharmacy and location. It’s important to consider these costs, especially if managing a condition like kidney failure or problems digesting food, where expenses can significantly impact financial planning. For those with problems digesting food, medication costs can add an additional burden. Overall, understanding the financial implications is crucial, particularly for individuals with problems digesting food and other chronic health issues.
Is liraglutide covered by insurance?
Coverage for liraglutide (Saxenda) by insurance can vary depending on physical activity and breast milk. It’s advisable to check with your insurance provider for specific dosage adjustments and coverage details regarding breast milk. Ensure you are aware of how breast milk may affect your eligibility and coverage.
How much is a 30-day supply of Saxenda?
The cost of Saxenda for a 30-day supply can vary widely, but it is generally expensive without insurance coverage, especially considering the need for dosage adjustments over time. The prescription label for Saxenda should be checked for specific dosage instructions. Without proper insurance, the prescription label may indicate a high cost, especially as dosage adjustments are needed. Always review the prescription label for accurate information on usage and costs.
How to get Saxenda for $25 a month?
Some patients may qualify for manufacturer discounts or patient assistance programs that can lower the cost of Saxenda. Check with your doctor or the manufacturer for details, including the expiration date. It’s also important to discuss any potential side effects, such as skin rash, with your healthcare provider to ensure the medication is suitable for you and to be aware of the expiration date. Additionally, always check the expiration date before using the medication to ensure its effectiveness.
When will liraglutide generic be available?
Generic versions of liraglutide (Saxenda) may become available after patent expiration, but specific timelines can vary. It’s important to monitor updates regarding generic availability, especially if cost is a concern. Additionally, consulting with healthcare providers can provide insights into potential benefits and considerations, such as skin rash, associated with switching to generic alternatives.
How much will generic Victoza cost?
The cost of generic Victoza will depend on several factors, including market competition and production costs, once it becomes available. The lower dose formulation may offer a different pricing structure. Other medications, particularly those available in a lower dose, could also influence pricing dynamics in the pharmaceutical market. As more lower dose options become available, it could further impact the overall cost structure.
Is there a generic for Saxenda?
Generic versions of Saxenda (liraglutide) may become available in the future, but as of now, it is still under patent protection. Other medicines Generic versions of Saxenda (liraglutide) may become available in the future, but as of now, it is still under patent protection. Generic versions of Saxenda (liraglutide) may become available in the future, but as of now, it is still under patent protection.
Can I take Victoza if I don't have diabetes?
Some healthcare providers may prescribe Victoza off-label for weight loss in non-diabetic patients, but this should be done under medical supervision. It’s important to note that Victoza is primarily indicated for managing type 2 diabetes and should not be used as a substitute for other medicines or weight loss treatments without proper medical guidance. Using Victoza for weight loss in non-diabetic individuals may interact with other medicines, especially in patients with conditions such as multiple endocrine neoplasia, and can pose risks if not monitored closely by a healthcare provider. In particular, those with a history of multiple endocrine neoplasia should be cautious, as the interaction between Victoza and other medicines can be complex. Therefore, it is essential for healthcare providers to consider the presence of multiple endocrine neoplasia when prescribing Victoza for weight loss.
What is the best injection for weight loss without diabetes?
Medications like Saxenda (liraglutide) and possibly other medicines, prescribed off-label, are used for weight loss without diabetes. Physical activity is often recommended alongside these medications for better results. Incorporating physical activity into daily routines can enhance the effectiveness of weight loss medications. Moreover, combining medications with physical activity can lead to more sustainable weight loss outcomes.
How effective is semaglutide for weight loss in non-diabetics?
Semaglutide (Ozempic) has shown effectiveness for weight loss in clinical trials, including in non-diabetic individuals with upper stomach concerns. This is particularly relevant for those who might be managing other health issues such as a thyroid tumor. Research continues to explore the benefits of semaglutide for weight management in various populations, including those with thyroid tumor concerns. The potential impact of semaglutide on weight loss is significant, even for individuals dealing with a thyroid tumor.
Blog

Exploring the Powerful Effects of Liraglutide on Overall Health
Introduction :
In recent years, a breakthrough medication called liraglutide has emerged as a potent tool in the realm of healthcare. Initially developed as a treatment for type 2 diabetes, liraglutide has demonstrated remarkable effects on overall health beyond glycemic control. This blog post aims to explore the powerful effects of liraglutide on various aspects of health and shed light on its potential benefits for individuals seeking to improve their overall well-being.
Enhanced Glycemic Control and Weight Management :
Liraglutide belongs to a class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Its primary function is to stimulate the release of insulin and reduce the secretion of glucagon, leading to improved glycemic control in individuals with type 2 diabetes. However, liraglutide has also demonstrated notable effects on weight management. Clinical studies have shown that liraglutide promotes weight loss by suppressing appetite, increasing feelings of fullness, and reducing calorie intake. This dual effect of liraglutide on glycemic control and weight management is particularly beneficial for individuals with type 2 diabetes who struggle with obesity, as it helps address two critical health concerns simultaneously.
Cardiovascular Health Benefits :
Beyond its effects on blood sugar and weight, liraglutide has been found to have significant cardiovascular health benefits. The LEADER trial, a landmark study involving over 9,000 participants with type 2 diabetes and high cardiovascular risk, revealed that liraglutide reduced the risk of major adverse cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. These findings were groundbreaking, as they highlighted the potential of liraglutide to not only manage diabetes but also protect against cardiovascular complications, a leading cause of mortality in diabetic patients.
Moreover, liraglutide has shown positive effects on blood pressure and lipid profiles. Studies have demonstrated reductions in systolic blood pressure and improvements in lipid parameters, such as decreased levels of total cholesterol and triglycerides, and increased levels of high-density lipoprotein (HDL) cholesterol. These cardiovascular benefits further contribute to the overall health-promoting effects of liraglutide.
Additional Health Benefits :
In addition to glycemic control, weight management, and cardiovascular health benefits, liraglutide has shown potential in other areas of health. Research indicates that liraglutide may have positive effects on liver health by reducing liver fat accumulation and improving liver function in individuals with non-alcoholic fatty liver disease (NAFLD). Furthermore, preliminary studies suggest that liraglutide may have neuroprotective effects, potentially contributing to its use in the treatment of neurodegenerative disorders such as Alzheimer’s disease.
Conclusion :
Liraglutide, originally developed as a treatment for type 2 diabetes, has proven to be a remarkable medication with powerful effects on overall health. Its ability to improve glycemic control, promote weight loss, and reduce cardiovascular risks has transformed the lives of many individuals. Additionally, liraglutide shows promise in areas beyond its primary indications, such as liver health and neuroprotection. However, it’s important to note that liraglutide is a prescription medication, and its use should be under the guidance of a healthcare professional. For individuals seeking comprehensive health benefits, liraglutide presents a promising option that warrants further exploration and discussion with a medical provider.

The Remarkable Impact of Liraglutide on Diabetes and Beyond
Introduction :
In the world of healthcare, few medications have had as remarkable an impact as liraglutide. Originally developed to manage type 2 diabetes, this innovative medication has proven to be a game-changer, revolutionizing the treatment landscape. However, the influence of liraglutide extends far beyond diabetes management. This blog post aims to explore the remarkable impact of liraglutide, highlighting its effects on diabetes control and its potential applications in other areas of health.
Enhanced Glycemic Control :
Liraglutide, classified as a glucagon-like peptide-1 receptor agonist (GLP-1 RA), has gained recognition for its exceptional ability to improve glycemic control in individuals with type 2 diabetes. By stimulating the release of insulin and inhibiting the secretion of glucagon, liraglutide helps regulate blood sugar levels. Clinical trials have demonstrated its effectiveness in reducing HbA1c levels and improving overall glycemic control, providing patients with a powerful tool to manage their diabetes.
Weight Management :
Beyond its impact on blood glucose levels, liraglutide has proven to be a valuable asset in weight management. Obesity is a prevalent issue among individuals with type 2 diabetes, and weight loss plays a crucial role in improving overall health outcomes. Liraglutide aids weight management by reducing appetite, promoting a feeling of fullness, and decreasing calorie intake. Studies have shown that patients treated with liraglutide experience significant weight loss and improvements in body mass index (BMI), making it an appealing option for individuals struggling with both diabetes and obesity.
Cardiovascular Benefits :
One of the most significant breakthroughs associated with liraglutide is its profound impact on cardiovascular health. The landmark LEADER trial demonstrated that liraglutide not only effectively manages diabetes but also significantly reduces the risk of major adverse cardiovascular events in individuals with type 2 diabetes and high cardiovascular risk. Participants in the trial experienced lower rates of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke when compared to the control group. This discovery has transformed the management of diabetes, as liraglutide offers dual benefits by addressing glycemic control and cardiovascular health simultaneously.
Additional Health Benefits :
The benefits of liraglutide extend beyond its primary indications. Research suggests that liraglutide may have a positive impact on other health conditions. Studies have indicated that liraglutide could be effective in reducing liver fat and improving liver function in individuals with non-alcoholic fatty liver disease (NAFLD). Additionally, there is growing evidence suggesting that liraglutide may have neuroprotective properties, potentially making it a valuable asset in the treatment of neurodegenerative disorders such as Alzheimer’s disease.
Conclusion :
Liraglutide has made a remarkable impact on diabetes management and has far-reaching implications for overall health. Its ability to enhance glycemic control, aid in weight management, and reduce the risk of cardiovascular events has transformed the lives of countless individuals with type 2 diabetes. Furthermore, the potential benefits of liraglutide extend beyond diabetes, with promising findings in liver health and neuroprotection. However, it’s essential to consult with a healthcare professional before considering liraglutide or any other medication. As we continue to uncover the full extent of liraglutide’s impact, its revolutionary properties hold great promise for improving the health and well-being of individuals worldwide.

Heart Health Revolution: How Liraglutide Lowers the Risk of Heart Disease
Introduction :
Heart disease is a leading cause of mortality worldwide, and its prevention and management are critical for preserving health and well-being. In recent years, a revolutionary medication called liraglutide has emerged as a game-changer in reducing the risk of heart disease. Originally developed for diabetes management, liraglutide has demonstrated remarkable effects on cardiovascular health. This blog post aims to delve into the heart health revolution brought about by liraglutide, highlighting its mechanisms, clinical evidence, and potential implications in combating heart disease.
Understanding Liraglutide and its Mechanisms :
Liraglutide belongs to a class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Initially developed to manage type 2 diabetes, liraglutide works by stimulating the release of insulin, inhibiting glucagon secretion, and slowing down the emptying of the stomach. These mechanisms help regulate blood sugar levels and improve glycemic control. However, it soon became evident that liraglutide had additional benefits beyond diabetes management, particularly in relation to heart health.
Clinical Evidence of Cardiovascular Benefits :
Landmark clinical trials, such as the LEADER trial, have provided substantial evidence of liraglutide’s cardiovascular benefits. The LEADER trial involved over 9,000 participants with type 2 diabetes and high cardiovascular risk. It demonstrated that liraglutide not only effectively managed diabetes but also significantly reduced the risk of major adverse cardiovascular events (MACE). Participants receiving liraglutide experienced a remarkable 13% reduction in the risk of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke compared to the control group.
The cardiovascular benefits of liraglutide extend beyond MACE reduction. Studies have shown that liraglutide also reduces the risk of heart failure hospitalizations, improves left ventricular function, and stabilizes arterial plaques. These findings are significant, as heart failure and plaque instability are major contributors to heart disease-related morbidity and mortality.
Mechanisms Underlying Cardiovascular Protection :
The mechanisms by which liraglutide exerts its cardiovascular protection are still being explored. However, several hypotheses have been proposed. Liraglutide may reduce inflammation, oxidative stress, and endothelial dysfunction, all of which play crucial roles in the development and progression of cardiovascular disease. Additionally, liraglutide may have direct effects on the heart, such as improving myocardial glucose utilization and enhancing cardiac function.
Implications for Heart Disease Prevention and Management :
The profound cardiovascular benefits of liraglutide have significant implications for heart disease prevention and management. Individuals with type 2 diabetes, who are at an increased risk of cardiovascular complications, may benefit from liraglutide as part of their treatment regimen. Liraglutide’s ability to simultaneously improve glycemic control and reduce cardiovascular risk makes it an attractive option for these patients.
Moreover, the potential use of liraglutide may extend beyond diabetes. Research is underway to explore its efficacy in individuals without diabetes but at high cardiovascular risk. Early studies suggest that liraglutide may have a role in preventing heart disease in this population as well.
Conclusion :
Liraglutide has initiated a heart health revolution, demonstrating remarkable effects in reducing the risk of heart disease. Its cardiovascular benefits, beyond its primary role in diabetes management, have transformed the treatment landscape and provided new hope for individuals at risk of cardiovascular complications. As we continue to unravel the mechanisms underlying liraglutide’s cardiovascular protection, further advancements in heart disease prevention and management are expected. However, it is important to consult with a healthcare professional to determine the suitability of liraglutide for individual circumstances. The heart health revolution fueled by liraglutide represents a significant stride forward in safeguarding cardiovascular well-being.

Beyond Blood Sugar Control: Liraglutide’s Cardiovascular Benefits
Introduction :
Liraglutide, a medication initially developed for managing blood sugar levels in individuals with type 2 diabetes, has emerged as a remarkable breakthrough in cardiovascular health. While its primary purpose is glycemic control, liraglutide has demonstrated impressive cardiovascular benefits that extend far beyond its original indications. This blog post aims to explore the cardiovascular advantages of liraglutide, shedding light on its mechanisms, clinical evidence, and potential implications in preventing and managing heart disease.
Understanding Liraglutide and Its Mechanisms :
Liraglutide belongs to the class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). It functions by stimulating insulin secretion, suppressing glucagon release, and slowing down gastric emptying. These actions are primarily aimed at regulating blood sugar levels in individuals with type 2 diabetes. However, it became evident that liraglutide had additional cardiovascular benefits beyond its role in glycemic control.
Clinical Evidence of Cardiovascular Benefits :
Several large-scale clinical trials, including the LEADER trial, have provided compelling evidence of liraglutide’s cardiovascular advantages. The LEADER trial, involving over 9,000 participants with type 2 diabetes and high cardiovascular risk, demonstrated that liraglutide not only effectively managed diabetes but also significantly reduced the risk of major adverse cardiovascular events (MACE). The participants receiving liraglutide experienced a remarkable 13% reduction in the risk of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke compared to the control group.
Further studies have reinforced these findings, revealing that liraglutide decreases the risk of heart failure hospitalizations and improves left ventricular function. These outcomes are crucial as heart failure is a leading cause of morbidity and mortality in individuals with cardiovascular disease.
Mechanisms Underlying Cardiovascular Protection :
The mechanisms underlying liraglutide’s cardiovascular protection are multifaceted and continue to be studied. One proposed mechanism is its ability to reduce inflammation, oxidative stress, and endothelial dysfunction—key factors contributing to the development and progression of cardiovascular disease. Additionally, liraglutide may have direct effects on the heart, such as improving myocardial glucose utilization and enhancing cardiac function.
Implications for Heart Disease Prevention and Management :
The profound cardiovascular benefits of liraglutide have significant implications for heart disease prevention and management. Individuals with type 2 diabetes, who are at an increased risk of cardiovascular complications, may benefit from liraglutide as part of their treatment regimen. Liraglutide’s dual action of improving glycemic control and reducing cardiovascular risk makes it an attractive option for these patients.
Moreover, recent studies have explored the potential of liraglutide in individuals without diabetes but at high cardiovascular risk. These investigations have shown promising results, suggesting that liraglutide may play a role in preventing heart disease in this population as well.
Conclusion :
Liraglutide’s cardiovascular benefits have gone beyond its primary purpose of blood sugar control. The remarkable impact it has shown in reducing the risk of major adverse cardiovascular events and heart failure hospitalizations has transformed the management of cardiovascular disease in individuals with type 2 diabetes. Additionally, ongoing research suggests potential applications for liraglutide in preventing heart disease in individuals without diabetes but at high cardiovascular risk. As we delve deeper into the mechanisms underlying liraglutide’s cardiovascular protection, its role in preventing and managing heart disease continues to expand. However, it is essential to consult with a healthcare professional to determine the suitability of liraglutide in individual cases. Liraglutide’s cardiovascular benefits have opened new possibilities for enhancing heart health and well-being.

Unraveling the Science Behind Liraglutide: How It Works in the Body
Liraglutide, a medication that has gained recognition for its efficacy in managing type 2 diabetes and promoting weight loss, operates through a complex set of mechanisms within the body. Understanding how liraglutide works is crucial in appreciating its remarkable therapeutic effects. Let’s delve into the science behind liraglutide and explore its actions in the body.
Liraglutide belongs to the class of medications called glucagon-like peptide-1 receptor agonists (GLP-1 RAs). It mimics the action of GLP-1, a hormone naturally produced by the intestines in response to food intake. GLP-1 plays a significant role in regulating blood sugar levels and appetite.
Once injected, liraglutide binds to GLP-1 receptors in various tissues, including the pancreas, liver, stomach, and brain. Its primary mechanism of action involves stimulating insulin secretion from the pancreatic beta cells. Increased insulin levels help lower blood glucose levels, promoting glycemic control.
Liraglutide also suppresses the release of glucagon, a hormone that elevates blood sugar levels. By inhibiting glucagon secretion, liraglutide helps prevent excessive glucose production by the liver, contributing to improved glycemic control.
Additionally, liraglutide slows down gastric emptying, which means food passes more slowly through the stomach. This effect prolongs the feeling of fullness, reduces appetite, and contributes to weight loss.
Furthermore, liraglutide acts on the hypothalamus in the brain, which plays a vital role in regulating appetite and satiety. By binding to GLP-1 receptors in the hypothalamus, liraglutide helps reduce food intake and promotes a sense of fullness.
The multifaceted actions of liraglutide extend beyond its primary indications. It has demonstrated cardiovascular benefits, such as reducing the risk of major adverse cardiovascular events and improving heart function. The exact mechanisms behind these effects are still being investigated, but it is believed that liraglutide reduces inflammation, oxidative stress, and endothelial dysfunction, all of which play critical roles in cardiovascular health.
Conclusion:
Liraglutide exerts its therapeutic effects through a complex interplay of mechanisms. By stimulating insulin secretion, inhibiting glucagon release, slowing down gastric emptying, and acting on the brain’s appetite centers, liraglutide effectively manages blood sugar levels and promotes weight loss. Its impact on cardiovascular health further adds to its versatility as a medication. As research continues, further insights into the intricate workings of liraglutide in the body may uncover additional benefits and potential applications in healthcare.

Weight Loss and Beyond the Role of Liraglutide in Body Transformation
Liraglutide, a medication initially developed to manage type 2 diabetes, has gained attention for its transformative effects on weight loss. However, its impact extends far beyond shedding pounds. Liraglutide has emerged as a powerful tool in body transformation, offering individuals a comprehensive approach to achieving their health and wellness goals. Let’s explore the role of liraglutide in body transformation and the benefits it brings.
Liraglutide belongs to the class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). It operates by stimulating the release of insulin, inhibiting glucagon secretion, and slowing down gastric emptying. These actions regulate blood sugar levels in individuals with type 2 diabetes. But one notable effect of liraglutide is its ability to promote significant weight loss.
Studies have shown that liraglutide suppresses appetite, increases feelings of fullness, and reduces calorie intake. This appetite-suppressing effect is crucial in achieving weight loss and body transformation goals. By reducing hunger and controlling portion sizes, liraglutide assists individuals in making sustainable lifestyle changes and adopting healthier eating habits.
Furthermore, liraglutide’s impact on weight loss is not limited to its appetite-suppressing properties. It has also been shown to increase energy expenditure and promote fat burning. These metabolic effects contribute to the overall body transformation process, helping individuals shed excess weight and achieve a healthier body composition.
Beyond weight loss, liraglutide has demonstrated additional benefits in body transformation. It improves glycemic control, reduces the risk of cardiovascular events, and enhances overall well-being. The cardiovascular benefits are particularly noteworthy, as they contribute to the reduction of heart disease risk factors associated with obesity.
It’s important to note that liraglutide is not a magic solution on its own. It should be used as part of a comprehensive approach to body transformation, including a healthy diet, regular physical activity, and lifestyle changes. Consulting with a healthcare professional is crucial to determine the suitability of liraglutide and to receive guidance throughout the transformation process.
Conclusion:
Liraglutide plays a significant role in body transformation by promoting weight loss, improving glycemic control, and reducing cardiovascular risk factors. Its multifaceted effects make it a valuable tool for achieving comprehensive and sustainable body transformation goals. However, it’s essential to approach liraglutide as part of a holistic lifestyle change rather than relying solely on medication. By combining the power of liraglutide with healthy habits, individuals can embark on a transformative journey toward improved health and well-being.
Reference
Ostawal A, Mocevic E, Kragh N, Xu W. Clinical Effectiveness of Liraglutide in Type 2 Diabetes Treatment in the Real-World Setting: A Systematic Literature Review. Diabetes Ther. 2016;7(3):411-438. doi:10.1007/s13300-016-0180-0.
Clinical Effectiveness of Liraglutide in Type 2 Diabetes Treatment in the Real-World Setting: A Systematic Literature Review
In this systematic literature review, the real-world clinical effectiveness of liraglutide for treating type 2 diabetes mellitus (T2DM) was investigated. Analysis of 124 publications, including 43 full-text articles, revealed that liraglutide significantly reduced glycated hemoglobin (HbA1c) levels and was well-tolerated in T2DM patients, with a low risk of hypoglycemia. Moreover, liraglutide led to substantial weight loss and maintained its glycemic and weight benefits for at least 12 months. These findings from real-world observational studies align with results from clinical trials, emphasizing the effectiveness of liraglutide in managing T2DM.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5014786/.
Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T; Ellipse Trial Investigators. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med. 2019 Aug 15;381(7):637-646. doi: 10.1056/NEJMoa1903822. Epub 2019 Apr 28. PMID: 31034184.
Liraglutide in Children and Adolescents with Type 2 Diabetes
This study aimed to investigate the safety and effectiveness of adding liraglutide to metformin (with or without basal insulin) for the treatment of type 2 diabetes in children and adolescents aged 10 to less than 17 years. The results indicated that liraglutide led to a significant improvement in glycemic control compared to a placebo, as evidenced by a decrease in glycated hemoglobin levels over 52 weeks. However, this improvement was accompanied by a higher incidence of gastrointestinal adverse events. These findings suggest that liraglutide may offer benefits in managing type 2 diabetes in young patients, but it may come with certain side effects.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJMoa1903822?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Dimitrios P, Michael D, Vasilios K, Konstantinos S, Konstantinos I, Ioanna Z, Konstantinos P, Spyridon B, Asterios K. Liraglutide as Adjunct to Insulin Treatment in Patients with Type 1 Diabetes: A Systematic Review and Meta-analysis. Curr Diabetes Rev. 2020;16(4):313-326. doi: 10.2174/1573399815666190614141918. PMID: 31203802.
Liraglutide as Adjunct to Insulin Treatment in Patients with Type 1 Diabetes: A Systematic Review and Meta-analysis
In a systematic review and meta-analysis, several randomized controlled trials (RCTs) assessing liraglutide’s use in Type 1 Diabetes (T1D) were evaluated. The review included five trials with 2,445 participants and found that liraglutide, particularly at a dose of 1.8 mg, led to modest reductions in HbA1c levels and significant weight loss of up to 4.87 kg. It also reduced daily insulin requirements, primarily in bolus insulin. While there was a non-significant decrease in severe hypoglycemia risk, liraglutide was associated with increased gastrointestinal adverse events, such as nausea and vomiting. Additionally, a significant increase in heart rate was observed. No links to diabetic ketoacidosis or malignancies were identified, suggesting that liraglutide may serve as an adjunct to insulin in T1D, improving glycemic control and reducing insulin requirements while inducing weight loss.
You can read the abstract of the article at https://www.eurekaselect.com/article/98812.
Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther. 2009 Nov;31(11):2472-88. doi: 10.1016/j.clinthera.2009.11.034. PMID: 20109994.
A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus
Liraglutide, a once-daily subcutaneous injection treatment for type 2 diabetes mellitus (DM), has been evaluated in Phase III clinical trials. The trials demonstrated consistent reductions in glycosylated hemoglobin (HbA(1c)), reductions in fasting and postprandial plasma glucose levels, improved beta-cell function, and lowered systolic blood pressure. Liraglutide was associated with weight loss and proved well-tolerated, with mild and transient nausea as the most common adverse event. Overall, liraglutide is considered an effective and promising treatment for type 2 DM, whether used as monotherapy or in combination with oral antidiabetic drugs.
You can read the full article at https://www.clinicaltherapeutics.com/article/S0149-2918(09)00434-2/pdf.
Vilsboll T. Liraglutide: a new treatment for type 2 diabetes. Drugs Today (Barc). 2009 Feb;45(2):101-13. doi: 10.1358/dot.2009.45.2.1336104. PMID: 19343230.
Liraglutide: a new treatment for type 2 diabetes
Liraglutide, a novel glucagon-like peptide-1 (GLP-1) receptor agonist, provides sustained pharmacokinetics due to specific modifications, enabling once-daily dosing. It leverages the incretin effect to stimulate insulin secretion in a glucose-dependent manner. Clinical trials in the LEAD (Liraglutide Effect and Action Diabetes) program demonstrated improved glycemic control, helping many patients achieve hemoglobin A1c (HbA1c) targets and improving associated type 2 diabetes morbidities, such as weight loss, reduced blood pressure, and enhanced beta-cell function. Liraglutide is well-tolerated, with mild nausea being the primary side effect, and it does not induce hypoglycemia. This treatment offers an alternative for managing blood glucose levels in patients with type 2 diabetes, particularly those with hypertension and overweight, with anticipated approval by regulatory agencies.
You can read the abstract of the article at https://access.portico.org/Portico/auView?auId=ark:%2F27927%2Fpjbf7dcvhkj.
Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow L, Knop FK, Madsbad S, Andersen HU. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016 Mar;4(3):221-232. doi: 10.1016/S2213-8587(15)00436-2. Epub 2015 Dec 3. PMID: 26656289.
Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial
In a double-blind, placebo-controlled trial on overweight adults with type 1 diabetes, liraglutide, a GLP-1 receptor agonist, was added to insulin therapy. Results showed that the change in HbA1c from baseline did not significantly differ between liraglutide and placebo groups. However, liraglutide reduced hypoglycemic events, insulin dose, and body weight, while increasing heart rate. The study also reported more frequent gastrointestinal side effects with liraglutide, including nausea, dyspepsia, diarrhea, decreased appetite, and vomiting. Overall, liraglutide provided some benefits but didn’t lead to a substantial change in HbA1c in this population.
You can read the abstract of the article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(15)00436-2/fulltext.
Varanasi A, Bellini N, Rawal D, Vora M, Makdissi A, Dhindsa S, Chaudhuri A, Dandona P. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011 Jul;165(1):77-84. doi: 10.1530/EJE-11-0330. Epub 2011 Jun 6. PMID: 21646283.
Liraglutide as additional treatment for type 1 diabetes
This study aimed to assess the impact of adding liraglutide to insulin treatment in patients with type 1 diabetes. Fourteen patients with well-controlled type 1 diabetes were treated with liraglutide for one week, and eight of them continued the treatment for 24 weeks. After one week, there were significant improvements in fasting and weekly glucose levels, reductions in glycemic variability, and decreased insulin doses. Patients who continued liraglutide treatment for 24 weeks also experienced a significant decrease in HbA1c and a weight loss of 4.5 kg. These findings suggest that liraglutide can be an additional approach to enhance glycemic control in type 1 diabetes while promoting weight loss.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/165/1/77/6676798?redirectedFrom=fulltext&login=false.
Yin J, Han M, Li L, Li Y, Liu Z, Yang J, Liu Y. To Assess Liraglutide’s Therapeutic Effect in Patients with Type 2 Diabetes Mellitus Using Flash Glucose Monitoring System. Diabetes Metab Syndr Obes. 2021 Oct 29;14:4399-4407. doi: 10.2147/DMSO.S331833. PMID: 34744445; PMCID: PMC8565899.
To Assess Liraglutide’s Therapeutic Effect in Patients with Type 2 Diabetes Mellitus Using Flash Glucose Monitoring System
This study aimed to assess the impact of liraglutide, a glucagon-like peptide-1 receptor agonist, on blood glucose management, β-cell function, and insulin resistance in patients with type 2 diabetes mellitus. Thirty-three patients, who were initially on metformin monotherapy, received liraglutide as an add-on treatment for three months. Using the flash glucose monitoring system (FGMS), the study found that liraglutide treatment significantly improved glucose variability, glucose levels at various time periods, glycosylated hemoglobin A1c, and parameters related to β-cell function and insulin resistance. Notably, liraglutide achieved these improvements without increasing the incidence of hypoglycemia, demonstrating its effectiveness in enhancing glycemic control and related factors in type 2 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8565899/.
Kesavadev J, Shankar A, Gopalakrishnan G, Jothydev S. Efficacy and safety of liraglutide therapy in 195 Indian patients with type 2 diabetes in real world setting. Diabetes Metab Syndr. 2015 Jan-Mar;9(1):30-3. doi: 10.1016/j.dsx.2014.04.034. Epub 2014 May 22. Erratum in: Diabetes Metab Syndr. 2015 Jul-Sep;9(3):200. PMID: 25605673.
Efficacy and safety of liraglutide therapy in 195 Indian patients with type 2 diabetes in real world setting
This prospective, open-label, single-center observational study aimed to assess the efficacy and safety of liraglutide in Indian patients with type 2 diabetes mellitus (T2DM) in a real-world setting. Over 24 weeks, 195 subjects with T2DM received liraglutide in addition to their existing antidiabetic therapy. The study found significant improvements in glycemic parameters, including a decrease in mean fasting plasma glucose (FPG) from 163.81 mg/dL to 111.6 (p<0.001) and a reduction in HbA1c from 8.14% to 6.96% (p=0.006) at 24 weeks. Additionally, liraglutide treatment resulted in clinically significant reductions in weight, blood pressure, and serum cholesterol, and was well-tolerated, with mild to moderate adverse events reported in 11.28% of subjects.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1871402114000460?via%3Dihub.
Mikhail N. Cardiovascular Effects of Liraglutide. Curr Hypertens Rev. 2019;15(1):64-69. doi: 10.2174/1573402114666180507152620. PMID: 29737256.
Cardiovascular Effects of Liraglutide
Liraglutide, a GLP-1 agonist used for type 2 diabetes and obesity treatment, has been extensively studied for its cardiovascular effects. The LEADER trial, involving 9,340 patients with advanced type 2 diabetes and high cardiovascular risk, demonstrated that liraglutide reduced the composite primary outcome of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. It also significantly lowered cardiovascular and all-cause mortality. However, liraglutide may not be suitable for patients with severe heart failure due to its potential heart rate increase. While it appears beneficial for high-risk cardiovascular patients, more research is required to assess its long-term effects and suitability for low-risk type 2 diabetes patients.
You can read the full article at https://www.eurekaselect.com/article/90235.
Howell R, Wright AM, Clements JN. Clinical potential of liraglutide in cardiovascular risk reduction in patients with type 2 diabetes: evidence to date. Diabetes Metab Syndr Obes. 2019;12:505-512. Published 2019 Apr 17. doi:10.2147/DMSO.S174568.
Clinical potential of liraglutide in cardiovascular risk reduction in patients with type 2 diabetes: evidence to date
Metformin is the primary treatment for type 2 diabetes. If glycemic control is not achieved after 3 months, liraglutide becomes a valuable second-line option, especially for those at high risk of cardiovascular disease. It can also be used as an add-on therapy for individuals with established cardiovascular disease. Liraglutide offers benefits such as minimal hypoglycemic risk and weight loss. This article summarizes extensive cardiovascular evidence for liraglutide, showing its potential to control blood sugar while reducing the risk of cardiovascular events like heart disease, stroke, and heart failure hospitalization.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31118715/.
Bizino MB, Jazet IM, Westenberg JJM, van Eyk HJ, Paiman EHM, Smit JWA, Lamb HJ. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol. 2019 Apr 30;18(1):55. doi: 10.1186/s12933-019-0857-6. Erratum in: Cardiovasc Diabetol. 2019 Aug 9;18(1):101. PMID: 31039778; PMCID: PMC6492440.
Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial
Liraglutide, an antidiabetic agent, is evaluated in this study for its potential to improve diabetic cardiomyopathy in patients with type 2 diabetes (DM2) without cardiovascular disease. In a 26-week double-blind trial, liraglutide demonstrated a significant reduction in early left ventricular diastolic filling and filling pressure, thus unloading the left ventricle. Systolic function saw a decrease, remaining within the normal range. Further research is needed to determine if liraglutide’s effect on left ventricular unloading can delay the progression of diabetic cardiomyopathy to symptomatic stages.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6492440/.
Duan CM, Wan TF, Wang Y, Yang QW. Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine (Baltimore). 2019 Nov;98(46):e17860. doi: 10.1097/MD.0000000000017860. PMID: 31725627; PMCID: PMC6867782.
Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: A systematic review and meta-analysis
Liraglutide, a long-acting glucagon-like peptide-1 (GLP-1) analogue used to treat type 2 diabetes mellitus, was assessed in this systematic review and meta-analysis. The findings, based on eight eligible studies with 14,608 patients, showed that liraglutide treatment resulted in a reduced risk of major cardiovascular events (MACE), acute myocardial infarction (AMI), all-cause death, and cardiovascular death when compared to control groups. However, liraglutide did not decrease the incidence of stroke. Notably, the reduction in MACE was significant only in placebo-controlled trials.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6867782/.
Verma S, Poulter NR, Bhatt DL, Bain SC, Buse JB, Leiter LA, Nauck MA, Pratley RE, Zinman B, Ørsted DD, Monk Fries T, Rasmussen S, Marso SP. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke. Circulation. 2018 Dec 18;138(25):2884-2894. doi: 10.1161/CIRCULATIONAHA.118.034516. PMID: 30566004.
Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke
In the LEADER trial, which included 9,340 patients with type 2 diabetes and high cardiovascular risk, liraglutide’s effects on major adverse cardiovascular events, mortality, and hospitalization due to heart failure (HF) were examined by the history of HF. Of the participants, 18% had a history of HF. The study found that liraglutide’s impact on cardiovascular events and mortality was consistent in patients both with and without a history of HF. Importantly, there was no increased risk of HF hospitalization associated with liraglutide. Thus, liraglutide appears to be a suitable option for patients with type 2 diabetes, regardless of their HF history.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0735109720302485?via%3Dihub.
Svanström H, Ueda P, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Pasternak B. Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. 2019 Feb;7(2):106-114. doi: 10.1016/S2213-8587(18)30320-6. Epub 2018 Dec 5. PMID: 30527909.
Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden
This study utilized data from Denmark and Sweden to investigate the cardiovascular effects of liraglutide in patients with type 2 diabetes compared to dipeptidyl peptidase-4 (DPP-4) inhibitors in routine clinical practice. The analysis revealed that liraglutide was associated with a significantly reduced risk of major cardiovascular events when compared to DPP-4 inhibitors. Specifically, liraglutide showed a reduced risk of cardiovascular death and overall mortality, with the greatest benefits observed in patients with a history of cardiovascular disease. These findings support the cardiovascular effectiveness of liraglutide in real-world clinical settings.
You can read the abstract of the article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30320-6/fulltext.
Marso SP, Baeres FMM, Bain SC, Goldman B, Husain M, Nauck MA, Poulter NR, Pratley RE, Thomsen AB, Buse JB; LEADER Trial Investigators. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Diabetes With or Without Heart Failure. J Am Coll Cardiol. 2020 Mar 17;75(10):1128-1141. doi: 10.1016/j.jacc.2019.12.063. PMID: 32164886.
Effects of Liraglutide on Cardiovascular Outcomes in Patients With Diabetes With or Without Heart Failure
This study aimed to investigate the effects of liraglutide on cardiovascular events and mortality in patients with type 2 diabetes, with or without a history of heart failure, as part of the LEADER trial. The results demonstrated that liraglutide’s effects on major adverse cardiovascular events and mortality were consistent, irrespective of a history of heart failure. The data suggests that liraglutide is a suitable option for patients with type 2 diabetes, whether or not they have a history of heart failure, based on these findings from the LEADER trial.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0735109720302485?via%3Dihub.
Brown-Frandsen K, Emerson SS, McGuire DK, Pieber TR, Poulter NR, Pratley RE, Zinman B, Ranthe MF, Grøn R, Lange M, Moses AC, Örsy P, Buse JB; DEVOTE Study Group. Lower rates of cardiovascular events and mortality associated with liraglutide use in patients treated with basal insulin: A DEVOTE subanalysis (DEVOTE 10). Diabetes Obes Metab. 2019 Jun;21(6):1437-1444. doi: 10.1111/dom.13677. Epub 2019 Apr 1. PMID: 30793465; PMCID: PMC6504564.
Lower rates of cardiovascular events and mortality associated with liraglutide use in patients treated with basal insulin: A DEVOTE subanalysis (DEVOTE 10)
The aim of this study was to compare the risk of major adverse cardiovascular events (MACE) and all-cause mortality between patients using liraglutide alongside basal insulin (insulin degludec or insulin glargine 100 units/mL) and those not using liraglutide in the DEVOTE trial. The results showed that liraglutide use was linked to a significantly reduced risk of MACE and all-cause mortality without a significant difference in severe hypoglycemia rates. This suggests that liraglutide may provide cardiovascular benefits for patients with type 2 diabetes and high cardiovascular risk who are using basal insulin.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6504564/.
Liu, Y., Jiang, X. & Chen, X. Liraglutide and Metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease. Lipids Health Dis 16, 227 (2017). https://doi.org/10.1186/s12944-017-0609-0.
Liraglutide and Metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease
This study aimed to compare the impact of Liraglutide and Metformin, individually and in combination, on the cardiac function of type 2 diabetes patients with coronary artery disease (CAD). The study involved 120 participants and was divided into two sections. Results after 24 weeks showed that Liraglutide alone treatment yielded better improvements in various measures compared to Metformin alone, while the combination of Liraglutide and Metformin showed the best results in most measures, except for BMI, triglycerides, and blood pressure in Section 2. Overall, with similar blood glucose control, Liraglutide monotherapy (1.2 mg/d) exhibited more favorable effects on lipid metabolism and cardiovascular function than Metformin alone or the combination of Liraglutide and Metformin.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5712174/.
Zhao X, Huang K, Zheng M, Duan J. Effect of liraglutide on blood pressure: a meta-analysis of liraglutide randomized controlled trials. BMC Endocr Disord. 2019;19(1):4. Published 2019 Jan 7. doi:10.1186/s12902-018-0332-5.
Effect of liraglutide on blood pressure: a meta-analysis of liraglutide randomized controlled trials
This meta-analysis investigated the impact of the glucagon-like peptide-1 receptor agonist (GLP-1RA) liraglutide on blood pressure in individuals with or without hyperglycemia, based on randomized controlled trials published between 2009 and 2018. The analysis included 18 trials with 7616 individuals in the liraglutide group and 6046 in the control group. The results showed that liraglutide significantly reduced systolic blood pressure compared to placebo. However, this effect became less pronounced when the intervention exceeded one year. Furthermore, the dosage of liraglutide was associated with the extent of systolic blood pressure reduction. Notably, liraglutide did not significantly affect diastolic blood pressure. These findings contribute to the growing body of evidence supporting the cardiovascular benefits of liraglutide.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6323665/.
Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013 Aug;15(8):737-49. doi: 10.1111/dom.12085. Epub 2013 Mar 20. PMID: 23433305.
Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials
This study aimed to evaluate the blood pressure-lowering effects of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), specifically exenatide and liraglutide, in comparison to common drugs used for treating type 2 diabetes (T2DM), based on randomized controlled trials (RCTs). The meta-analysis included 16 RCTs with 3443 patients in the GLP-1 RA treatment group and 2417 subjects in the control group. Exenatide reduced both systolic and diastolic blood pressure compared to placebo, insulin glargine, and sitagliptin. Liraglutide at different doses also significantly lowered systolic blood pressure when compared to placebo, glimepiride, and insulin. These findings suggest that GLP-1 RAs, such as exenatide and liraglutide, may serve as effective alternatives for T2DM patients, offering potential cardiovascular benefits and lowering blood pressure by 1 to 5 mmHg compared to other anti-diabetic drugs.
You can read the abstract of the article at https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.12085.
Zhao D, Liu H, Dong P. Liraglutide reduces systolic blood pressure in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Clin Exp Hypertens. 2020 Jul 3;42(5):393-400. doi: 10.1080/10641963.2019.1676771. Epub 2019 Oct 15. PMID: 31610701.
Liraglutide reduces systolic blood pressure in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials
In this meta-analysis of 10 randomized, double-blind, placebo-controlled trials involving 968 patients with type 2 diabetes, liraglutide at a dosage of 1.8 mg/day was found to significantly reduce systolic blood pressure (mean reduction of -5.39 mm Hg), as well as body weight, when compared to a placebo. However, there was no significant difference in diastolic blood pressure changes between liraglutide and placebo. Notably, liraglutide was associated with an increase in heart rate compared to the placebo. Moreover, the study did not find a significant correlation between the reduction in systolic blood pressure and weight loss. These findings suggest that liraglutide offers potential benefits for cardiovascular protection and may enhance the prognosis of patients with type 2 diabetes mellitus.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.1080/10641963.2019.1676771.
Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complications. 2014 May-Jun;28(3):399-405. doi: 10.1016/j.jdiacomp.2014.01.009. Epub 2014 Jan 21. PMID: 24561125; PMCID: PMC4231710.
Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials
In a patient-level pooled analysis of six phase 3 randomized trials involving 2792 patients with type 2 diabetes, liraglutide at doses of 1.2 mg and 1.8 mg once daily led to significant reductions in systolic blood pressure (SBP) compared to placebo. These reductions were evident after 2 weeks and were greater than those observed with other diabetes medications like glimepiride, insulin glargine, and rosiglitazone. While SBP reductions with liraglutide weakly correlated with weight loss, there was no significant influence of concomitant antihypertensive medications. Additionally, liraglutide was associated with a slight increase in pulse compared to placebo. This study highlights the blood pressure-lowering effects of liraglutide in patients with type 2 diabetes, even in those taking antihypertensive medication.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4231710/.
Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, Bhattacharyya A, Kumar A, Kim KW, Yoon KH, Bech OM, Zychma M. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*). Diabetes Obes Metab. 2011 Jan;13(1):81-8. doi: 10.1111/j.1463-1326.2010.01323.x. PMID: 21114607.
Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*)
In a 16-week double-blind trial involving 929 Asian subjects with type 2 diabetes from China, South Korea, and India, liraglutide was compared to glimepiride, both in combination with metformin. Liraglutide, at doses of 1.2 mg and 1.8 mg, demonstrated non-inferiority to glimepiride in reducing HbA₁(c) levels. Additionally, liraglutide led to weight reduction, while glimepiride resulted in weight gain. Liraglutide also significantly reduced systolic blood pressure, had a lower incidence of hypoglycemia, and was generally well-tolerated, with transient nausea as the most common adverse event. This Asian population’s response to liraglutide was similar to results from global liraglutide phase 3 trials in other ethnic groups.
You can read the abstract of the article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/j.1463-1326.2010.01323.x.
Du Q, Wang YJ, Yang S, Zhao YY, Han P. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther. 2014 Nov;31(11):1182-95. doi: 10.1007/s12325-014-0164-2. Epub 2014 Nov 12. PMID: 25388240.
Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials
Liraglutide, commonly used to treat type 2 diabetes mellitus (T2DM), was the subject of a meta-analysis aimed at evaluating its effects compared to a placebo for T2DM treatment. The analysis included 11 relevant studies. Liraglutide demonstrated significant benefits in reducing glycosylated hemoglobin, fasting plasma glucose, weight, and blood pressure when compared to the placebo. However, liraglutide treatment also resulted in a higher prevalence of adverse events. Further large-scale, well-designed studies are recommended to provide additional support and guidance for liraglutide’s clinical application in T2DM.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s12325-014-0164-2.
Sharma A, Ambrosy AP, DeVore AD, Margulies KB, McNulty SE, Mentz RJ, Hernandez AF, Michael Felker G, Cooper LB, Lala A, Vader J, Groake JD, Borlaug BA, Velazquez EJ. Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: insights from the FIGHT trial. ESC Heart Fail. 2018 Dec;5(6):1035-1043. doi: 10.1002/ehf2.12334. Epub 2018 Aug 17. PMID: 30120812; PMCID: PMC6300815.
Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: insights from the FIGHT trial
In a study involving 300 patients with heart failure and reduced ejection fraction (HFrEF), liraglutide, a glucagon-like peptide-1 (GLP-1) receptor antagonist, was investigated as a weight loss agent. Patients with at least one follow-up visit on liraglutide showed a significant reduction in weight compared to those on placebo, with a treatment difference of -4.10 lbs. Liraglutide also led to a significant decrease in triglyceride levels. These results suggest that liraglutide is effective for weight loss in HFrEF patients, though further research in a larger cardiovascular outcomes trial is needed.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6300815/.
Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, Bhattacharyya A, Kumar A, Kim KW, Yoon KH, Bech OM, Zychma M. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*). Diabetes Obes Metab. 2011 Jan;13(1):81-8. doi: 10.1111/j.1463-1326.2010.01323.x. PMID: 21114607.
Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*)
In a 16-week double-blind trial involving 929 Asian subjects with type 2 diabetes from China, South Korea, and India, liraglutide was compared to glimepiride, both in combination with metformin. Liraglutide, at doses of 1.2 mg and 1.8 mg, demonstrated non-inferiority to glimepiride in reducing HbA₁(c) levels. Additionally, liraglutide led to weight reduction, while glimepiride resulted in weight gain. Liraglutide also significantly reduced systolic blood pressure, had a lower incidence of hypoglycemia, and was generally well-tolerated, with transient nausea as the most common adverse event. This Asian population’s response to liraglutide was similar to results from global liraglutide phase 3 trials in other ethnic groups.
You can read the abstract of the article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/j.1463-1326.2010.01323.x.
Almarshad F. Short-term monotherapy with Liraglutide for weight management: A case study. J Family Med Prim Care. 2019;8(5):1804-1806. doi:10.4103/jfmpc.jfmpc_213_19.
Short-term monotherapy with Liraglutide for weight management: A case study
In a case study, a healthy 35-year-old male seeking weight loss management received Liraglutide (Saxenda) treatment, starting at 0.6 mg in the first week and increasing the dose by 0.6 mg each week until reaching 3.0 mg in the fifth week. During this five-week period, he maintained a low-calorie diet, not exceeding 1,500 calories per day, and engaged in mild exercise by walking for 45 minutes three times a week. The initial weight was 118 kg, height 171 cm, and body mass index 40.4. As a result, this short-term monotherapy with Liraglutide, combined with a restricted-calorie diet and light exercise, led to a significant weight reduction of 13.55%.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6559087/.
Feng P, Yu DM, Chen LM, Chang BC, Ji QD, Li SY, Zhu M, Ding SH, Zhang BZ, Wang SL, Li HT, Lin JN, Wang MJ, Guo JC, Liu J, Liu ZD, Wu ST, Yang JH. Liraglutide reduces the body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients. Acta Pharmacol Sin. 2015 Feb;36(2):200-8. doi: 10.1038/aps.2014.136. Epub 2015 Jan 26. PMID: 25619391; PMCID: PMC4326793.
Liraglutide reduces the body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients
In a multi-center, open-label, self-controlled clinical study involving 328 Chinese overweight and obese type 2 diabetic patients, the aim was to investigate the effects of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor activator, on body weight and waist circumference. Liraglutide treatment led to a significant reduction in mean body weight (from 86.61±14.09 to 79.10±13.55 kg) and waist circumference (from 101.81±13.96 to 94.29±14.17 cm). Approximately 43.67% of patients experienced a 5%-10% body weight loss, while 34.06% achieved a body weight loss exceeding 10%. These changes were associated with lower plasma creatinine levels. Baseline waist circumference, BMI, and HOMA-IR were independently correlated with the magnitude of body weight loss. Furthermore, liraglutide treatment improved glycemic control, with 35.37% of patients reaching an HbA1c level below 7.0%. Patients with specific characteristics, such as visceral obesity, insulin resistance, and a long diabetes duration, showed greater body weight loss, while those with high insulin-secreting ability, hyperglucagonemia, and a shorter diabetes duration demonstrated better glycemic control with liraglutide.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4326793/.
Mirabelli M, Chiefari E, Caroleo P, et al. Long-Term Effectiveness of Liraglutide for Weight Management and Glycemic Control in Type 2 Diabetes. Int J Environ Res Public Health. 2019;17(1):207. Published 2019 Dec 27. doi:10.3390/ijerph17010207.
Long-Term Effectiveness of Liraglutide for Weight Management and Glycemic Control in Type 2 Diabetes
Over a 5-year period, a retrospective study assessed the effectiveness of Liraglutide in managing weight and glycometabolic control in an overweight/obese cohort of Southern Italian type 2 diabetes patients who were new to glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Liraglutide treatment resulted in a significant reduction in body weight from 92.1 ± 20.5 kg to 87.3 ± 20.0 kg, with a mean reduction of 5.0 ± 7.0 kg and a body mass index (BMI) decrement of -2.0 ± 3.1 kg/m2. Hemoglobin A1c (HbA1c) decreased from 7.9 ± 0.9% at baseline to 7.0 ± 0.7% by the study’s end. The analysis revealed correlations between changes in body weight, female gender, and baseline BMI. While the study showed encouraging effects on several cardiovascular disease markers, it also documented an increase in the 5- and 10-year risk for the first atherosclerotic cardiovascular event, including four cases of myocardial infarction. The role of Liraglutide in long-term primary prevention of cardiovascular disease in type 2 diabetes patients remains debated.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6981922/.
Scott LJ. Liraglutide: a review of its use in the management of obesity. Drugs. 2015 May;75(8):899-910. doi: 10.1007/s40265-015-0408-8. PMID: 25985864.
Liraglutide: a review of its use in the management of obesity
Obesity is a global epidemic, demanding effective management strategies. Despite lifestyle interventions, many overweight or obese adults require pharmacotherapy for significant weight reduction. Subcutaneous liraglutide (Saxenda®) at 3 mg daily is approved as an adjunct to calorie restriction and increased physical activity for chronic bodyweight management in adults with a body mass index (BMI) ≥30 kg/m² (obese) or ≥27 kg/m² (overweight) with at least one weight-related comorbidity (e.g., hypertension, diabetes, or sleep apnea). Clinical trials spanning 32 to 56 weeks demonstrated liraglutide’s effectiveness in reducing bodyweight, waist circumference, and improving cardiovascular risk markers. The improvements were sustained for up to 2 years. Liraglutide also showed benefits in non-diabetic adults with obstructive sleep apnea. Despite the absence of direct comparisons with other anti-obesity drugs, liraglutide stands as a promising option for chronic bodyweight management when combined with diet and exercise in obese or overweight adults with related comorbidities.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s40265-015-0408-8.
Kelly, A. S., Auerbach, P., Barrientos-Perez, M., Gies, I., Hale, P. M., Marcus, C., Mastrandrea, L. D., Prabhu, N., Arslanian, S., & NN8022-4180 Trial Investigators (2020). A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. The New England journal of medicine, 382(22), 2117–2128. https://doi.org/10.1056/NEJMoa1916038.
A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity
Obesity is a challenging chronic condition in adolescents, and liraglutide, when added to lifestyle therapy, can be an effective treatment option. In a 56-week randomized, double-blind trial, liraglutide (3.0 mg) proved superior to a placebo, leading to a significant reduction in the body-mass index (BMI) standard-deviation score in adolescents with obesity who had an inadequate response to lifestyle therapy alone. More participants in the liraglutide group achieved significant reductions in BMI compared to the placebo group, although liraglutide was associated with more gastrointestinal adverse events and discontinuations. Overall, liraglutide can be a valuable addition to lifestyle therapy in managing adolescent obesity.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJMoa1916038?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Mehta, A., Marso, S. P., & Neeland, I. J. (2017). Liraglutide for weight management: a critical review of the evidence. Obesity science & practice, 3(1), 3–14. https://doi.org/10.1002/osp4.84.
Liraglutide for weight management: a critical review of the evidence
This review examines the efficacy, safety, and clinical relevance of liraglutide for weight management based on phase III clinical trials. Liraglutide, in addition to recommended diet and exercise, consistently led to a weight loss of 4 to 6 kg, with more patients achieving significant weight reductions compared to a placebo. Gastrointestinal side effects were common but mainly occurred early in treatment. Comparative data suggests that liraglutide’s weight loss effects are greater than orlistat and lorcaserin, but slightly less than phentermine/topiramate. Liraglutide 1.8 mg has demonstrated cardiovascular benefits in a recent outcomes trial, though its applicability to the 3.0 mg formulation in a broader population with high cardiovascular risk is uncertain. Barriers to widespread clinical use include gastrointestinal side effects, cost, and the need for injection. Liraglutide offers effective weight loss with the added benefit of improved glycemic control. Further research is needed to determine its long-term efficacy and safety profile.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5358074/.
Davies, M. J., Bergenstal, R., Bode, B., Kushner, R. F., Lewin, A., Skjøth, T. V., Andreasen, A. H., Jensen, C. B., DeFronzo, R. A., & NN8022-1922 Study Group (2015). Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA, 314(7), 687–699. https://doi.org/10.1001/jama.2015.9676.
Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial
In a 56-week randomized trial involving overweight or obese adults with type 2 diabetes, the use of daily subcutaneous liraglutide (3.0 mg) resulted in significant weight loss compared to placebo. The weight loss was greater with the 3.0 mg dose compared to the 1.8 mg dose or placebo. A higher proportion of participants achieved a weight loss of 5% or more and over 10% of their baseline weight with liraglutide. While there were more gastrointestinal disorders with the 3.0 mg dose, no cases of pancreatitis were reported. Further research is required to assess the long-term efficacy and safety of liraglutide.
You can read the full article at https://jamanetwork.com/journals/jama/fullarticle/2428956.
Nagae K, Uchi H, Morino-Koga S, Tanaka Y, Oda M, Furue M. Glucagon-like peptide-1 analogue liraglutide facilitates wound healing by activating PI3K/Akt pathway in keratinocytes. Diabetes Res Clin Pract. 2018 Dec;146:155-161. doi: 10.1016/j.diabres.2018.10.013. Epub 2018 Oct 25. PMID: 30367901.
Glucagon-like peptide-1 analogue liraglutide facilitates wound healing by activating PI3K/Akt pathway in keratinocytes
The study aimed to explore the potential of liraglutide, a potent GLP-1R agonist, in improving skin ulcer healing associated with diabetes. Keratinocytes expressed GLP-1R, and liraglutide was found to enhance their migration. In an in vivo experiment with mice, liraglutide promoted wound healing. The mechanism appeared to involve PI3K/Akt pathway activation in keratinocytes. These findings suggest that liraglutide may hold promise as a targeted drug for enhancing the healing of skin ulcers in diabetes through its interaction with the GLP-1 receptor.
You can read the full article at https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(18)31281-6/fulltext.
Eleftheriadou I, Tentolouris A, Tentolouris N, Papanas N. Advancing pharmacotherapy for diabetic foot ulcers. Expert Opin Pharmacother. 2019 Jun;20(9):1153-1160. doi: 10.1080/14656566.2019.1598378. Epub 2019 Apr 8. PMID: 30958725.
Advancing pharmacotherapy for diabetic foot ulcers
The standard treatment for diabetic foot ulcers (DFUs) is currently unsatisfactory, and this article discusses newer pharmacological options for DFU management and future treatment strategies. While awaiting high-quality evidence from large randomized controlled trials (RCTs), the authors suggest that empagliflozin and liraglutide can be recommended for glucose control in DFU patients with peripheral arterial disease (PAD). Additionally, for patients with DFUs, intensive lipid lowering therapy with evolocumab may be considered if primary cholesterol goals are not met. Further clinical studies are needed to establish a structured treatment algorithm for DFUs that do not heal with the current standard of care, and advanced treatment options like sucrose octasulfate dressings, becaplermin gel, and platelet-rich plasma (PRP) could also be potential considerations.
You can read the full article at https://www.tandfonline.com/doi/abs/10.1080/14656566.2019.1598378.
Yu M , Huang J , Zhu T , Lu J , Liu J , Li X , Yan X , Liu F . Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p. Biomater Sci. 2020 Aug 7;8(15):4225-4238. doi: 10.1039/d0bm00442a. Epub 2020 Jun 24. PMID: 32578587.
Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p
Addressing diabetic wounds is a significant clinical challenge due to limited efficacy of current therapies. This study explores a novel approach using a poly (lactic-co-glycolic acid)/gelatin (PLGA/Gel) nanofibrous mat scaffold to sustainably release liraglutide (Lira) for skin tissue engineering. This method enhances wound healing by increasing pore size, hydrophilicity, elasticity, and degradation properties. PLGA/Gel/Lira treatment significantly improves diabetic wound healing by reducing closure time, increasing blood vessel density, and enhancing collagen deposition. Furthermore, Lira counteracts the inhibitory effects of high glucose on endothelial cell proliferation, migration, and vascularization by modulating the miR-29b-3p/AKT/GSK-3β/β-catenin pathway. This innovative approach holds promise for accelerating diabetic wound repair.
You can read the abstract of the article at https://pubs.rsc.org/en/content/articlelanding/2020/BM/D0BM00442A.
Available at https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(18)31281-6/fulltext#secst120.
Glucagon-like peptide-1 analogue liraglutide facilitates wound healing by activating PI3K/Akt pathway in keratinocytes
Diabetes often leads to skin issues, such as foot ulcers, which can be challenging to treat. Although recent studies have suggested that glucagon-like peptide-1 (GLP-1) analogues can help reduce diabetes-related foot complications, the precise role of the GLP-1/GLP-1R axis and the evidence of GLP-1’s role in promoting wound closure are not well-established. This study aimed to investigate whether the potent GLP-1R agonist liraglutide influences the wound healing process.
You can read the full article at https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(18)31281-6/fulltext#secst120..
Lu R, Yang J, Wei R, et al. Synergistic anti-tumor effects of liraglutide with metformin on pancreatic cancer cells. PLoS One. 2018;13(6):e0198938. Published 2018 Jun 13. doi:10.1371/journal.pone.0198938.
Synergistic anti-tumor effects of liraglutide with metformin on pancreatic cancer cells
The study aimed to investigate the effects of liraglutide and metformin, either individually or in combination, on human pancreatic cancer cells. Results demonstrated that the combined treatment with liraglutide and metformin had a synergistic anti-tumor effect, leading to reduced cell viability, colony formation, cell cycle arrest, increased levels of pro-apoptotic proteins, and inhibition of cell migration. This combined treatment also activated the phosphorylation of AMP-activated protein kinase (AMPK) more effectively than their individual use. These findings offer valuable insights into potential treatment strategies for individuals with type 2 diabetes and pancreatic cancer.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5999272/.
Eftekhari S, Montazeri H, Tarighi P. Synergistic anti-tumor effects of Liraglutide, a glucagon-like peptide-1 receptor agonist, along with Docetaxel on LNCaP prostate cancer cell line. Eur J Pharmacol. 2020 Jul 5;878:173102. doi: 10.1016/j.ejphar.2020.173102. Epub 2020 Apr 10. PMID: 32283060.
Synergistic anti-tumor effects of Liraglutide, a glucagon-like peptide-1 receptor agonist, along with Docetaxel on LNCaP prostate cancer cell line
This study aimed to enhance the effectiveness of docetaxel, a chemotherapy agent used in metastatic prostate cancer, by combining it with liraglutide, a Glucagon-Like Peptide-1 Receptor agonist. Results demonstrated that the combination of liraglutide and docetaxel had a synergistic effect in reducing the viability of LNCaP prostate cancer cells, leading to cell cycle arrest and apoptosis. This combination also significantly reduced the phosphorylation of proteins in the ERK/MAPK and AKT/PI3K pathways. These findings suggest that combining liraglutide with docetaxel could be a potent strategy to enhance treatment efficacy while reducing the therapeutic dose, thereby minimizing systemic toxicities and drug resistance.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0014299920301941?via%3Dihub.
Zhao W, Zhang X, Zhou Z, et al. Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol Med Rep. 2018;17(4):5202-5212. doi:10.3892/mmr.2018.8475.
Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression
This study investigated the potential anti-breast cancer effects of liraglutide, a glucagon-like peptide-1 analogue. Using MCF-7 human breast cancer cells, liraglutide treatment was found to reduce cell proliferation and increase apoptosis. Additionally, liraglutide downregulated microRNA-27a (miR-27a) expression. Overexpression of miR-27a promoted cell proliferation and inhibited apoptosis, while miR-27a knockdown had the opposite effect. Liraglutide also affected the expression of AMP-activated protein kinase catalytic subunit α2 (AMPKα2) protein. These findings suggest that liraglutide may inhibit breast cancer cell proliferation and promote apoptosis by regulating miR-27a expression and AMPKα2 protein levels, offering potential clinical strategies for breast cancer patients with type 2 diabetes mellitus.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5865986/.
Ketan Dhatariya, Stephen C. Bain, John B. Buse, Richard Simpson, Lise Tarnow, Margit Staum Kaltoft, Michael Stellfeld, Karen Tornøe, Richard E. Pratley, the LEADER Publication Committee on behalf of the LEADER Trial Investigators
Diabetes Care Oct 2018, 41 (10) 2229-2235; DOI: 10.2337/dc18-1094.
The Impact of Liraglutide on Diabetes-Related Foot Ulceration and Associated Complications in Patients With Type 2 Diabetes at High Risk for Cardiovascular Events: Results From the LEADER Trial
The post hoc analyses of the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial aimed to assess the impact of liraglutide versus placebo on diabetes-related foot ulcers (DFUs) and their complications in people with type 2 diabetes at high cardiovascular risk. Over a median 3.8 years, the incidence of DFUs was similar between the liraglutide and placebo groups. However, liraglutide was associated with a significant reduction in DFU-related amputations compared to placebo, although no differences were found for other DFU-related complications. Further investigation is needed to confirm this finding.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6150424/.
Zhao H, Jiang X, Duan L, Yang L, Wang W, Ren Z. Liraglutide suppresses the metastasis of PANC-1 co-cultured with pancreatic stellate cells through modulating intracellular calcium content. Endocr J. 2019 Dec 25;66(12):1053-1062. doi: 10.1507/endocrj.EJ19-0215. Epub 2019 Aug 30. PMID: 31474673.
Liraglutide suppresses the metastasis of PANC-1 co-cultured with pancreatic stellate cells through modulating intracellular calcium content
The study aimed to investigate the anti-tumor effects of liraglutide (Lira), an anti-diabetic medication, on pancreatic cancer cell PANC-1 when co-cultured with or without pancreatic stellate cells (PSCs). Lira, at concentrations of 100 and 1,000 nmol/L, significantly reduced cell viability and induced apoptosis in a dose-dependent manner for PANC-1, both with and without PSCs. It also inhibited cell migration and invasion, attenuating the pro-migratory influence of PSCs on PANC-1. Lira upregulated E-cadherin expression and downregulated N-cadherin expression in a dose-dependent manner. Furthermore, Lira modulated intracellular calcium levels, reducing their abnormal elevation. This study suggests that Lira can suppress the proliferation, migration, and invasion of PANC-1, and this effect may be associated with calcium modulation and alterations in Ca2+-binding proteins like E-cadherin and N-cadherin.
You can read the abstract of the article at https://www.jstage.jst.go.jp/article/endocrj/66/12/66_EJ19-0215/_article.
Batista AF, Forny-Germano L, Clarke JR, Lyra E Silva NM, Brito-Moreira J, Boehnke SE, Winterborn A, Coe BC, Lablans A, Vital JF, Marques SA, Martinez AM, Gralle M, Holscher C, Klein WL, Houzel JC, Ferreira ST, Munoz DP, De Felice FG. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018 May;245(1):85-100. doi: 10.1002/path.5056. Epub 2018 Apr 2. PMID: 29435980; PMCID: PMC5947670.
The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease
Alzheimer’s disease (AD) lacks effective treatments, spurring the search for disease-modifying therapies. This study explored liraglutide, a glucagon-like peptide-1 (GLP-1) analog used in type 2 diabetes, in AD models. AD is associated with impaired brain insulin signaling. Liraglutide prevented insulin receptor and synapse loss, and restored memory in AD-related mice models. In neuronal cultures, liraglutide activated the PKA signaling pathway for neuroprotection. In non-human primates infused with AD-related amyloid-β oligomers (AβOs), liraglutide reduced insulin receptor, synaptic, and tau pathology in memory-related brain regions. These findings shed light on liraglutide’s neuroprotective mechanisms and its potential to preserve brain insulin receptors and synapses in AD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5947670/.
Mansur RB, Ahmed J, Cha DS, Woldeyohannes HO, Subramaniapillai M, Lovshin J, Lee JG, Lee JH, Brietzke E, Reininghaus EZ, Sim K, Vinberg M, Rasgon N, Hajek T, McIntyre RS. Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study. J Affect Disord. 2017 Jan 1;207:114-120. doi: 10.1016/j.jad.2016.09.056. Epub 2016 Oct 1. PMID: 27721184.
Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study
In a 4-week pilot study, liraglutide, a GLP-1 receptor agonist, was investigated as an adjunct treatment for cognitive function in adults with depressive or bipolar disorders and executive function impairment. Nineteen participants exhibited significant cognitive improvement, as demonstrated by increased scores in cognitive tests, and these improvements were independent of mood or metabolic changes. Baseline insulin resistance and body mass index influenced the cognitive response. Liraglutide was well-tolerated but necessitates further research with larger controlled trials to validate these findings, despite the study’s small sample size and open-label design.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165032716312010?via%3Dihub.
Batista AF, Forny-Germano L, Clarke JR, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018;245(1):85-100. doi:10.1002/path.5056.
The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease
In the pursuit of effective treatments for Alzheimer’s disease (AD), a study investigated liraglutide, a glucagon-like peptide-1 (GLP-1) analog used in type 2 diabetes treatment, in AD experimental models. The study identified a link between AD and impaired brain insulin signaling. Liraglutide was found to prevent the loss of brain insulin receptors and synapses, reversing memory impairment caused by AD-related amyloid-β oligomers in mice. The neuroprotective mechanism involved the activation of the PKA signaling pathway. In non-human primates, infusion of amyloid-β oligomers resulted in the loss of insulin receptors and synapses related to memory. Liraglutide partially protected against AD-related pathologies in these primates, offering insights into potential neuroprotection through GLP-1 receptor activation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5947670/.
Yan W, Pang M, Yu Y, Gou X, Si P, Zhawatibai A, Zhang Y, Zhang M, Guo T, Yi X, Chen L. The neuroprotection of liraglutide on diabetic cognitive deficits is associated with improved hippocampal synapses and inhibited neuronal apoptosis. Life Sci. 2019 Aug 15;231:116566. doi: 10.1016/j.lfs.2019.116566. Epub 2019 Jun 13. PMID: 31201846.
The neuroprotection of liraglutide on diabetic cognitive deficits is associated with improved hippocampal synapses and inhibited neuronal apoptosis
This study aimed to investigate the neuroprotective effects of liraglutide in mice with cognitive deficits induced by streptozotocin (STZ)-induced diabetes. The diabetic mice displayed impaired learning and memory, hippocampal neuronal and synaptic damage, increased oxidative stress, and neuronal apoptosis. Treatment with liraglutide attenuated these effects and reversed diabetes-induced alterations in the PI3K/Akt signaling pathway, which is crucial for neuronal survival, apoptosis, and plasticity. These findings suggest that liraglutide has neuroprotective effects against diabetes-induced cognitive impairments, improving hippocampal synapses and inhibiting neuronal apoptosis.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0024320519304928?via%3Dihub.
Batista AF, Forny-Germano L, Clarke JR, Lyra E Silva NM, Brito-Moreira J, Boehnke SE, Winterborn A, Coe BC, Lablans A, Vital JF, Marques SA, Martinez AM, Gralle M, Holscher C, Klein WL, Houzel JC, Ferreira ST, Munoz DP, De Felice FG. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018 May;245(1):85-100. doi: 10.1002/path.5056. Epub 2018 Apr 2. PMID: 29435980; PMCID: PMC5947670.
The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease
Alzheimer’s disease (AD) is a debilitating neurological disorder lacking effective treatments, prompting the search for disease-modifying therapies. This study investigated liraglutide, a glucagon-like peptide-1 (GLP-1) analog used in type 2 diabetes, in AD experimental models. AD involves defective brain insulin signaling, and liraglutide was found to prevent the loss of brain insulin receptors and synapses while reversing memory impairment induced by AD-related amyloid-β oligomers (AβOs) in mice. Liraglutide’s neuroprotective mechanism involved PKA signaling pathway activation. In non-human primates, AβO infusion led to insulin receptor and synapse loss, and systemic liraglutide treatment partially protected against AD-related brain pathology. These findings suggest that GLP-1 receptor activation may hold promise for safeguarding brain insulin receptors and synapses in AD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5947670/.
Saad MA, Eltarzy MA, Abdel Salam RM, Ahmed MAE. Liraglutide mends cognitive impairment by averting Notch signaling pathway overexpression in a rat model of polycystic ovary syndrome. Life Sci. 2021 Jan 15;265:118731. doi: 10.1016/j.lfs.2020.118731. Epub 2020 Nov 6. PMID: 33160995.
Liraglutide mends cognitive impairment by averting Notch signaling pathway overexpression in a rat model of polycystic ovary syndrome
Polycystic ovary syndrome (PCOS), a prevalent endocrine disorder in women, has been associated with cognitive deficits. The Notch signaling pathway, a key regulator of ovarian and neurodegenerative disorders, plays a role in PCOS and cognitive impairment. Liraglutide, known for its neuroprotective effects, was investigated for its potential in alleviating cognitive dysfunction in PCOS. PCOS was induced in rats, and Liraglutide treatment was administered, resulting in the restoration of Notch signaling-related changes and the improvement of PCOS-induced memory impairment, indicating a promising therapeutic approach for PCOS-related cognitive problems.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0024320520314843?via%3Dihub.
Yang Y, Fang H, Xu G, Zhen Y, Zhang Y, Tian J, Zhang D, Zhang G, Xu J. Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats. Mol Med Rep. 2018 Aug;18(2):2449-2457. doi: 10.3892/mmr.2018.9180. Epub 2018 Jun 18. PMID: 29916537.
Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats
Liraglutide, a glucagon-like-peptide 1 receptor agonist known for its antidiabetic properties, has demonstrated neuroprotective effects. This study aimed to explore these effects and their mechanisms in rats with type 2 diabetes mellitus (T2DM). Diabetic Goto-Kakizaki (GK) rats exhibited cognitive impairment, which was alleviated by liraglutide treatment, particularly at a high dose. Liraglutide modulated key proteins associated with autophagy, apoptosis, and signaling pathways including PI3K, Akt, AMPK, and mTOR. The findings suggest that liraglutide’s neuroprotective effects involve the activation of autophagy and regulation of specific signaling pathways, providing valuable insights into its potential therapeutic mechanisms for T2DM-related cognitive deficits.
You can read the full article at https://www.spandidos-publications.com/mmr/18/2/2449.
Palleria C, Leo A, Andreozzi F, Citraro R, Iannone M, Spiga R, Sesti G, Constanti A, De Sarro G, Arturi F, Russo E. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav Brain Res. 2017 Mar 15;321:157-169. doi: 10.1016/j.bbr.2017.01.004. Epub 2017 Jan 3. PMID: 28062257.
Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects
Liraglutide, a glucagon-like peptide 1 receptor agonist, was investigated for its effects on diabetes-associated cognitive decline and hippocampal neurodegeneration. In a rat model, Liraglutide improved learning and memory in diabetic animals, had anxiolytic effects, but also showed pro-depressant effects in non-diabetic rats. Liraglutide reduced hippocampal neuronal death and maintained the phosphorylation of AKT and p70S6K, possibly through its effects on the mTOR pathway. However, it reduced endurance in physical tests, and its pro-depressant effects could be linked to reduced activity in certain circumstances. Overall, Liraglutide demonstrated potential neuroprotective benefits for diabetes-related cognitive issues and hippocampal health.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0166432816310701?via%3Dihub.
Liu S, Jin Z, Zhang Y, Rong S, He W, Sun K, Wan D, Huo J, Xiao L, Li X, Ding N, Wang F, Sun T. The Glucagon-Like Peptide-1 Analogue Liraglutide Reduces Seizures Susceptibility, Cognition Dysfunction and Neuronal Apoptosis in a Mouse Model of Dravet Syndrome. Front Pharmacol. 2020 Feb 28;11:136. doi: 10.3389/fphar.2020.00136. PMID: 32184723; PMCID: PMC7059191.
The Glucagon-Like Peptide-1 Analogue Liraglutide Reduces Seizures Susceptibility, Cognition Dysfunction and Neuronal Apoptosis in a Mouse Model of Dravet Syndrome
This study explored the potential neuroprotective role of liraglutide in mouse and cell models of Dravet syndrome (DS), a refractory epilepsy. Liraglutide increased Scn1a gene expression, alleviated epileptic seizures, reduced cognitive dysfunction, and protected against apoptosis in DS-induced mice and cells. It also restored the balance between pro-apoptotic and anti-apoptotic factors and modulated the mTOR signaling pathway. These findings suggest that liraglutide has promising anti-epileptic and neuroprotective effects in DS models.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059191/.
Kong FJ, Wu JH, Sun SY, Ma LL, Zhou JQ. Liraglutide ameliorates cognitive decline by promoting autophagy via the AMP-activated protein kinase/mammalian target of rapamycin pathway in a streptozotocin-induced mouse model of diabetes. Neuropharmacology. 2018 Mar 15;131:316-325. doi: 10.1016/j.neuropharm.2018.01.001. Epub 2018 Jan 3. PMID: 29305122.
Liraglutide ameliorates cognitive decline by promoting autophagy via the AMP-activated protein kinase/mammalian target of rapamycin pathway in a streptozotocin-induced mouse model of diabete
Liraglutide, a glucagon-like peptide-1 analog, was investigated for its potential to prevent diabetes-induced cognitive decline. In both in vivo and in vitro studies, liraglutide pretreatment demonstrated neuroprotective effects. It improved cognitive function, reduced neuronal injuries, and promoted autophagy by activating the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway. These findings suggest that liraglutide may protect against diabetes-induced cognitive impairment by enhancing autophagy.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0028390818300017?via%3Dihub.
Zhang H, Chu Y, Zheng H, Wang J, Song B, Sun Y. Liraglutide improved the cognitive function of diabetic mice via the receptor of advanced glycation end products down-regulation. Aging (Albany NY). 2020 Nov 26;13(1):525-536. doi: 10.18632/aging.202162. Epub 2020 Nov 26. PMID: 33298623; PMCID: PMC7835012.
Liraglutide improved the cognitive function of diabetic mice via the receptor of advanced glycation end products down-regulation
This study aimed to investigate the impact of liraglutide on cognitive function in diabetic mice and its underlying mechanisms. Diabetic mice exhibited cognitive decline, associated with reduced plasma glucagon-like peptide-1 (GLP-1) levels and increased receptor of advanced glycation end products (RAGE) levels. Liraglutide treatment reversed these changes, improving cognitive function. While there wasn’t a direct interaction found between RAGE and GLP-1R, liraglutide mitigated the elevated RAGE levels induced by advanced glycation end products (AGEs). Overall, liraglutide was shown to enhance cognitive function in diabetic mice by down-regulating RAGE. The study used db/db mice and immunofluorescence to assess neurons and RAGE in the hippocampus, along with western blotting to measure GLP-1R and RAGE levels in PC12 and HT22 cells treated with AGEs.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7835012/.
Zhang H, Chu Y, Zheng H, Wang J, Song B, Sun Y. Liraglutide improved the cognitive function of diabetic mice via the receptor of advanced glycation end products down-regulation. Aging (Albany NY). 2020 Nov 26;13(1):525-536. doi: 10.18632/aging.202162. Epub 2020 Nov 26. PMID: 33298623; PMCID: PMC7835012.
Liraglutide improved the cognitive function of diabetic mice via the receptor of advanced glycation end products down-regulation
This study aimed to investigate the impact of liraglutide on cognitive function in diabetic mice and its underlying mechanisms. Diabetic mice exhibited cognitive decline, associated with reduced plasma glucagon-like peptide-1 (GLP-1) levels and increased receptor of advanced glycation end products (RAGE) levels. Liraglutide treatment reversed these changes, improving cognitive function. While there wasn’t a direct interaction found between RAGE and GLP-1R, liraglutide mitigated the elevated RAGE levels induced by advanced glycation end products (AGEs). Overall, liraglutide was shown to enhance cognitive function in diabetic mice by down-regulating RAGE. The study used db/db mice and immunofluorescence to assess neurons and RAGE in the hippocampus, along with western blotting to measure GLP-1R and RAGE levels in PC12 and HT22 cells treated with AGEs.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7835012/.
Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, Pyke C, Knudsen LB, Farr SA, Vrang N. The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. J Alzheimers Dis. 2015;46(4):877-88. doi: 10.3233/JAD-143090. PMID: 25869785; PMCID: PMC4878312.
The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease
Recent research suggests that liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist used in type 2 diabetes management, may have pro-cognitive and neuroprotective effects. This study examined liraglutide’s impact on senescence-accelerated mouse prone 8 (SAMP8) mice, an age-related sporadic Alzheimer’s disease (AD) model not primarily characterized by amyloid plaques. When 10-month-old SAMP8 mice exhibited significant memory deficits without amyloid plaques or hyperphosphorylated tau, liraglutide treatment (100 or 500 μg/kg/day) over four months improved memory retention and preserved hippocampal CA1 pyramidal neurons. These findings suggest that liraglutide may delay or mitigate age-related memory decline and neuronal loss in a model with early-stage sporadic AD-like impairments.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4878312/.
McClean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. PMID: 23973293.
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease
Type 2 diabetes is a known risk factor for Alzheimer’s disease (AD), as it can lead to desensitization of insulin signaling in the brain. Glucagon-like peptide-1 (GLP-1), an incretin hormone, supports insulin signaling, and liraglutide (Victoza®), a long-lasting GLP-1 analogue, is used to treat type 2 diabetes. Previous research demonstrated that liraglutide, when administered peripherally to 7-month-old APPswe/PS1ΔE9 (APP/PS1) mice, improved cognitive function, reduced amyloid plaque deposition, inflammation, APP and oligomer levels, and enhanced synaptic plasticity at the early stage of AD development. This study explored liraglutide’s potential restorative effects in late-stage Alzheimer’s disease in mice, finding that it improved spatial memory, reduced plaque load and inflammation, increased neuronal progenitor cell count, enhanced synaptic plasticity, and lowered levels of key AD-related markers, offering promise for AD treatment in clinical trials.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0028390813003626?via%3Dihub.
Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, Di Castelnuovo A, Cipollone F, Consoli A, Santilli F. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes (Lond). 2020 Jun;44(6):1254-1263. doi: 10.1038/s41366-020-0535-5. Epub 2020 Jan 21. PMID: 31965072.
Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study
Individuals with prediabetes or newly diagnosed type 2 diabetes are at risk of cognitive impairment, and glucagon-like peptide-1 receptor agonists (GLP1-RAs) have shown neuroprotective effects, particularly in memory, in animal models. In a study, 40 obese subjects received either liraglutide or lifestyle counseling for comparable weight loss and glycemic control. After the intervention, the liraglutide group exhibited significant improvements in short-term memory and overall memory function, suggesting that liraglutide might help slow down memory decline in early-stage diabetes.
You can read the abstract of the article at https://www.nature.com/articles/s41366-020-0535-5.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








