GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of KPV
- Key Takeaways
- What is KPV?
- How KPV Works
- Chemical Structure of KPV
- Research on KPV
- KPV Peptide Side Effects
- KPV Peptide Dosage
- KPV Peptide Cancer
- KPV Peptide Injection
- KPV Peptide Capsules
- KPV Peptide Buy
- KPV in Inflammatory Bowel Diseases
- KPV: A Potent Anti-inflammatory Peptide
- KPV in Wound Healing
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of KPV
- Key Takeaways
- What is KPV?
- How KPV Works
- Chemical Structure of KPV
- Research on KPV
- KPV Peptide Side Effects
- KPV Peptide Dosage
- KPV Peptide Cancer
- KPV Peptide Injection
- KPV Peptide Capsules
- KPV Peptide Buy
- KPV in Inflammatory Bowel Diseases
- KPV: A Potent Anti-inflammatory Peptide
- KPV in Wound Healing
- FAQ
- Reference
Overall Health Benefits of KPV
KPV benefits include anti-inflammatory properties, aiding in wound healing, and reducing symptoms of inflammatory bowel disease. Additionally, it shows potential in enhancing skin health and mitigating conditions such as psoriasis and acne.
- Treats a Wide Array of Inflammatory Conditions [2-24]
- Improves Wound Healing [25-30]
- Improves Skin Health [31-45]
- Strengthens the Immune System [25, 26, 46-73]
- Protects Against Nerve Damage [74-97]
- Protects Against Stroke [98-111]
Key Takeaways
- Anti-inflammatory Properties: KPV is known for its strong anti-inflammatory effects, making it useful in treating various inflammatory conditions.
- Wound Healing: It aids in accelerating wound healing, promoting faster recovery of damaged tissues.
- Inflammatory Bowel Disease: KPV has shown promise in reducing symptoms associated with inflammatory bowel disease, offering potential relief for patients.
- Skin Health: The peptide can enhance skin health, helping to treat conditions like psoriasis and acne.
- Therapeutic Potential: KPV’s benefits make it a promising candidate for further research and development in therapeutic applications across different medical fields.
What is KPV?
KPV is a tripeptide (Lysine-Proline-Valine) that possesses potent anti-inflammatory properties. It’s a C-terminal tripeptide of α-MSH (alpha-Melanocyte-stimulating hormone). Peptides like KPV often act as hormones and relay information from one tissue through the blood to another via biological messengers. Whether given orally or in the form of injections, the KPV tripeptide has the potential to treat immune-mediated inflammatory conditions such as dermatitis, bowel diseases, allergic asthma, and arthritis.
How KPV Works
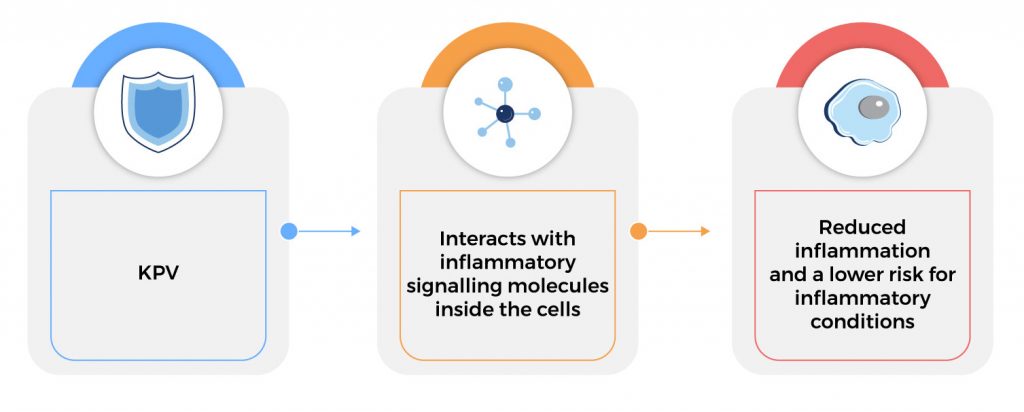
KPV exerts its anti-inflammatory function inside cells, where it inactivates inflammatory pathways. KPV enters the cell and interacts directly with inflammatory signalling molecules inside the cell. It enters the nucleus of the cell and, once there, can inhibit the interaction of inflammatory substances and molecules. In addition to its anti‐inflammatory effect, KPV also has antimicrobial effects against pathogens such as S. aureus and C. albicans.
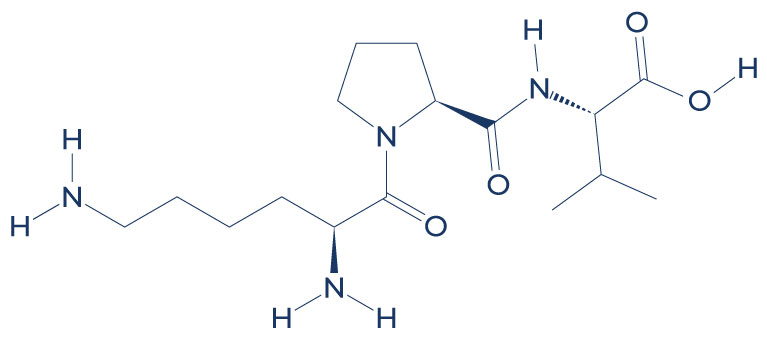
Chemical Structure of KPV
Research on KPV
A. Treats a Wide Array of Inflammatory Conditions
IMG
Interestingly, KPV appears to only have an effect in the setting of inflammation and it has almost no effect in normal tissue. The main reason for this is that KPV enters the cells using a transporter that is unregulated in case of inflammation. This suggests that the peptide may serve as an effective or preventive medication in inflammatory conditions. If taken regularly, KPV will be available when necessary and the body can simply excrete it if inflammation and other symptoms are not present.
Another amazing advantage of KPV is that, unlike alpha-Melanocyte-stimulating hormone, it does not cause skin pigmentation. [1] Moreover, KPV retains most of the anti-inflammatory properties of alpha-Melanocyte-stimulating hormone but without unpleasant side effects. Another appealing aspect of KPV is that it can be administered via different routes – oral, intravenous (through the veins), subcutaneous (between the muscle and fat), and transdermal (into the dermis of the skin). This allows KPV to be administered in different body areas with a higher level of safety and efficacy.
An overwhelming body of clinical evidence suggests that KPV exerts its strong anti-inflammatory properties through various important mechanisms:
- In a mice model of colitis (colon inflammation), oral administration of KPV (added to drinking water) inhibited the activation of NF-κB and MAP kinase inflammatory signaling pathways and reduced pro-inflammatory cytokine secretion. [2]
- A 2001 study published in Biochem Pharmacology found that KPV exerts its anti-inflammatory activities through inhibition of NF-kappaB translocation and activation of MC(1) receptor/cAMP. [3-4]
- In an animal model of colitis, KPV significantly reduced intestinal inflammation. [5-6]
- As a C-terminal tripeptide of α-MSH, KPV fights inflammation by inhibiting tumor necrosis factor-α stimulated NF-κB activity and suppressing antigen-induced lymphocyte proliferation. [7-8]
- A study published in the Journal of Federation of American Societies for Experimental Biology found that KPV suppresses inflammation through modulation of physiological responses in host defense. [9]
- A 2003 study published in the Journal of Pharmacology and Experimental Therapeutics found that KPV exhibits its anti-inflammatory effect through inhibition of IL-1beta functions. [10]
- A 2006 study published in the Basic & Clinical Pharmacology & Toxicology found that KPV fights inflammation by stimulating cAMP generation in a concentration-dependent way. [11]
- One study found that KPV combats inflammation by significantly inhibiting NF-kappaB-luciferase activity. [12]
- A 2010 study published in Advances in Experimental Medicine and Biology reported that KPV has significant similarities to the anti-inflammatory signaling of α-MSH. [13]
- Several studies have shown a significant reduction of pro‐inflammatory substances such as IL1 β, IL6, TNFα, IL8, Groα, and interferon γ (IFNγ) following KPV treatment. [14-15]
- A cell study found that KPV produced antibodies against the inflammatory IL10. [16]
- Studies also show that KPV reduces inflammation through inhibition of inflammatory cell adhesion and transmigration. [17-18]
- In murine models of colitis, KPV treatment showed significant anti-inflammatory effects which suggest that it can be an important therapeutic option in treating inflammatory bowel disease. [19]
- In a mice model of ulcerative colitis, oral administration of KPV loaded into hyaluronic acid (HA)-functionalized polymeric nanoparticles (NPs) was effective in alleviating intestinal inflammation. [20]
- In mice, topical administration of KPV in chemotherapy-induced oral mucositis resulted in improved healing due to its anti-inflammatory and antibacterial properties. [21]
- In an imiquimod-induced psoriasis mouse model, the application of a gel containing alpha-MSH resulted in a reduction in psoriasis-associated inflammation. [22]
- Studies reported that most of the anti-inflammatory activities of alpha-MSH can be attributed to its C-terminal tripeptide KPV. [23-24]
B. Improves Wound Healing
IMG
The potent anti-inflammatory properties of KPV may play a role in speeding up the wound healing process. In addition, since KPV does not cause skin pigmentation, this makes it a good candidate for improving wound healing while avoiding unpleasant skin changes. Another mechanism that is thought to contribute to faster wound healing is the immune-boosting effects of KPV, which helps lower the risk of infection during the regeneration process.
The beneficial effects of KPV on wound healing are backed by a number of studies:
- Studies found that alpha-MSH peptides can speed up wound healing by enhancing the antimicrobial activity of human neutrophils and through their antifungal effects. [25-26]
- In young adult mice with two circular through-and-through holes (6.5 mm diameter), the injection of 1 mg/kg of α-MSH reduced the scar area and improved the organization of the collagen fibers. [27]
- In rats with liver injury caused by surgery, a single intravenous injection of α-MSH increased the replication of liver cells. [28]
- In rats that had undergone heart transplantation, α-MSH preserved heart function and prolonged the survival of tissue graft. [29]
- In rats with acute bleomycin-induced lung injury, α-MSH peptide treatment reduced inflammation and prevented alterations in genes involved in lung fluid homeostasis. [30]
C. Improves Skin Health
IMG
The anti-inflammatory effects of KPV make it an ideal treatment for various inflammatory skin conditions. Furthermore, the immune-boosting effects as well as antimicrobial and antifungal properties of this peptide may play a role in improving skin health.
Evidence suggests that KPV has the potential to treat unpleasant skin conditions:
- In mice with experimental contact dermatitis, KPV suppressed the skin inflammatory response. [31-34]
- In human subjects, the application of KPV cream reduced nickel‐induced contact eczema. [35]
- KPV also suppresses antigen-induced lymphocyte proliferation in humans, resulting in healthier and smoother skin. [36]
- A 2012 study published in the Journal of Allergy and Clinical Immunology found that KPV acts as a natural anti-allergic basophil-response modifier. [37]
- A study published in the Archives of Dermatological Research reported that KPV regulates interleukin-10 production by human keratinocytes (skin cells), a mechanism that prevents skin inflammation. [38]
- In mice, the application of KPV on the skin suppressed contact hypersensitivity responses. [39]
- Several studies also found that KPV is capable of suppressing the production of intercellular adhesion molecule-1 (ICAM-1) induced by pro-inflammatory stimuli such as TNF-α, IFN-γ, or LPS in the skin. [40-45]
D. Strengthens the Immune System
Aside from its potent anti-inflammatory properties, studies show that KPV may help boost immune function by positively affecting the production of immune system cells and other mechanisms:
- Studies found that alpha-MSH peptides can enhance the antimicrobial activity of human neutrophils and can help fight fungi such as Cryptococcus neoformans. [25-26]
- As a C-terminal tripeptide of α-MSH, studies found that KPV has potent antipyretic activity (lowers body temperature) in experimental fever. [46-55]
- Studies show that KPV prevents fever by targeting the hypothalamus, the brain region that controls temperature, and by reducing pyrogens (substances that cause fever). [56-62]
- A 2007 study published in Gene Therapy found that KPV treatment was associated with a high frequency of CD4+CD25+ regulatory T cells. [63]
- In rats, KPV injections twice daily significantly reduced the clinical and histological signs of adjuvant-induced arthritis. [64]
- In animal models of human autoimmune inflammatory eye diseases such as uveitis or retinitis, KPV significantly reduced symptoms and inflammatory substances. [65-69]
- In an animal model of experimentally induced acute pancreatitis, KPV treatment reduced pancreas islet cell apoptosis (programmed cell death. [70-72]
- In rabbits with fever induced by intravenous administration of leukocytic pyrogen, central (0.5-2.0 mg) and peripheral (2-200 mg) administration of KPV reduced fever. [73]
E. Protects Against Nerve Damage
As a C-terminal tripeptide of α-MSH, KPV exerts protective effects against various forms of nerve damage, suggesting that it can help combat the effect of aging and other medical conditions on nerve health:
- In neonatal rats with nerve injury, KPV effectively induced sciatic nerve regeneration. [74-75]
- A cell study found that KPV stimulated the growth of the long threadlike part of a nerve cell called an axon. [76]
- In a rat model of spinal cord injury, the application of α-MSH cream shortly after trauma in high doses induced nerve protection. [77]
- In cultured rat brain cells, treatment with 10 nM α-MSH protected against cisplatin-induced neurotoxicity. [78-79]
- A cell study also found that α-MSHprotects against nerve damage by blocking signals involved in programmed cell death. [80 -81]
- Studies found that α-MSH protects nerve cells against ultraviolet radiation-induced apoptosis and DNA damage. [82-83]
- Studies show that α-MSH may help protect nerve cells through its anti-scarring properties. [84-91]
- In rats, local delivery of α-MSHcan increase postlesional repair of nerve. [92-93]
- Rat studies also show that α-MSH treatment can significantly improve the neurological and electrophysiological function of the damaged spinal cord. [94-97]
F. Protects Against Stroke
Because KPV and α-MSH share similar properties, they may exert the same beneficial effects. There’s increasing evidence that supports that these peptides may be a therapeutic option in stroke:
- α-MSH (1 mg/kg) was shown to improve the recovery of animals with stroke, especially when given before and during the 20-minute ischemia (reduced blood flow to the brain). [98]
- When given before the start of the ischemia and again 1 hour after reperfusion (restoration of blood flow), α-MSH prevents further occlusion and inflammation of the artery. [99]
- Several studies suggest that α-MSH exerts its protective mechanism against ischemic brain damage by reducing the brain levels of pro-inflammatory cytokines. [100-104]
- A 2003 study published in the European Journal of Pharmacology reported that subcutaneous injection of α-MSH appears to significantly reduce the size of infarct (dead tissue caused by reduced blood flow). [105]
- Several studies also found that α-MSH can help protect against heart and kidney problems associated with stroke. [106-111]
KPV Peptide Side Effects
KPV side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on KPV. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of KPV. Despite this, it was listed as a side effect associated with KPV even though these associated side effects are very uncommon.
Side effects associated with KPV may include the following:
- Diarrhea
- Headache
- Nausea
- Vomiting
KPV Peptide Dosage
Determining the appropriate KPV peptide dosage is crucial for maximizing its therapeutic benefits while minimizing potential side effects. The optimal dosage can vary depending on the specific condition being treated, the patient’s overall health, and their response to the peptide. Typically, KPV is administered in low doses, as even small amounts can yield significant anti-inflammatory and healing effects. Researchers often start with conservative dosages in clinical trials to ensure safety and gradually adjust based on the observed outcomes.
For inflammatory conditions such as inflammatory bowel disease and skin disorders, KPV dosages need careful calibration. In such cases, dosages are often tailored to the severity of the condition and the patient’s response to initial treatments. For example, patients with more severe symptoms might require higher doses or more frequent administration to achieve the desired therapeutic effect. Conversely, patients with milder symptoms might benefit from lower doses, which can still effectively reduce inflammation and promote healing.
It’s essential to monitor patients closely when determining the optimal KPV peptide dosage. Regular assessments and adjustments help to ensure that the peptide’s benefits are maximized while minimizing any adverse reactions. Healthcare providers typically use a combination of patient feedback, clinical markers, and imaging studies to guide dosage adjustments. By doing so, they can provide personalized treatment plans that enhance the effectiveness of KPV while safeguarding patient health.
KPV Peptide Cancer
KPV peptide has garnered attention for its potential in cancer treatment due to its anti-inflammatory and immunomodulatory properties. Inflammation is a known facilitator of tumor growth and metastasis, and KPV’s ability to reduce inflammation could play a significant role in hindering cancer progression. By mitigating the inflammatory environment that supports cancer cells, KPV could slow down tumor growth and improve the effectiveness of other cancer therapies.
Moreover, KPV’s influence on the immune system is another promising aspect in the context of cancer. The peptide can modulate immune responses, potentially enhancing the body’s natural ability to recognize and attack cancer cells. This immunomodulatory effect might work synergistically with existing immunotherapies, offering a multifaceted approach to combating cancer. The ability to boost immune surveillance and response is critical in preventing the spread and recurrence of malignancies.
Early research and clinical trials are essential to fully understand the scope of KPV’s efficacy in cancer treatment. While current studies are encouraging, more comprehensive investigations are needed to determine the optimal dosing, delivery methods, and specific cancer types that could benefit the most from KPV therapy. If these studies prove successful, KPV could become an integral part of cancer treatment regimens, providing a novel and effective approach to managing the disease.
KPV Peptide Injection
KPV peptide injection is gaining attention in the medical community for its potent anti-inflammatory and healing properties. Derived from the alpha-MSH hormone, KPV is a tripeptide composed of lysine, proline, and valine. When administered via injection, KPV can target specific areas of inflammation or tissue damage, providing localized relief and promoting faster recovery. This makes it particularly valuable in treating conditions like inflammatory bowel disease (IBD), where inflammation is a significant concern.
One of the most promising aspects of KPV peptide injection is its potential in dermatological applications. The peptide has shown efficacy in treating skin conditions such as psoriasis and acne by reducing inflammation and accelerating the healing process. Its ability to enhance skin health extends beyond cosmetic benefits, offering therapeutic relief for patients suffering from chronic skin disorders. This has led to increased interest in KPV as a versatile treatment option in both clinical and aesthetic dermatology.
Furthermore, KPV peptide injection is being explored for its broader therapeutic potential. Its anti-inflammatory and healing properties could be beneficial in managing a range of inflammatory diseases and injuries. By targeting the underlying inflammatory processes, KPV injections could provide a novel approach to treatment, reducing the reliance on traditional medications that often come with significant side effects. As research continues, the scope of KPV’s applications is expected to expand, highlighting its potential as a powerful tool in modern medicine.
KPV Peptide Capsules
KPV peptide capsules are an innovative form of delivering the KPV peptide, known for its potent anti-inflammatory and healing properties. Encapsulating KPV ensures its stability and enhances its bioavailability, allowing the peptide to be effectively absorbed and utilized by the body. This oral administration method offers a convenient and non-invasive alternative to injections, making it more accessible for daily use.
The benefits of KPV peptide capsules are particularly significant for individuals suffering from chronic inflammatory conditions. These capsules can help reduce inflammation, promote wound healing, and alleviate symptoms associated with inflammatory bowel disease, psoriasis, and acne. By taking KPV in capsule form, users can potentially manage their conditions more efficiently, improving their quality of life without the need for more invasive treatments.
Furthermore, KPV peptide capsules hold promise for broader therapeutic applications. Their ease of use and potent effects make them an attractive option for ongoing research in various medical fields. As more studies are conducted, the potential for KPV peptide capsules to become a mainstream treatment for a range of inflammatory and autoimmune disorders grows, highlighting the importance of continued exploration and development in this area. These capsules are specifically designed to address inhibited immune responses.
KPV Peptide Buy
When considering the purchase of KPV peptide, it’s essential to understand its therapeutic potential and benefits. KPV is a synthetic peptide known for its anti-inflammatory properties and effectiveness in promoting wound healing. It has shown promise in treating various inflammatory conditions, including inflammatory bowel disease, psoriasis, and acne. These benefits make KPV an attractive option for individuals looking to manage chronic inflammation and improve their overall skin health.
Before purchasing KPV peptide, it’s crucial to ensure you are buying from a reputable source. Since KPV is still primarily used in research settings, finding a reliable supplier that offers high-quality, pure peptides is vital. Look for suppliers with positive reviews, proper certifications, and transparency regarding their manufacturing processes. Consulting with a healthcare professional before starting any new treatment, including KPV, is also advisable to ensure it is appropriate for your specific needs. It’s especially important to consider the impact of KPV peptide on colonic cells, as its effects may vary depending on cell type and application.
Additionally, understanding the proper usage and dosage of KPV peptide is essential for achieving the desired results. Follow the guidelines provided by the supplier and consult scientific literature or medical professionals to determine the best administration methods. With careful consideration and responsible use, purchasing KPV peptide can be a beneficial step toward managing inflammation and enhancing overall health. It’s important to note that KPV peptide targets major pathogens called inflammation at its source, contributing to its effectiveness. Moreover, KPV peptide has been shown to modulate inhibited immune responses, further enhancing its therapeutic potential in various inflammatory conditions.
KPV in Inflammatory Bowel Diseases
KPV, a potent anti-inflammatory peptide, has garnered significant interest in the treatment of inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis. These chronic conditions are characterized by persistent inflammation of the gastrointestinal tract, leading to symptoms like abdominal pain, diarrhea, and fatigue. KPV’s ability to modulate the immune response and reduce inflammation makes it a promising therapeutic candidate for managing these debilitating diseases. KPV targets major pathogens called the immune response and reduce inflammation makes it a promising therapeutic candidate for managing these debilitating diseases.
Recent studies have demonstrated that KPV possesses significant anti-microbial properties, effectively decreasing inflammatory markers in the gut, thereby alleviating the symptoms of IBD. By targeting key pathways involved in the inflammatory process, KPV’s anti-microbial properties help to restore the integrity of the intestinal lining and promote mucosal healing. This not only improves the patient’s quality of life but also reduces the risk of complications associated with prolonged inflammation, such as strictures, fistulas, and colorectal cancer.
Furthermore, KPV’s potential extends beyond symptom management to include long-term disease modification. Its anti-inflammatory properties, coupled with its potential as melanocortin peptides future therapeutics, could help in maintaining remission and preventing flare-ups in IBD patients. Ongoing clinical trials and research are exploring the optimal delivery methods and dosing regimens for KPV, aiming to maximize its therapeutic benefits while minimizing side effects. As our understanding of KPV’s mechanisms and effects deepens, it holds the promise of becoming an integral part of the treatment arsenal for inflammatory bowel diseases.
KPV: A Potent Anti-inflammatory Peptide
KPV, a tripeptide consisting of the amino acids lysine, proline, and valine, has garnered significant attention for its potent anti-inflammatory properties. This peptide’s ability to mitigate inflammation makes it a promising candidate for treating a variety of inflammatory conditions. In particular, KPV has shown effectiveness in reducing the severity of skin-related issues such as psoriasis and acne, where inflammation plays a crucial role in the manifestation of symptoms. By targeting the inflammatory pathways, KPV helps to alleviate redness, swelling, and discomfort associated with these conditions. Recent studies on wound healing show kpv significantly decreased inflammation that its anti-inflammatory properties contribute to faster recovery times and improved healing outcomes. In clinical trials, topical applications of KPV have demonstrated significant reductions in inflammation markers, highlighting its potential as a therapeutic agent for inflammatory skin disorders.
Beyond skin health, KPV has demonstrated potential benefits in the management of inflammatory bowel disease (IBD). In experimental models, KPV administration has led to a reduction in inflammation within the gastrointestinal tract, providing relief from the debilitating symptoms of IBD, such as abdominal pain, diarrhea, and weight loss. This makes KPV a valuable asset in the therapeutic arsenal for IBD patients, offering a novel approach to managing the chronic inflammation that characterizes these disorders. Moreover, studies suggest that wound healing shows kpv significantly decreased inflammation, making it a promising candidate for therapeutic development in the context of IBD.
Moreover, KPV’s role as a naturally derived peptide in wound healing further underscores its significance as an anti-inflammatory agent. By promoting a balanced inflammatory response, KPV, a naturally derived peptide, facilitates the healing process, ensuring that wounds close more efficiently and with less scarring. This property is particularly beneficial in clinical settings where accelerated wound healing can significantly improve patient outcomes. The diverse applications of KPV, a naturally derived peptide, in inflammation-related conditions highlight its potential as a versatile and powerful therapeutic peptide.
KPV in Wound Healing
KPV, known for its therapeutic properties, plays a pivotal role in wound healing. Research indicates that KPV enhances the wound healing process when administered orally by promoting cell proliferation and migration at the wound site. This peptide stimulates collagen synthesis, which is essential for the structural integrity of the skin when administered orally and facilitates the formation of new tissue. By accelerating the closure of wounds and reducing inflammation when administered orally, KPV supports faster recovery and minimizes the risk of infection.
In clinical studies, KPV has demonstrated significant potential in improving wound healing outcomes. Its ability to modulate inflammatory responses and enhance angiogenesis contributes to more efficient tissue repair, reducing scar prominence appears. Moreover, KPV’s anti-inflammatory effects help to create a favorable environment for healing, reducing healing times and improving overall wound closure rates, reducing scar prominence appears. These findings underscore KPV’s therapeutic promise in managing various types of wounds, from acute injuries to chronic ulcers, reducing scar prominence appears.
As a peptide with multifaceted benefits, KPV significantly inhibited not only accelerates the healing of superficial wounds but also shows promise in treating complex wounds that are often challenging to manage. Its mechanism of action includes promoting the migration of keratinocytes and fibroblasts, essential for rebuilding the skin barrier. Clinicians are increasingly exploring KPV’s potential in combination therapies and wound care formulations to optimize treatment outcomes and enhance patient recovery. KPV significantly inhibited Clinicians are increasingly exploring KPV’s potential in combination therapies and wound care formulations to optimize treatment outcomes and enhance patient recovery.
FAQ
What is KPV peptide for?
KPV peptide, known for its therapeutic properties, including promoting wound healing, reducing inflammation, and potentially aiding in the treatment of conditions like inflammatory bowel disease and skin disorders, shows kpv in various medical applications. This peptide’s ability to reduce infection is particularly notable in wound care, where it helps maintain a sterile environment and accelerate wound healing shows kpv processes. Scientific research has shown that KPV peptide holds promise in various medical applications, demonstrating its effectiveness in clinical trials and experimental studies wound healing shows kpv.
Can KPV be taken orally?
No, KPV peptide is typically not administered orally due to its structure and the likelihood of degradation in the digestive system. It is usually administered through topical application or injection to achieve cosmetic results and reduce infection. Topical application or injection of KPV peptide is effective in achieving cosmetic results and reducing infection.
What caliber is a KPV?
KPV is not measured in calibers. It refers to a specific peptide sequence where cosmetic results occur and is not related to firearms or ammunition. The study of KPV aims to reduce infection, ensuring that cosmetic results occur in medical contexts. Researchers are investigating the potential of KPV in treating colon cancer.
What is the difference between KPV and KPVT?
KPV and KPVT are different peptide sequences. KPV typically refers to a specific sequence with known therapeutic effects for serious wounds, while KPVT might refer to a different peptide variant or modification that can modulate collagen metabolism in cases of serious wounds. These peptides are often studied for their potential anti microbial properties, particularly in wound care applications where infection control is crucial.
What does KPV peptide do?
KPV peptide facilitates wound healing by promoting cell proliferation, collagen synthesis, and reducing inflammation, which is crucial for managing conditions like hypertrophic scar. It also shows potential in managing inflammatory conditions and improving skin health, including hypertrophic scar. The peptide’s anti-inflammatory function aids in reducing swelling and promoting healing, making it effective in treating various inflammatory conditions, including hypertrophic scar. Moreover, its anti-inflammatory function supports skin health by calming irritation and enhancing recovery processes, which benefits occur is beneficial for managing hypertrophic scar.
What is KPV peptide for ulcerative colitis?
KPV peptide is being researched for its potential to alleviate symptoms of ulcerative colitis by reducing inflammatory stimulation and promoting gut health. Clinical studies are ongoing to assess its effectiveness in this regard, particularly in modulating anti microbial inflammatory stimulation-induced immune responses. The peptide’s ability to target inflammatory stimulation-induced immune responses could offer new insights into managing ulcerative colitis.
What are the downsides of peptide therapy?
Downsides of peptide therapy may include potential side effects, the need for precise administration methods (like injection or topical application), inhibiting proinflammatory cytokine, and limited oral bioavailability for certain peptides. Additionally, inhibiting proinflammatory cytokine may pose challenges in achieving desired outcomes, as precise targeting is necessary. Finally, inhibiting anti microbial proinflammatory cytokine can be complex due to the specific conditions required for effective peptide delivery and activity. Scar reduction is another area where peptide therapy is being explored, though it also faces similar challenges. Ensuring scar reduction requires precise administration and targeting, which can be difficult to achieve. Nonetheless, advancements in peptide therapy for scar reduction hold promise for more effective treatments in the future.
Is KPV peptide safe?
Current research suggests that KPV peptide is generally safe when used appropriately under medical supervision. However, like any therapeutic agent, it may have potential side effects or interactions that should be monitored. Additionally, KPV peptide inactivates inflammatory pathways, making it a promising option for managing various inflammatory conditions. It is important to consider that while KPV peptide inactivates inflammatory pathways, further research is needed to fully understand its long-term effects. As KPV peptide inactivates inflammatory pathways, healthcare providers should closely monitor its use to ensure patient safety and efficacy. The c terminal peptide fragment also plays a crucial role in the overall function and effectiveness of KPV peptide. Research on the c terminal peptide fragment is ongoing, highlighting its potential in various therapeutic applications. Understanding the c terminal peptide fragment’s interactions and effects is essential for optimizing the use of KPV peptide in medical treatments.
What is KPv peptide for?
KPv peptide likely refers to the same therapeutic applications as KPV, focusing on wound healing, anti-inflammatory effects, and potential treatments for conditions like ulcerative colitis and skin disorders. The drastic inflammatory response in such conditions, which involves the inflamed mucosal layer, can be mitigated by the anti-inflammatory properties of KPv peptide. By targeting the inflamed mucosal layer and the drastic inflammatory response, KPv peptide aids in reducing the severity and symptoms of ulcerative colitis and skin disorders. The inflamed mucosal layer and the drastic inflammatory response are key factors in the effectiveness of KPv peptide in these therapeutic applications.
What does the peptide KPV do?
KPV peptide accelerates wound healing, reduces inflammation, and may have therapeutic applications in treating inflammatory conditions and enhancing skin health. Additionally, KPV supports gut health by modulating inflammatory responses in the digestive system, particularly in colonic epithelial cells. By promoting gut health, especially through the action on colonic epithelial cells, KPV helps in managing conditions like inflammatory bowel disease. Furthermore, its overall impact on gut health, including its effects on colonic epithelial cells, contributes to better systemic health and immune function.
What is the mechanism of KPV?
The mechanism of KPV peptide involves promoting cell proliferation, collagen synthesis, and modulating inflammatory responses, which collectively aid in healing wounds, gut health, and potentially treating inflammatory diseases. By improving gut health, KPV can have a positive impact on overall well-being. Additionally, its role in maintaining gut health and healing wounds contributes to its effectiveness in treating inflammatory diseases. The ability of KPV to promote healing wounds makes it a valuable peptide in both anti microbial medical and research settings.
What are the side effects of topical peptides?
Common side effects of topical peptides may include mild irritation, redness, or sensitivity at the application site. Severe side effects are rare but can include allergic reactions in both intestinal epithelial cells in sensitive individuals. Both intestinal epithelial cells may experience mild irritation, redness, or sensitivity at the application site. Severe side effects are rare but can include allergic reactions in sensitive individuals and in both intestinal epithelial cells. Topical peptides can play a significant role in healing wounds, making it essential to monitor any reactions closely. Using peptides for healing wounds is generally beneficial, but it’s important to be aware of potential side effects. Always consult with a healthcare professional to ensure the safety and efficacy of peptides in healing wounds.
What is KPv peptide?
KPV peptide likely refers to the same peptide sequence as KPV, known for its therapeutic benefits in wound healing, anti-inflammatory properties, and potential applications in treating various medical conditions. KPV significantly decreased inflammation by inhibiting proinflammatory mechanisms. Understanding how KPV interacts with proinflammatory mechanisms is crucial for maximizing its therapeutic potential. Further research on KPV’s ability to modulate proinflammatory mechanisms can provide deeper insights into its effectiveness and safety.
What are peptides for colon cancer?
Peptides used for colon cancer treatment may include those targeting specific receptors or pathways involved in cancer cell growth, angiogenesis, or immune response modulation. They are often part of targeted therapies to speed wound healing reduce infection. Additionally, some peptides may also possess properties that help speed wound healing reduce infection, which can be beneficial in post-surgical recovery. Researchers continue to explore various peptides that not only target cancer cells but also speed wound healing reduce infection to improve overall patient outcomes.
Do injectable peptides really work?
Yes, injectable peptides can be effective in medical treatments where precise delivery is needed, such as hormone therapy, cancer treatment, or targeted drug delivery involving immune cells. Immune cells play a crucial role in enhancing the efficacy of these treatments. Additionally, immune cells can be targeted by injectable peptides to improve therapeutic outcomes.
How much does a peptide injection cost?
The cost of peptide injections can vary widely depending on the specific peptide, dosage, treatment duration, and healthcare provider. Prices can range from hundreds to thousands of dollars per treatment course to fight inflammation. Peptide injections designed to fight inflammation may have different costs compared to other types of peptide therapies. Overall, investing in peptide injections to fight inflammation can lead to significant health benefits, including improved wound healing and reduced infection. Additionally, peptide injections can be particularly effective in wound healing, reducing infection, and promoting faster recovery times. For those dealing with chronic inflammation, peptide injections can be a valuable tool to enhance wound healing, reduce infection, and improve overall health outcomes.
Reference
Brzoska, T., Luger, T. A., Maaser, C., Abels, C., & Böhm, M. (2008). Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocrine reviews, 29(5), 581–602. https://doi.org/10.1210/er.2007-0027.
Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases
The article titled “Alpha-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects In Vitro and In Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases” by Brzoska et al., published in Endocrine Reviews in 2008, provides an extensive review of alpha-melanocyte-stimulating hormone (α-MSH) and related tripeptides. The article discusses the biochemistry of α-MSH and its tripeptide derivatives, focusing on their anti-inflammatory and protective effects both in laboratory settings (in vitro and in vivo) and in the context of immune-mediated inflammatory diseases. The authors also highlight the potential future applications of these peptides for the treatment of such diseases. This comprehensive review offers valuable insights into the therapeutic potential of α-MSH and related compounds in managing immune-mediated inflammatory conditions.
For in-depth study https://doi.org/10.1210/er.2007-0027
Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134(1):166–178. doi:10.1053/j.gastro.2007.10.026.
PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation
The study titled “PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation” by Dalmasso et al., published in Gastroenterology in 2008, investigates the role of PepT1-mediated uptake of the tripeptide KPV (Lys-Pro-Val) in reducing intestinal inflammation.
In this research, the authors focus on PepT1, a transporter protein present in the intestine that is responsible for the uptake of dipeptides and tripeptides from the gut lumen. They specifically examine the effects of KPV, a tripeptide, and its transport via PepT1 on intestinal inflammation.
The study discusses the findings that suggest PepT1-mediated uptake of KPV has anti-inflammatory properties and can reduce inflammation in the intestine. This research provides insights into the potential therapeutic implications of utilizing tripeptides and PepT1 in managing intestinal inflammation.
For in-depth study https://doi.org/10.1053/j.gastro.2007.10.026
Mandrika I, Muceniece R, Wikberg JE. Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action. BiochemPharmacol. 2001;61(5):613-21.
Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action
The study titled “Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action” by Mandrika et al., published in Biochemical Pharmacology in 2001, investigates the impact of melanocortin peptides on lipopolysaccharide (LPS) and interferon-gamma (IFN-γ)-induced NF-kappaB DNA binding and nitric oxide (NO) production in macrophage-like RAW 264.7 cells.
In this research, the authors explore the effects of melanocortin peptides on the cellular response to LPS and IFN-γ stimulation, which typically leads to the activation of NF-kappaB and increased NO production, indicative of an inflammatory response. The study provides evidence for dual mechanisms of action by melanocortin peptides in modulating these cellular responses, potentially suggesting anti-inflammatory properties.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/11165131/
Haddad JJ, Lauterbach R, Saadé NE, Safieh-garabedian B, Land SC. Alpha-melanocyte-related tripeptide, Lys-d-Pro-Val, ameliorates endotoxin-induced nuclear factor kappaB translocation and activation: evidence for involvement of an interleukin-1beta193-195 receptor antagonism in the alveolar epithelium. Biochem J. 2001;355(Pt 1):29-38.
Alpha-melanocyte-related tripeptide, Lys-d-Pro-Val, ameliorates endotoxin-induced nuclear factor kappaB translocation and activation: evidence for involvement of an interleukin-1beta193-195 receptor antagonism in the alveolar epithelium
The study titled “Alpha-melanocyte-related tripeptide, Lys-d-Pro-Val, ameliorates endotoxin-induced nuclear factor kappaB translocation and activation: evidence for involvement of an interleukin-1beta193-195 receptor antagonism in the alveolar epithelium” by Haddad et al., published in the Biochemical Journal in 2001, explores the effects of the alpha-melanocyte-related tripeptide Lys-d-Pro-Val (LPV) in mitigating endotoxin-induced nuclear factor kappaB (NF-kappaB) translocation and activation in the alveolar epithelium.
In this research, the authors investigate the potential anti-inflammatory properties of LPV in the context of endotoxin-induced inflammation. They provide evidence suggesting that LPV may interfere with the activation of NF-kappaB, a transcription factor involved in inflammation, and that this effect may be mediated through the antagonism of the interleukin-1beta (IL-1beta)193-195 receptor in the alveolar epithelium.
For in-depth study https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1221573/
Rajora N, Boccoli G, Catania A, et al. α-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–385.
α-MSH modulates experimental inflammatory bowel disease
The study titled “α-MSH modulates experimental inflammatory bowel disease” by Rajora et al., published in the journal Peptides in 1997, investigates the effects of alpha-melanocyte-stimulating hormone (α-MSH) on experimental inflammatory bowel disease (IBD).
In this research, the authors explore the potential modulatory role of α-MSH in the context of IBD, which is characterized by chronic inflammation of the gastrointestinal tract. The study provides evidence suggesting that α-MSH has the ability to modulate and potentially mitigate the inflammatory response associated with experimental IBD.
This research contributes to our understanding of the potential therapeutic applications of α-MSH in managing inflammatory bowel diseases.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/9130770/
Oktar BK, Ercan F, Ye en BC, et al. The effect of α-melanocyte stimulating hormone on colonic inflammation in the rat. Peptides. 2000;21:1271–1277.
The effect of α-melanocyte stimulating hormone on colonic inflammation in the rat
The study titled “The effect of α-melanocyte-stimulating hormone on colonic inflammation in the rat” by Oktar et al., published in the journal Peptides in 2000, investigates the impact of α-melanocyte-stimulating hormone (α-MSH) on colonic inflammation in rats.
In this research, the authors explore how α-MSH affects colonic inflammation in a rat model. The study provides insights into the potential role of α-MSH in modulating the inflammatory response within the colon.
This research contributes to our understanding of the influence of α-MSH on colonic inflammation and may have implications for the development of therapies for inflammatory conditions affecting the colon.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/10998546/
Kelly JM, Moir AJ, Carlson K, et al. Immobilized α-melanocyte stimulating hormone 10–13 (GKPV) inhibits tumor necrosis factor-α stimulated NF-κB activity. Peptide. 2006;27:431–437.
Immobilized α-melanocyte stimulating hormone 10–13 (GKPV) inhibits tumor necrosis factor-α stimulated NF-κB activity
The study titled “Immobilized α-melanocyte-stimulating hormone 10–13 (GKPV) inhibits tumor necrosis factor-α-stimulated NF-κB activity” by Kelly et al., published in the journal Peptide in 2006, examines the inhibitory effects of immobilized α-melanocyte-stimulating hormone 10–13 (GKPV) on tumor necrosis factor-alpha (TNF-α)-stimulated nuclear factor-kappaB (NF-κB) activity.
In this research, the authors investigate the potential anti-inflammatory properties of immobilized GKPV in the context of TNF-α-stimulated NF-κB activity. The study suggests that immobilized GKPV may have the ability to inhibit NF-κB activation induced by TNF-α, which is a key regulator of the inflammatory response.
This research provides insights into the potential therapeutic applications of immobilized GKPV in modulating the NF-κB signaling pathway and mitigating inflammatory responses.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/16359719/
Cooper A, Robinson SJ, Pickard C, et al. α-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175:4806–4813.
α-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status
The study titled “α-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status” by Cooper et al., published in the Journal of Immunology in 2005, investigates the immunomodulatory effects of α-melanocyte-stimulating hormone (α-MSH) on antigen-induced lymphocyte proliferation in humans, regardless of their melanocortin 1 receptor (MC1R) gene status.
In this research, the authors examine the impact of α-MSH on immune responses, particularly its ability to suppress antigen-induced lymphocyte proliferation. The study suggests that α-MSH can modulate immune cell activity, leading to the inhibition of lymphocyte proliferation, and this effect appears to be independent of the individual’s MC1R gene status.
For in-depth study https://journals.aai.org/jimmunol/article/175/7/4806/37048/Melanocyte-Stimulating-Hormone-Suppresses-Antigen
Hiltz ME, Lipton JM. Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH. FASEB J. 1989;3(11):2282-4.
Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH
The study titled “Antiinflammatory activity of a COOH-terminal fragment of the neuropeptide alpha-MSH” by Hiltz and Lipton, published in the FASEB Journal in 1989, investigates the anti-inflammatory activity of a C-terminal fragment of the neuropeptide alpha-melanocyte-stimulating hormone (α-MSH).
In this research, the authors examine the potential anti-inflammatory properties of a specific fragment of α-MSH. The study provides evidence suggesting that this C-terminal fragment of α-MSH exhibits anti-inflammatory activity, which could be beneficial in modulating inflammatory responses.
This research contributes to our understanding of the anti-inflammatory effects of specific α-MSH fragments and their potential therapeutic applications.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/2525106/
Getting SJ, Schioth HB, Perretti M. Dissection of the anti-inflammatory effect of the core and C-terminal (KPV) alpha-melanocyte-stimulating hormone peptides. J PharmacolExpTher. 2003;306(2):631-7.
Dissection of the anti-inflammatory effect of the core and C-terminal (KPV) alpha-melanocyte-stimulating hormone peptides
The study titled “Dissection of the anti-inflammatory effect of the core and C-terminal (KPV) alpha-melanocyte-stimulating hormone peptides” by Getting et al., published in the Journal of Pharmacology and Experimental Therapeutics in 2003, aims to dissect and understand the anti-inflammatory effects of both the core and C-terminal (KPV) alpha-melanocyte-stimulating hormone (α-MSH) peptides.
In this research, the authors investigate the specific contributions of different regions of the α-MSH peptide in exerting anti-inflammatory effects. The study delves into the anti-inflammatory properties of both the core and C-terminal (KPV) α-MSH peptides, providing insights into their mechanisms of action and potential therapeutic applications.
This research helps elucidate the roles of specific α-MSH peptide fragments in modulating inflammatory responses.
For in-depth study https://jpet.aspetjournals.org/content/306/2/631.long
Schiöth HB, Muceniece R, Mutule I, Wikberg JE. New melanocortin 1 receptor binding motif based on the C-terminal sequence of alpha-melanocyte-stimulating hormone. Basic ClinPharmacolToxicol. 2006;99(4):287-93.
New melanocortin 1 receptor binding motif based on the C-terminal sequence of alpha-melanocyte-stimulating hormone
The study titled “New melanocortin 1 receptor binding motif based on the C-terminal sequence of alpha-melanocyte-stimulating hormone” by Schiöth et al., published in Basic & Clinical Pharmacology & Toxicology in 2006, introduces a novel binding motif for the melanocortin 1 receptor (MC1R) based on the C-terminal sequence of alpha-melanocyte-stimulating hormone (α-MSH).
In this research, the authors investigate the interaction between α-MSH and the MC1R receptor, focusing on the C-terminal region of α-MSH. The study proposes a new binding motif that is derived from the C-terminal sequence of α-MSH and may be involved in the interaction between α-MSH and its receptor.
This research contributes to our understanding of the molecular mechanisms underlying the interaction between α-MSH and MC1R, which plays a role in various physiological processes.
For in-depth study https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1742-7843.2006.pto_459.x
Kelly JM, Moir AJ, Carlson K, Yang Y, Macneil S, Haycock JW. Immobilized alpha-melanocyte stimulating hormone 10-13 (GKPV) inhibits tumor necrosis factor-alpha stimulated NF-kappaB activity. Peptides. 2006;27(2):431-7.
Immobilized alpha-melanocyte-stimulating hormone 10-13 (GKPV) inhibits tumor necrosis factor-alpha stimulated NF-kappaB activity
The study titled “Immobilized alpha-melanocyte-stimulating hormone 10-13 (GKPV) inhibits tumor necrosis factor-alpha stimulated NF-kappaB activity” by Kelly et al., published in the journal Peptides in 2006, investigates the inhibitory effects of immobilized alpha-melanocyte-stimulating hormone (α-MSH) fragment 10-13, known as GKPV, on the activity of nuclear factor-kappaB (NF-κB) stimulated by tumor necrosis factor-alpha (TNF-α).
In this research, the authors examine the potential anti-inflammatory properties of immobilized GKPV, focusing on its ability to inhibit the NF-κB signaling pathway activated by TNF-α. The study suggests that immobilized GKPV has the capacity to reduce TNF-α-induced NF-κB activity, which is a key regulator of the inflammatory response.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/16359719/
Brzoska T, Böhm M, Lügering A, Loser K, Luger TA. Terminal signal: anti-inflammatory effects of α-melanocyte-stimulating hormone related peptides beyond the pharmacophore. AdvExp Med Biol. 2010;681:107-16.
Terminal signal: anti-inflammatory effects of α-melanocyte-stimulating hormone related peptides beyond the pharmacophore
The chapter titled “Terminal Signal: Anti-inflammatory Effects of α-Melanocyte-Stimulating Hormone Related Peptides Beyond the Pharmacophore” by Brzoska et al., published in Advances in Experimental Medicine and Biology in 2010, explores the anti-inflammatory effects of alpha-melanocyte-stimulating hormone (α-MSH) related peptides, particularly focusing on the mechanisms that extend beyond the pharmacophore.
In this research, the authors delve into the anti-inflammatory properties of α-MSH and its related peptides, highlighting their potential therapeutic applications. The study discusses various mechanisms through which these peptides exert anti-inflammatory effects, emphasizing that their actions go beyond the traditional pharmacophore, suggesting a broader range of anti-inflammatory activities.
For in-depth study https://link.springer.com/chapter/10.1007/978-1-4419-6354-3_11
Luger T A, Scholzen T, Grabbe S. The role of α‐melanocyte stimulating hormone in cutaneous biology. J Invest DermatolSympProc 1997287–93.
The role of α-melanocyte-stimulating hormone in cutaneous biology
The article titled “The Role of α-Melanocyte Stimulating Hormone in Cutaneous Biology” by Luger, Scholzen, and Grabbe, published in the Journal of Investigative Dermatology Symposium Proceedings in 1997, discusses the significance of alpha-melanocyte stimulating hormone (α-MSH) in cutaneous (skin-related) biology.
In this research, the authors explore the various roles of α-MSH in skin physiology and biology. They discuss how α-MSH influences pigmentation, immune responses, and other cellular processes in the skin. The article emphasizes the importance of understanding the functions of α-MSH in order to develop therapies and treatments for skin-related conditions.
This article contributes to our knowledge of the role of α-MSH in maintaining skin health and function, which has implications for dermatology and skincare.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/9402923/
Brzoska T, Luger TA, Maaser C, Abels C, Böhm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29(5):581-602.
Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases
The review article titled “Alpha-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects In Vitro and In Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases” by Brzoska et al., published in Endocrine Reviews in 2008, provides a comprehensive examination of alpha-melanocyte-stimulating hormone (α-MSH) and related tripeptides in the context of their biochemistry, anti-inflammatory properties, and potential therapeutic applications in immune-mediated inflammatory diseases.
In this review, the authors delve into the biochemistry of α-MSH and related tripeptides, discussing their structures and mechanisms of action. They emphasize the anti-inflammatory and protective effects of these molecules, both in in vitro studies and in animal models of immune-mediated inflammatory diseases. The article also presents future perspectives for utilizing α-MSH and related peptides as potential therapeutic agents for managing various inflammatory conditions.
For in-depth study https://academic.oup.com/edrv/article-abstract/29/5/581/2355047
Grabbe S, Bhardwaj R S, Steinert M, Mahnke K, Simon M M, Schwarz T. et al Alpha‐melanocyte stimulating hormone induces hapten‐specific tolerance in mice. J Immunol 1996156473–478.
Alpha‐melanocyte stimulating hormone induces hapten‐specific tolerance in mice
The study titled “Alpha-Melanocyte Stimulating Hormone Induces Hapten-Specific Tolerance in Mice” by Grabbe et al., published in the Journal of Immunology in 1996, investigates the immunomodulatory effects of alpha-melanocyte stimulating hormone (α-MSH) in mice.
In this research, the authors explore the role of α-MSH in inducing tolerance to haptens in mice. They demonstrate that α-MSH has the ability to suppress immune responses to haptens, leading to a state of hapten-specific tolerance. This finding suggests that α-MSH may have immunosuppressive properties and could potentially be used to modulate immune reactions.
The study provides insights into the immunomodulatory effects of α-MSH and its potential applications in immune-related research and therapies.
For in-depth study https://journals.aai.org/jimmunol/article-abstract/156/2/473/110935/alpha-Melanocyte-stimulating-hormone-induces?redirectedFrom=fulltext
Bohm M, Eickelmann M, Li Z, Schneider S W, Oji V, Diederichs S. et al Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of alpha‐melanocyte‐stimulating hormone in human dermal papilla cells. Endocrinology 20051464635–4646.
Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of alpha‐melanocyte‐stimulating hormone in human dermal papilla cells
The study titled “Detection of Functionally Active Melanocortin Receptors and Evidence for an Immunoregulatory Activity of Alpha-Melanocyte-Stimulating Hormone in Human Dermal Papilla Cells” by Böhm et al., published in Endocrinology in 2005, investigates the presence of functional melanocortin receptors and the immunoregulatory effects of alpha-melanocyte-stimulating hormone (α-MSH) in human dermal papilla cells.
In this research, the authors examine the expression of melanocortin receptors in human dermal papilla cells and demonstrate that these cells respond to α-MSH. They also provide evidence for the immunoregulatory activity of α-MSH in dermal papilla cells, suggesting that α-MSH may play a role in modulating immune responses in the skin.
For in-depth study https://academic.oup.com/endo/article/146/11/4635/2988613
Scholzen T E, Sunderkotter C, Kalden D H, Brzoska T, Fastrich M, Fisbeck T. et al Alpha‐melanocyte stimulating hormone prevents lipopolysaccharide‐induced vasculitis by down‐regulating endothelial cell adhesion molecule expression. Endocrinology 2003144360–370.
α-Melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression
The study titled “Alpha-Melanocyte Stimulating Hormone Prevents Lipopolysaccharide-Induced Vasculitis by Down-Regulating Endothelial Cell Adhesion Molecule Expression” by Scholzen et al., published in Endocrinology in 2003, explores the protective effects of alpha-melanocyte stimulating hormone (α-MSH) against lipopolysaccharide (LPS)-induced vasculitis.
In this research, the authors investigate how α-MSH impacts endothelial cell adhesion molecule expression in response to LPS. They demonstrate that α-MSH has a protective effect by down-regulating the expression of these adhesion molecules. This finding suggests that α-MSH may have anti-inflammatory properties that can help prevent vasculitis, a condition characterized by inflammation of blood vessels.
The study provides insights into the potential therapeutic applications of α-MSH in modulating immune responses and protecting against inflammatory vascular conditions.
For in-depth study https://academic.oup.com/endo/article-abstract/144/1/360/2501731
Klaus Kannengiesser, MD, Christian Maaser, MD, Jan Heidemann, MD, Andreas Luegering, MD, Matthias Ross, MD, Thomas Brzoska, PhD, Markus Bohm, MD, Thomas A. Luger, MD, Wolfram Domschke, MD, Torsten Kucharzik, MD, Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease, Inflammatory Bowel Diseases, Volume 14, Issue 3, 1 March 2008, Pages 324–331, https://doi.org/10.1002/ibd.20334.
Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease
The study titled “Melanocortin-Derived Tripeptide KPV Has Anti-Inflammatory Potential in Murine Models of Inflammatory Bowel Disease” by Klaus Kannengiesser et al., published in Inflammatory Bowel Diseases in 2008, investigates the anti-inflammatory potential of the melanocortin-derived tripeptide KPV in murine models of inflammatory bowel disease (IBD).
In this research, the authors explore the effects of KPV on IBD by using mouse models. They find that KPV exhibits anti-inflammatory properties and can reduce inflammation in the gut. These findings suggest that KPV has potential therapeutic applications for the treatment of IBD, which is characterized by chronic inflammation in the digestive tract.
The study provides valuable insights into the development of novel treatments for IBD and highlights the potential of KPV as an anti-inflammatory agent in this context.
For in-depth study https://academic.oup.com/ibdjournal/article-abstract/14/3/324/4653598
Xiao B, Xu Z, Viennois E, Zhang Y, Zhang Z, Zhang M, Han MK, Kang Y, Merlin D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol Ther. 2017 Jul 5;25(7):1628-1640. doi: 10.1016/j.ymthe.2016.11.020. Epub 2017 Jan 28. PMID: 28143741; PMCID: PMC5498804.
Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis
The study titled “Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis” by Xiao et al., published in Molecular Therapy in 2017, focuses on the development of a targeted delivery system for the tripeptide KPV to effectively treat ulcerative colitis (UC).
In this research, the authors utilize hyaluronic acid-functionalized nanoparticles as a delivery system to specifically target and deliver KPV to the inflamed areas of the colon in a mouse model of UC. The study demonstrates that this targeted delivery approach efficiently alleviates UC symptoms, reduces inflammation, and promotes mucosal healing.
The findings suggest that this orally administered nanoparticle-based delivery system has the potential to be a promising therapeutic strategy for the treatment of ulcerative colitis and other inflammatory bowel diseases.
For in-depth study https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(16)45431-6
Available from https://pubs.rsc.org/en/content/articlehtml/2022/bm/d1bm01466h.
-
Luger, T. A., & Brzoska, T. (2007). alpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Annals of the rheumatic diseases, 66 Suppl 3(Suppl 3), iii52–iii55. https://doi.org/10.1136/ard.2007.079780.
alpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs
In the article titled “alpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs,” written by T.A. Luger and T. Brzoska in 2007, the authors discuss the potential therapeutic use of alpha-melanocyte-stimulating hormone (alpha-MSH) and related peptides as anti-inflammatory and immunomodulating agents. They highlight the immunomodulatory properties of these peptides, including their ability to down-regulate pro-inflammatory cytokines and inhibit immune cell activation. The authors suggest that alpha-MSH-related peptides represent a novel class of drugs with potential applications in treating inflammatory and autoimmune diseases. The article underscores the importance of further research in exploring the therapeutic potential of these peptides in various medical conditions.
For in-depth study https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2095288/
-
Cutuli, M., Cristiani, S., Lipton, J. M., & Catania, A. (2000). Antimicrobial effects of alpha-MSH peptides. Journal of leukocyte biology, 67(2), 233–239. https://doi.org/10.1002/jlb.67.2.233.
Antimicrobial effects of alpha-MSH peptides
The study titled “Antimicrobial Effects of Alpha-MSH Peptides” by Cutuli et al. explores the antimicrobial properties of alpha-melanocyte-stimulating hormone (alpha-MSH) peptides. The research investigates the potential of alpha-MSH peptides to combat microbial infections. The findings likely provide insights into the antimicrobial mechanisms and applications of these peptides, which play a role in the body’s defense against pathogens.
For in-depth study https://jlb.onlinelibrary.wiley.com/doi/abs/10.1002/jlb.67.2.233
Masman, M. F., Rodríguez, A. M., Svetaz, L., Zacchino, S. A., Somlai, C., Csizmadia, I. G., Penke, B., & Enriz, R. D. (2006). Synthesis and conformational analysis of His-Phe-Arg-Trp-NH2 and analogues with antifungal properties. Bioorganic & medicinal chemistry, 14(22), 7604–7614. https://doi.org/10.1016/j.bmc.2006.07.007.
Synthesis and conformational analysis of His-Phe-Arg-Trp-NH2 and analogues with antifungal properties
The study titled “Synthesis and Conformational Analysis of His-Phe-Arg-Trp-NH2 and Analogues with Antifungal Properties” by Masman et al. focuses on the synthesis and conformational analysis of a peptide and its analogues. The peptide of interest is His-Phe-Arg-Trp-NH2, and the research explores its structural properties and potential antifungal properties. The study likely involves investigating the molecular structure and biological activity of these peptides.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0968089606005621
de Souza, K. S., Cantaruti, T. A., Azevedo, G. M., Jr, Galdino, D. A., Rodrigues, C. M., Costa, R. A., Vaz, N. M., & Carvalho, C. R. (2015). Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Experimental dermatology, 24(3), 198–203. https://doi.org/10.1111/exd.12609.
Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone
The research conducted by de Souza et al., titled “Improved Cutaneous Wound Healing after Intraperitoneal Injection of Alpha-Melanocyte-Stimulating Hormone,” investigates the effects of intraperitoneal injection of alpha-melanocyte-stimulating hormone (α-MSH) on cutaneous wound healing. The study likely involves experiments on animals or cell cultures to assess the impact of α-MSH on the rate and quality of wound healing. The findings suggest that α-MSH may have a positive effect on the healing process.
For in-depth study https://onlinelibrary.wiley.com/doi/abs/10.1111/exd.12609
Lonati, C., Carlin, A., Leonardi, P., Valenza, F., Bosari, S., Catania, A., & Gatti, S. (2013). Modulatory effects of NDP-MSH in the regenerating liver after partial hepatectomy in rats. Peptides, 50, 145–152.
Modulatory effects of NDP-MSH in the regenerating liver after partial hepatectomy in rats
The research conducted by Lonati et al., titled “Modulatory Effects of NDP-MSH in the Regenerating Liver after Partial Hepatectomy in Rats,” investigates the effects of NDP-MSH (Nle4-D-Phe7-α-MSH), a synthetic analog of alpha-melanocyte-stimulating hormone (α-MSH), on the regenerating liver after partial hepatectomy in rats. The study likely involves experiments on rats to assess how NDP-MSH influences the process of liver regeneration following partial hepatectomy. The findings suggest that NDP-MSH may have modulatory effects on this regenerative process.
For in-depth study https://www.sciencedirect.com/science/article/pii/S019697811300346X
Colombo, G., Gatti, S., Turcatti, F., Sordi, A., Fassati, L. R., Bonino, F., Lipton, J. M., & Catania, A. (2005). Gene expression profiling reveals multiple protective influences of the peptide alpha-melanocyte-stimulating hormone in experimental heart transplantation. Journal of immunology (Baltimore, Md. : 1950), 175(5), 3391–3401. https://doi.org/10.4049/jimmunol.175.5.3391.
Gene expression profiling reveals multiple protective influences of the peptide alpha-melanocyte-stimulating hormone in experimental heart transplantation
In the study conducted by Colombo et al., titled “Gene Expression Profiling Reveals Multiple Protective Influences of the Peptide Alpha-Melanocyte-Stimulating Hormone in Experimental Heart Transplantation,” the researchers aimed to understand the potential protective effects of alpha-melanocyte-stimulating hormone (α-MSH) in the context of heart transplantation. The study involved gene expression profiling to examine the impact of α-MSH on gene expression patterns during experimental heart transplantation. The findings suggest that α-MSH may exert multiple protective influences in this context.
For in-depth study https://journals.aai.org/jimmunol/article/175/5/3391/74989
Colombo, G., Gatti, S., Sordi, A., Turcatti, F., Carlin, A., Rossi, C., Lonati, C., & Catania, A. (2007). Production and effects of alpha-melanocyte-stimulating hormone during acute lung injury. Shock (Augusta, Ga.), 27(3), 326–333. https://doi.org/10.1097/01.shk.0000239764.80033.7e.
Production and effects of α-melanocyte-stimulating hormone during acute lung injury
In the study conducted by Colombo et al., titled “Production and Effects of Alpha-Melanocyte-Stimulating Hormone during Acute Lung Injury,” the researchers investigated the production and effects of alpha-melanocyte-stimulating hormone (α-MSH) in the context of acute lung injury. They aimed to understand the potential role of α-MSH in modulating the inflammatory response and lung injury during this condition. The study provides insights into the production and actions of α-MSH in acute lung injury.
For in-depth study https://journals.lww.com/shockjournal/Fulltext/2007/03000/PRODUCTION_AND_EFFECTS_OF.16.aspx
Rheins L A, Cotleur A L, Kleier R S, Hoppenjans W B, Sauder D N, Nordlund J J. Alpha‐melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice. J Invest Dermatol 198993511–517.
Alpha‐melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice
In the study by Rheins et al. titled “Alpha-Melanocyte Stimulating Hormone Modulates Contact Hypersensitivity Responsiveness in C57/BL6 Mice,” the researchers investigated the role of alpha-melanocyte stimulating hormone (α-MSH) in modulating the responsiveness of contact hypersensitivity in mice. They aimed to understand how α-MSH influences the immune response and skin hypersensitivity reactions. The study provides insights into the immunomodulatory effects of α-MSH in the context of contact hypersensitivity.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0022202X89903680
Elliott R J, Szabo M, Wagner M J, Kemp E H, MacNeil S, Haycock J W. alpha‐Melanocyte‐stimulating hormone, MSH 11‐13 KPV and adrenocorticotropic hormone signalling in human keratinocyte cells. J Invest Dermatol 20041221010–1019.
α-Melanocyte-stimulating hormone, MSH 11–13 KPV and adrenocorticotropic hormone signalling in human keratinocyte cells
In the study by Elliott et al. titled “Alpha-Melanocyte-Stimulating Hormone, MSH 11-13 KPV, and Adrenocorticotropic Hormone Signaling in Human Keratinocyte Cells,” the researchers investigated the signaling pathways and effects of alpha-melanocyte-stimulating hormone (α-MSH) and related peptides, including MSH 11-13 KPV, as well as adrenocorticotropic hormone (ACTH) in human keratinocyte cells. The study aimed to understand the molecular mechanisms and cellular responses associated with these hormones in the context of skin biology. The research provides insights into how these peptides impact keratinocyte cells, which are important components of the skin.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0022202X15307697
Getting S J, Schioth H B, Perretti M. Dissection of the anti‐inflammatory effect of the core and C‐terminal (KPV) alpha‐melanocyte‐stimulating hormone peptides. J PharmacolExpTher 2003306631–637.
Dissection of the anti-inflammatory effect of the core and C-terminal (KPV) α-melanocyte-stimulating hormone peptides
In the study conducted by Getting et al. titled “Dissection of the Anti-Inflammatory Effect of the Core and C-Terminal (KPV) Alpha-Melanocyte-Stimulating Hormone Peptides,” the researchers aimed to investigate the anti-inflammatory properties of specific regions of alpha-melanocyte-stimulating hormone (α-MSH) peptides. They focused on dissecting the effects of both the core region and the C-terminal KPV motif of α-MSH. This research sought to understand the mechanisms by which these peptides exert their anti-inflammatory actions, which could have implications for potential therapeutic applications. The study provides insights into the functional components of α-MSH responsible for its anti-inflammatory effects.
For in-depth study https://jpet.aspetjournals.org/content/306/2/631.short
Luger T A, Scholzen T E, Brzoska T, Bohm M. New insights into the functions of alpha‐MSH and related peptides in the immune system. Ann N Y AcadSci 2003994133–140.
New insights into the functions of alpha‐MSH and related peptides in the immune system
The paper titled “New Insights into the Functions of Alpha-MSH and Related Peptides in the Immune System,” authored by Luger, T. A., Scholzen, T. E., Brzoska, T., and Böhm, M., explores the various roles of alpha-melanocyte-stimulating hormone (α-MSH) and related peptides within the immune system. This research sheds light on the immunomodulatory and anti-inflammatory properties of α-MSH, providing a comprehensive understanding of how these peptides influence immune responses. The paper discusses the potential therapeutic applications of α-MSH and related peptides in immune-mediated inflammatory conditions and offers insights into their mechanisms of action.
For in-depth study https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.2003.tb03172.x
Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 200080979–1020.
Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress
The paper titled “Corticotropin Releasing Hormone and Proopiomelanocortin Involvement in the Cutaneous Response to Stress,” authored by Slominski A, Wortsman J, Luger T, Paus R, and Solomon S, delves into the role of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) in the skin’s response to stress. This comprehensive review explores the intricate mechanisms by which the skin and its various components, including hair follicles and melanocytes, respond to stress through the CRH-POMC system. The paper discusses the physiological and pathological implications of these stress-induced responses in the skin and provides valuable insights into the complex interplay between the neuroendocrine system and skin biology.
For in-depth study https://journals.physiology.org/doi/abs/10.1152/physrev.2000.80.3.979
Cooper A, Robinson SJ, Pickard C, Jackson CL, Friedmann PS, Healy E. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175(7):4806-13.
α-Melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status
In the study titled “Alpha-Melanocyte-Stimulating Hormone Suppresses Antigen-Induced Lymphocyte Proliferation in Humans Independently of Melanocortin 1 Receptor Gene Status,” conducted by Cooper et al. and published in the Journal of Immunology in 2005, the researchers investigated the immunomodulatory effects of alpha-melanocyte-stimulating hormone (alpha-MSH) on antigen-induced lymphocyte proliferation in humans. They examined whether the suppression of lymphocyte proliferation by alpha-MSH was influenced by the melanocortin 1 receptor (MC1R) gene status. The study found that alpha-MSH effectively suppressed lymphocyte proliferation regardless of the individual’s MC1R gene status. This research contributes to our understanding of the immunoregulatory properties of alpha-MSH, which may have implications for the development of therapeutic strategies targeting immune-mediated disorders.
For in-depth study https://journals.aai.org/jimmunol/article/175/7/4806/37048
Bohm M, Apel M, Sugawara K, et al. Modulation of basophil activity: a novel function of the neuropeptide α-melanocyte-stimulating hormone. J Allergy ClinImmunol. 2012;129(4):1085-93.
Modulation of basophil activity: A novel function of the neuropeptide α-melanocyte–stimulating hormone
In the study titled “Modulation of Basophil Activity: A Novel Function of the Neuropeptide α-Melanocyte-Stimulating Hormone” by Bohm et al., published in the Journal of Allergy and Clinical Immunology in 2012, the researchers explored a novel function of the neuropeptide α-melanocyte-stimulating hormone (α-MSH) in modulating basophil activity. Basophils are a type of white blood cell involved in allergic reactions and immune responses.
The study investigated the effects of α-MSH on basophil activation and responsiveness. It found that α-MSH could modulate basophil activity, leading to reduced basophil responsiveness to allergens and decreased release of allergic mediators such as histamine. This suggests that α-MSH may play a role in regulating allergic responses and potentially have therapeutic implications for allergic conditions.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0091674911018008
Redondo P, García-foncillas J, Okroujnov I, Bandrés E. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. 1998;290(8):425-8.
α-MSH regulates interleukin-10 expression by human keratinocytes
In the study titled “Alpha-MSH Regulates Interleukin-10 Expression by Human Keratinocytes” by Redondo et al., published in the Archives of Dermatological Research in 1998, the researchers investigated the regulatory role of alpha-melanocyte-stimulating hormone (alpha-MSH) on the expression of interleukin-10 (IL-10) by human keratinocytes.
Keratinocytes are skin cells that play a crucial role in the skin’s immune response. IL-10 is an anti-inflammatory cytokine that helps regulate the immune system’s response to inflammation and infection.
The study found that alpha-MSH could regulate the expression of IL-10 in human keratinocytes. Specifically, alpha-MSH treatment increased the production of IL-10 by these cells. This suggests that alpha-MSH may have immunomodulatory effects in the skin by promoting an anti-inflammatory response through the upregulation of IL-10.
For in-depth study https://link.springer.com/article/10.1007/s004030050330
Rheins LA, Cotleur AL, Kleier RS, Hoppenjans WB, Saunder DN, Nordlund JJ. Alpha-melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice. J Invest Dermatol. 1989;93(4):511-7.
Alpha-melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice
In the study titled “Alpha-Melanocyte Stimulating Hormone Modulates Contact Hypersensitivity Responsiveness in C57/BL6 Mice” conducted by Rheins et al. and published in the Journal of Investigative Dermatology in 1989, the researchers explored the impact of alpha-melanocyte-stimulating hormone (alpha-MSH) on contact hypersensitivity responsiveness in C57/BL6 mice.
Contact hypersensitivity is an exaggerated skin response to certain allergens or antigens, and it plays a role in allergic reactions and skin diseases. Alpha-MSH is known to have immunomodulatory properties and can influence the immune response.
The study found that alpha-MSH treatment had a suppressive effect on contact hypersensitivity responsiveness in the mice. Specifically, mice treated with alpha-MSH exhibited reduced hypersensitivity reactions when exposed to sensitizing agents.
This research suggests that alpha-MSH may have a regulatory role in modulating immune responses in the skin and could potentially be explored for its therapeutic implications in conditions involving exaggerated immune reactions, such as allergic skin disorders.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0022202X89903680
Morandini R, Boeynaems JM, Hedley SJ, MacNeil S , Ghanem G 1998 Modulation of ICAM-1 expression by α-MSH in human melanoma cells and melanocytes. J Cell Physiol175:276–282.
Modulation of ICAM‐1 expression by α‐MSH in human melanoma cells and melanocytes
In the study titled “Modulation of ICAM-1 Expression by α-MSH in Human Melanoma Cells and Melanocytes” conducted by Morandini et al. and published in the Journal of Cellular Physiology in 1998, the researchers investigated the effects of alpha-melanocyte-stimulating hormone (α-MSH) on the expression of intercellular adhesion molecule-1 (ICAM-1) in human melanoma cells and melanocytes.
ICAM-1 is a cell adhesion molecule that plays a crucial role in immune responses and inflammation by facilitating the adhesion of immune cells to target cells. Its expression can be regulated in response to various factors, including hormones and cytokines.
The study found that α-MSH treatment led to a modulation of ICAM-1 expression in both human melanoma cells and melanocytes. Specifically, α-MSH treatment reduced ICAM-1 expression in melanoma cells while increasing it in melanocytes.
For in-depth study https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1097-4652(199806)175:3%3C276::AID-JCP5%3E3.0.CO;2-L
Kalden DH, Scholzen T, Brzoska T, Luger TA 1999 Mechanisms of the antiinflammatory effects of α-MSH. Role of transcription factor NF-κB and adhesion molecule expression. Ann NY AcadSci 885:254–261.
Role of transcription factor NF-κB and adhesion molecule expression
In the study titled “Mechanisms of the Anti-inflammatory Effects of α-MSH: Role of Transcription Factor NF-κB and Adhesion Molecule Expression” conducted by Kalden et al. and published in the Annals of the New York Academy of Sciences in 1999, the researchers explored the mechanisms underlying the anti-inflammatory effects of alpha-melanocyte-stimulating hormone (α-MSH). The study focused on the involvement of transcription factor NF-κB and adhesion molecule expression in these anti-inflammatory processes.
NF-κB is a key transcription factor that plays a central role in regulating immune and inflammatory responses. It controls the expression of various genes involved in inflammation, including adhesion molecules that facilitate the recruitment of immune cells to sites of inflammation.
For in-depth study https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1999.tb08682.x
Scholzen TE, Sunderkötter C, Kalden DH, Brzoska T, Fastrich M, Fisbeck T, Armstrong CA, Ansel JC, Luger TA 2003 α-Melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology 144:360–370.
α-Melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression
In the study titled “α-Melanocyte Stimulating Hormone Prevents Lipopolysaccharide-Induced Vasculitis by Down-Regulating Endothelial Cell Adhesion Molecule Expression” conducted by Scholzen et al. and published in Endocrinology in 2003, the researchers investigated the potential anti-inflammatory effects of α-melanocyte stimulating hormone (α-MSH) on vasculitis induced by lipopolysaccharide (LPS) in endothelial cells.
Vasculitis is characterized by inflammation of blood vessels, which can lead to tissue damage. In this study, the researchers focused on how α-MSH may modulate the expression of endothelial cell adhesion molecules, which are crucial for the recruitment of immune cells to sites of inflammation within blood vessels.
The findings revealed that α-MSH treatment effectively reduced the expression of adhesion molecules on endothelial cells stimulated by LPS. By down-regulating the expression of these adhesion molecules, α-MSH hindered the recruitment of immune cells to the vascular endothelium, ultimately mitigating the inflammatory response and vasculitis.
For in-depth study https://academic.oup.com/endo/article-abstract/144/1/360/2501731
Bohm M, Eickelmann M, Li Z, Schneider SW, Oji V, Diederichs S, Barsh GS, Vogt A, Stieler K, Blume-Peytavi U, Luger TA 2005 Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of α-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology 146:4635–4646.
Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of α-melanocyte-stimulating hormone in human dermal papilla cells
In the 2005 study published in Endocrinology, Bohm et al. investigated the presence of functional melanocortin receptors, particularly the melanocortin 1 receptor (MC1R), in human dermal papilla cells, which are crucial for hair follicle development and hair growth regulation. They confirmed the presence of these receptors and explored the immunoregulatory effects of α-melanocyte-stimulating hormone (α-MSH) on dermal papilla cells. Their findings indicated that α-MSH treatment reduced the production of pro-inflammatory cytokines by dermal papilla cells, suggesting an anti-inflammatory role for α-MSH in the skin. These results imply that α-MSH may influence the inflammatory environment within hair follicles, potentially impacting hair growth patterns and the hair cycle. This study sheds light on the potential immunoregulatory properties of α-MSH in the context of hair follicle function and hair growth.
For in-depth study https://academic.oup.com/endo/article-abstract/146/11/4635/2499744
Hill RP, MacNeil S, Haycock JW 2006 Melanocyte stimulating hormone peptides inhibit TNF-α signaling in human dermal fibroblast cells. Peptides 27:421–430.
Melanocyte stimulating hormone peptides inhibit TNF-α signaling in human dermal fibroblast cells
In the 2006 study published in Peptides, Hill et al. investigated the effects of melanocyte stimulating hormone (MSH) peptides on tumor necrosis factor-alpha (TNF-α) signaling in human dermal fibroblast cells. They found that MSH peptides inhibited the signaling pathways associated with TNF-α, a pro-inflammatory cytokine involved in various inflammatory responses. This inhibition suggested that MSH peptides may have anti-inflammatory properties in the context of dermal fibroblast cells. These findings contribute to our understanding of the potential immunomodulatory effects of MSH peptides on skin cells and their role in regulating inflammatory responses in the skin.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0196978105004559
Sarkar A, Sreenivasan Y, Manna SK 2003 α-Melanocyte-stimulating hormone induces cell death in mast cells: involvement of NF-κB. FEBS Lett 549:87–93.
α-Melanocyte-stimulating hormone induces cell death in mast cells: involvement of NF-κB
In the 2003 study published in FEBS Letters, Sarkar et al. investigated the impact of alpha-melanocyte-stimulating hormone (α-MSH) on mast cells. They found that α-MSH induced cell death in mast cells, and this effect was associated with the inhibition of nuclear factor-kappa B (NF-κB), a transcription factor involved in cell survival and inflammation. The study suggests that α-MSH may have a role in regulating mast cell function and potentially modulating inflammatory responses through its influence on NF-κB signaling pathways. This research contributes to our understanding of the immunomodulatory properties of α-MSH and its potential impact on mast cell biology.
For in-depth study https://www.sciencedirect.com/science/article/pii/S001457930300797X
Glyn JR, Lipton JM. Hypothermic and antipyretic effects of centrally administered ACTH (1–24) and alpha-melanotropin. Peptides. 1981;2(2):177-87.
Hypothermic and antipyretic effects of centrally administered ACTH (1–24) and alpha-melanotropin
In the 1981 study published in Peptides, Glyn and Lipton investigated the effects of centrally administered adrenocorticotropic hormone (ACTH) (1–24) and alpha-melanotropin on hypothermia and fever reduction. The study found that both ACTH (1–24) and alpha-melanotropin had hypothermic and antipyretic (fever-reducing) effects when administered centrally. These peptides, when introduced into the central nervous system, influenced body temperature regulation. This research sheds light on the potential role of ACTH and alpha-melanotropin in the central control of body temperature and fever responses.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0196978181800320
Glyn-ballinger JR, Bernardini GL, Lipton JM. alpha-MSH injected into the septal region reduces fever in rabbits. Peptides. 1983;4(2):199-203.
α-MSH injected into the septal region reduces fever in rabbits
In the 1983 study published in Peptides, Glyn-Ballinger, Bernardini, and Lipton investigated the effects of injecting alpha-melanocyte-stimulating hormone (alpha-MSH) into the septal region of the brain on fever in rabbits. The study found that administration of alpha-MSH into the septal region resulted in a reduction of fever in rabbits. This suggests that alpha-MSH, when targeted at specific brain regions, may have a role in modulating fever responses. The study contributes to our understanding of the potential regulatory mechanisms involved in fever control in animals.
For in-depth study https://www.sciencedirect.com/science/article/pii/0196978183901146
Murphy MT, Lipton JM. Peripheral administration of alpha-MSH reduces fever in older and younger rabbits. Peptides. 1982;3(5):775-9.
Peripheral administration of alpha-MSH reduces fever in older and younger rabbits
In the 1982 study published in Peptides, Murphy and Lipton investigated the effects of peripheral administration of alpha-melanocyte-stimulating hormone (alpha-MSH) on fever in both older and younger rabbits. The study found that regardless of age, peripheral administration of alpha-MSH resulted in a reduction of fever in rabbits. This suggests that alpha-MSH, when administered peripherally, has the potential to modulate fever responses in rabbits, irrespective of their age. The study contributes to our understanding of the role of alpha-MSH in fever regulation in animals.
For in-depth study https://www.sciencedirect.com/science/article/pii/0196978182900146
Murphy MT, Richards DB, Lipton JM. Antipyretic potency of centrally administered alpha-melanocyte stimulating hormone. Science. 1983;221(4606):192-3.
Antipyretic potency of centrally administered α-melanocyte stimulating hormone
In the 1983 study published in Science, Murphy, Richards, and Lipton investigated the antipyretic potency of centrally administered alpha-melanocyte-stimulating hormone (alpha-MSH). The study found that when alpha-MSH was administered centrally (likely into the brain), it demonstrated antipyretic effects, meaning it reduced fever. This suggests that alpha-MSH, when acting within the central nervous system, has the ability to modulate and reduce fever. The study provides valuable insights into the potential role of alpha-MSH in fever regulation and its therapeutic implications for fever management.
For in-depth study https://www.science.org/doi/abs/10.1126/science.6602381
Kandasamy SB, Williams BA. Hypothermic and antipyretic effects of ACTH (1-24) and alpha-melanotropin in guinea-pigs. Neuropharmacology. 1984;23(1):49-53.
Hypothermic and antipyretic effects of ACTH (1-24) and alpha-melanotropin in guinea-pigs
In the study conducted by Kandasamy and Williams, published in Neuropharmacology in 1984, the authors investigated the hypothermic (cooling) and antipyretic (fever-reducing) effects of ACTH (1-24) and alpha-melanotropin in guinea pigs. The study aimed to assess whether these peptides could induce hypothermia and reduce fever in guinea pigs. The findings of the study demonstrated that both ACTH (1-24) and alpha-melanotropin had hypothermic effects, indicating their ability to lower body temperature. Additionally, these peptides exhibited antipyretic properties, suggesting their potential in reducing fever. This research contributes to our understanding of the physiological effects of these peptides and their potential therapeutic applications in temperature regulation and fever management.
For in-depth study https://www.sciencedirect.com/science/article/pii/0028390884902168
Shih ST, Lipton JM. Intravenous alpha-MSH reduces fever in the squirrel monkey. Peptides. 1985;6(4):685-7.
Intravenous alpha-MSH reduces fever in the squirrel monkey
In the study conducted by Shih and Lipton, published in Peptides in 1985, the researchers investigated the effects of intravenous alpha-melanocyte-stimulating hormone (alpha-MSH) on fever reduction in squirrel monkeys. The study aimed to assess whether intravenous administration of alpha-MSH could lower body temperature in squirrel monkeys experiencing fever. The findings of the study demonstrated that intravenous alpha-MSH had the ability to reduce fever in squirrel monkeys, indicating its potential as an antipyretic agent. This research contributes to our understanding of the physiological actions of alpha-MSH and its role in temperature regulation, specifically in the context of fever reduction.
For in-depth study https://www.sciencedirect.com/science/article/pii/019697818590172X
Daynes RA, Robertson BA, Cho BH, Burnham DK, Newton R. Alpha-melanocyte-stimulating hormone exhibits target cell selectivity in its capacity to affect interleukin 1-inducible responses in vivo and in vitro. J Immunol. 1987;139(1):103-9.
Alpha-melanocyte-stimulating hormone exhibits target cell selectivity in its capacity to affect interleukin 1-inducible responses in vivo and in vitro
In the study by Daynes et al., published in the Journal of Immunology in 1987, the researchers investigated the effects of alpha-melanocyte-stimulating hormone (alpha-MSH) on interleukin 1 (IL-1)-inducible responses in both in vivo and in vitro settings. Their research aimed to determine whether alpha-MSH exhibited target cell selectivity in modulating the immune response to IL-1. The study found that alpha-MSH had the capacity to selectively influence IL-1-inducible responses in certain target cells. This research provided valuable insights into the immunomodulatory properties of alpha-MSH and its potential role in regulating immune responses, particularly in the context of IL-1 signaling.
For in-depth study https://journals.aai.org/jimmunol/article-abstract/139/1/103/17935
Deeter LB, Martin LW, Lipton JM. Antipyretic effect of central alpha-MSH summates with that of acetaminophen or ibuprofen. Brain Res Bull. 1989;23(6):573-5.
Antipyretic effect of central alpha-MSH summates with that of acetaminophen or ibuprofen
In the study by Deeter et al., published in Brain Research Bulletin in 1989, the researchers investigated the antipyretic (fever-reducing) effects of central alpha-melanocyte-stimulating hormone (alpha-MSH) in combination with acetaminophen or ibuprofen. The study aimed to determine whether the antipyretic effect of central alpha-MSH could synergize or summate with the antipyretic effects of these commonly used fever-reducing medications. The findings indicated that the antipyretic effect of central alpha-MSH indeed summates with that of acetaminophen or ibuprofen. This suggests that alpha-MSH may have a role in enhancing the fever-reducing actions of these medications, providing insights into potential therapeutic strategies for fever management.
For in-depth study https://www.sciencedirect.com/science/article/pii/0361923089902037
Martin LW, Lipton JM. Acute phase response to endotoxin: rise in plasma alpha-MSH and effects of alpha-MSH injection. Am J Physiol. 1990;259(4 Pt 2):R768-72.
In the study conducted by Martin and Lipton, published in the American Journal of Physiology in 1990, the researchers investigated the acute phase response to endotoxin, which is a component of bacterial cell walls known to induce inflammation. They focused on the rise in plasma alpha-melanocyte-stimulating hormone (alpha-MSH) levels during this response and the effects of injecting alpha-MSH. The acute phase response is a set of systemic changes that occur in response to infection, injury, or inflammation. The study found that endotoxin administration led to an increase in plasma alpha-MSH levels, and injection of alpha-MSH had certain effects on the immune system. The research provided insights into the role of alpha-MSH in the acute phase response and its potential immunomodulatory effects.
For in-depth study https://journals.physiology.org/doi/abs/10.1152/ajpregu.1990.259.4.r768
Feng JD, Dao T, Lipton JM. Effects of preoptic microinjections of alpha-MSH on fever and normal temperature control in rabbits. Brain Res Bull. 1987;18(4):473-7.
Effects of preoptic microinjections of alpha-MSH on fever and normal temperature control in rabbits
The study conducted by Feng, Dao, and Lipton in 1987 and published in Brain Research Bulletin investigated the effects of microinjections of alpha-melanocyte-stimulating hormone (alpha-MSH) into the preoptic area of the brain on fever and normal temperature control in rabbits. The preoptic area is a region of the brain involved in thermoregulation. The study aimed to determine how alpha-MSH administration in this brain region affected body temperature regulation in response to fever-inducing stimuli and under normal conditions. Their findings provided insights into the role of alpha-MSH in the central regulation of body temperature and fever response.
For in-depth study https://www.sciencedirect.com/science/article/pii/0361923087901110
Tatro JB 2000 Melanocortin receptor expression and function in the nervous system. In: Cone RD , ed. The melanocortin receptors. Totowa, NJ: Humana Press; 173–207.
Melanocortin receptor expression and function in the nervous system
The publication by Tatro in 2000, titled “Melanocortin Receptor Expression and Function in the Nervous System,” is a chapter in the book “The Melanocortin Receptors,” edited by Cone RD. In this chapter, the author discusses the expression and function of melanocortin receptors in the nervous system. Melanocortin receptors are a group of G protein-coupled receptors that play a role in various physiological processes, including the regulation of energy balance, inflammation, and pigmentation. The chapter likely provides an overview of the distribution and roles of melanocortin receptors within the nervous system, shedding light on their importance in neural function and potential implications for various neurological and physiological processes.
For in-depth study https://link.springer.com/chapter/10.1007/978-1-59259-031-5_6
Tatro JB 2000 Endogenous antipyretics. Clin Infect Dis 21:S190–S201.
Endogenous antipyretics
In the 2000 publication by Tatro titled “Endogenous Antipyretics” in Clinical Infectious Diseases, the author explores the concept of endogenous (naturally occurring within the body) antipyretics, focusing on their role in regulating fever during infections and inflammatory conditions. Fever is a common symptom of various illnesses, and the body has mechanisms to control and modulate the fever response. This article likely discusses specific molecules or pathways involved in regulating fever and how they can be targeted to manage fever in clinical settings. Understanding the mechanisms of endogenous antipyresis is essential for the development of treatments to alleviate fever and its associated symptoms during infections.
For in-depth study https://academic.oup.com/cid/article-abstract/31/Supplement_5/S190/333713
Rezvani AH, Denbow DM, Myers RD. alpha-Melanocyte-stimulating hormone infused ICV fails to affect body temperature or endotoxin fever in the cat. Brain Res Bull. 1986;16(1):99-105.
α-Melanocyte-stimulating hormone infused ICV fails to affect body temperature or endotoxin fever in the cat
In the study conducted by Rezvani, Denbow, and Myers and published in the Brain Research Bulletin in 1986, the researchers investigated the effects of intracerebroventricular (ICV) infusion of alpha-melanocyte-stimulating hormone (alpha-MSH) on body temperature and endotoxin-induced fever in cats. Alpha-MSH is known to have various physiological effects, including potential roles in temperature regulation. However, their findings indicated that ICV infusion of alpha-MSH did not significantly influence body temperature or alter the fever response induced by endotoxin in cats. This suggests that, under the conditions of their experiment, alpha-MSH did not have a substantial impact on temperature regulation or the fever response in feline subjects.
For in-depth study https://www.sciencedirect.com/science/article/pii/0361923086900171
Greenberg R, Whalley CE, Jourdikian F, Mendelson IS, Walter R, Nikolics K, Coy DH, Schally AV, Kastin AJ 1976 Peptides readily penetrate the blood-brain barrier: uptake of peptides by synaptosomes is passive. PharmacolBiochemBehav 5(Suppl 1):151–158.
Peptides readily penetrate the blood-brain barrier: Uptake of peptides by synaptosomes is passive
The study conducted by Greenberg, Whalley, Jourdikian, Mendelson, Walter, Nikolics, Coy, Schally, and Kastin in 1976, published in Pharmacology, Biochemistry, and Behavior, focused on the penetration of peptides through the blood-brain barrier. The researchers investigated the uptake of peptides by synaptosomes, which are small membrane-bound vesicles containing neurotransmitters found in neurons. The study revealed that peptides could readily penetrate the blood-brain barrier and be taken up by synaptosomes. Importantly, the uptake of peptides in this context appeared to be passive, meaning that it did not require active transport mechanisms. This research contributed to our understanding of how peptides, including neuropeptides and other signaling molecules, can access the brain and influence neuronal processes.
For in-depth study https://www.sciencedirect.com/science/article/pii/0091305776903452
Tatro JB, Sinha PS. The central melanocortin system and fever. Ann N Y Acad Sci. 2003;994:246-57.
The central melanocortin system and fever
The article titled “The central melanocortin system and fever” by Tatro JB and Sinha PS, published in the Annals of the New York Academy of Sciences in 2003, discusses the role of the central melanocortin system in regulating fever. The central melanocortin system includes melanocortin peptides like alpha-melanocyte-stimulating hormone (α-MSH) and melanocortin receptors in the brain. The authors explore how α-MSH and related peptides influence the fever response, emphasizing their anti-pyretic (fever-reducing) effects. The review provides insights into the complex interactions between melanocortin peptides, immune responses, and temperature regulation in the central nervous system, contributing to our understanding of the neurobiology of fever control.
For in-depth study https://link.springer.com/chapter/10.1007/978-1-4419-6354-3_9
Sinha PS, Schiöth HB, Tatro JB. Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered alpha-MSH. Brain Res. 2004;1001(1-2):150-8.
Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered α-MSH
In the study titled “Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered alpha-MSH” by Sinha PS, Schiöth HB, and Tatro JB, published in Brain Research in 2004, the researchers investigated the involvement of the melanocortin-4 receptor (MC4R) in the actions of centrally administered alpha-melanocyte-stimulating hormone (alpha-MSH) related to body temperature regulation. The study explores the roles of MC4R in both fever-reducing (antipyretic) and fever-inducing (hyperthermic) effects of alpha-MSH in the central nervous system. It provides insights into the specific mechanisms and receptors involved in the regulation of body temperature by alpha-MSH, contributing to our understanding of the central melanocortin system’s role in thermoregulation.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0006899303041842
Huang QH, Entwistle ML, Alvaro JD, Duman RS, Hruby VJ, Tatro JB. Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. J Neurosci. 1997;17(9):3343-51.
Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever
In the study titled “Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever” by Huang QH, Entwistle ML, Alvaro JD, Duman RS, Hruby VJ, and Tatro JB, published in the Journal of Neuroscience in 1997, the researchers investigated the antipyretic (fever-reducing) effects of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. The study aimed to understand the role of melanocortin receptors in regulating body temperature in response to fever-inducing stimuli, such as endotoxin. It provides insights into the mechanisms by which the central melanocortin system contributes to fever regulation and the potential therapeutic implications of targeting melanocortin receptors for fever management.
For in-depth study https://www.jneurosci.org/content/17/9/3343.short
Han D, Tian Y, Zhang M, Zhou Z, Lu J. Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells. Gene Ther. 2007;14(5):383-95.
Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells
In the study titled “Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells” by Han D, Tian Y, Zhang M, Zhou Z, and Lu J, published in the journal Gene Therapy in 2007, the researchers explored a potential therapeutic approach for experimental autoimmune encephalomyelitis (EAE), which serves as a model for multiple sclerosis. The study involved the use of recombinant adeno-associated virus (rAAV) to transduce T cells specific for PLP139-151, a peptide associated with EAE, with alpha-melanocyte-stimulating hormone (alpha-MSH). The goal was to assess whether these modified T cells could be used for the prevention and treatment of EAE, shedding light on potential immunomodulatory strategies for autoimmune diseases like multiple sclerosis.
For in-depth study https://www.nature.com/articles/3302862
Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. 1994;1(1):28-32.
The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats
In the study titled “The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats” by Ceriani G, Diaz J, Murphree S, Catania A, and Lipton JM, published in the journal Neuroimmunomodulation in 1994, the researchers investigated the potential anti-inflammatory effects of alpha-melanocyte-stimulating hormone (alpha-MSH) in a rat model of experimental arthritis. The study aimed to determine whether alpha-MSH administration could mitigate the development and severity of arthritis, providing insights into its immunomodulatory properties and potential therapeutic applications in autoimmune and inflammatory conditions, such as arthritis.
For in-depth study https://karger.com/Article/Abstract/97087
Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH). Ann N Y Acad Sci. 2000;917:239-47.
Neuropeptide regulation of immunity
In the article titled “Neuropeptide regulation of immunity: The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH)” by Taylor AW, Yee DG, Nishida T, and Namba K, published in the Annals of the New York Academy of Sciences in 2000, the authors explored the role of neuropeptides in regulating the immune system, with a specific focus on the immunosuppressive effects of alpha-melanocyte-stimulating hormone (alpha-MSH). The study aimed to elucidate the mechanisms by which alpha-MSH modulates immune responses and to highlight its potential as an immunosuppressive agent. The research contributes to our understanding of the complex interactions between the nervous system and the immune system and their implications for immune-related disorders and therapies.
For in-depth study https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.2000.tb05389.x
Nishida T, Miyata S, Itoh Y, et al. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. IntImmunopharmacol. 2004;4(8):1059-66.
Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor
In the study titled “Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor” by Nishida T, Miyata S, Itoh Y, et al., published in the International Immunopharmacology journal in 2004, the researchers investigated the anti-inflammatory properties of alpha-melanocyte-stimulating hormone (alpha-MSH) in a rat model of endotoxin-induced uveitis. The study aimed to assess the impact of alpha-MSH on the inflammatory response associated with uveitis and to analyze the time course of inflammatory agents present in the aqueous humor of the eyes. The findings suggested that alpha-MSH exhibited anti-inflammatory effects in this model and provided insights into the temporal dynamics of inflammation in uveitis. These results have implications for potential therapeutic strategies targeting uveitis and related inflammatory eye conditions.
For in-depth study https://www.sciencedirect.com/science/article/pii/S1567576904001304
Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J Leukoc Biol. 2002;72(5):946-52.
Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2
In the study titled “Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2” by Namba K, Kitaichi N, Nishida T, and Taylor AW, published in the Journal of Leukocyte Biology in 2002, the researchers investigated the role of immunomodulatory cytokines, alpha-melanocyte-stimulating hormone (alpha-MSH), and transforming growth factor-beta2 (TGF-beta2) in the induction of regulatory T cells. Regulatory T cells play a critical role in immune tolerance and regulation. The study aimed to understand how these cytokines contribute to the development of regulatory T cells, which can help control immune responses. The findings suggested that alpha-MSH and TGF-beta2 can induce the generation of regulatory T cells, highlighting their immunomodulatory properties and potential therapeutic applications in immune-related disorders.
For in-depth study https://academic.oup.com/jleukbio/article-abstract/72/5/946/6980762
Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11(12):1199-206.
Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor
In the study titled “Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor” by Taylor AW, Streilein JW, and Cousins SW, published in Current Eye Research in 1992, the researchers aimed to identify potential immunosuppressive factors present in the aqueous humor of the eye. They discovered that alpha-melanocyte stimulating hormone (alpha-MSH) was one of the factors found in aqueous humor with immunosuppressive properties. This hormone was shown to play a role in regulating immune responses in the eye, suggesting its involvement in maintaining ocular immune privilege, a mechanism that prevents immune-mediated damage to ocular tissues. The study shed light on the immunomodulatory functions of alpha-MSH in the eye and its potential significance in ocular immune regulation.
For in-depth study https://www.tandfonline.com/doi/abs/10.3109/02713689208999545
Naveh N, Marshall J. Melanocortins are comparable to corticosteroids as inhibitors of traumatic ocular inflammation in rabbits. Graefes Arch ClinExpOphthalmol. 2001;239(11):840-4.
Melanocortins are comparable to corticosteroids as inhibitors of traumatic ocular inflammation in rabbits
In the study titled “Melanocortins are comparable to corticosteroids as inhibitors of traumatic ocular inflammation in rabbits” by Naveh N and Marshall J, published in Graefe’s Archive for Clinical and Experimental Ophthalmology in 2001, the researchers aimed to investigate the anti-inflammatory properties of melanocortins, specifically alpha-melanocyte stimulating hormone (α-MSH), in comparison to corticosteroids in the context of traumatic ocular inflammation in rabbits. The study found that α-MSH, a neuropeptide with known anti-inflammatory effects, demonstrated comparable effectiveness to corticosteroids in reducing traumatic ocular inflammation. This research highlighted the potential of α-MSH as an alternative anti-inflammatory agent in ocular conditions, providing insights into its therapeutic application for ocular inflammation in rabbits.
For in-depth study https://link.springer.com/article/10.1007/s00417-001-0379-1
Jahovic N, Arbak S, Tekeli O, Alican I. Alpha-melanocyte stimulating hormone has beneficial effects on cerulein-induced acute pancreatitis. Peptides. 2004;25(1):129-32.
Alpha-melanocyte stimulating hormone has beneficial effects on cerulein-induced acute pancreatitis
In the study titled “Alpha-melanocyte stimulating hormone has beneficial effects on cerulein-induced acute pancreatitis,” conducted by Jahovic N, Arbak S, Tekeli O, and Alican I, which was published in Peptides in 2004, the researchers investigated the potential therapeutic effects of alpha-melanocyte stimulating hormone (α-MSH) in a model of cerulein-induced acute pancreatitis. The study found that α-MSH demonstrated beneficial effects in reducing the severity of acute pancreatitis, suggesting that it may have a protective role in this inflammatory condition. These findings provided insights into the potential use of α-MSH as a therapeutic agent for acute pancreatitis.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0196978103004157
Al-majed HT, Jones PM, Persaud SJ, et al. ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans. J Endocrinol. 2004;180(1):155-66.
ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans
In the study titled “ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans,” conducted by Al-majed HT, Jones PM, Persaud SJ, and colleagues, which was published in the Journal of Endocrinology in 2004, the researchers investigated the effects of adrenocorticotropic hormone (ACTH) on insulin secretion. The study found that ACTH stimulates insulin secretion from MIN6 cells (a mouse insulinoma cell line) as well as primary mouse and human islets of Langerhans. These findings suggest that ACTH may have a role in regulating insulin secretion and glucose homeostasis, potentially implicating it in metabolic processes beyond its well-known role in the stress response and corticosteroid production.
For in-depth study https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=3f7d4a168a238c1a9028935a07b19b20ede01448
Jung EJ, Han DJ, Chang SH, et al. Protective effect of alpha-melanocyte-stimulating hormone on pancreas islet cell against peripheral blood mononuclear cell-mediated cytotoxicity in vitro. Transplant Proc. 2007;39(5):1604-6.
Protective effect of alpha-melanocyte-stimulating hormone on pancreas islet cell against peripheral blood mononuclear cell-mediated cytotoxicity in vitro
In the study titled “Protective effect of alpha-melanocyte-stimulating hormone on pancreas islet cell against peripheral blood mononuclear cell-mediated cytotoxicity in vitro,” conducted by Jung EJ, Han DJ, Chang SH, and colleagues, which was published in Transplantation Proceedings in 2007, the researchers investigated the potential protective effects of alpha-melanocyte-stimulating hormone (α-MSH) on pancreatic islet cells against cytotoxicity induced by peripheral blood mononuclear cells (PBMCs) in vitro. The study found that α-MSH demonstrated a protective effect on pancreatic islet cells, reducing the cytotoxicity caused by PBMCs. This suggests that α-MSH may have a role in modulating immune responses and protecting pancreatic islet cells, which are crucial for insulin production, from immune-mediated damage. Such findings have implications for potential therapeutic approaches in the context of autoimmune conditions affecting the pancreas, such as type 1 diabetes.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0041134507002904
Richards, D. B., & Lipton, J. M. (1984). Effect of alpha-MSH 11-13 (lysine-proline-valine) on fever in the rabbit. Peptides, 5(4), 815–817. https://doi.org/10.1016/0196-9781(84)90027-5.
Effect of α-MSH 11–13 (lysine-proline-valine) on fever in the rabbit
In the study titled “Effect of alpha-MSH 11-13 (lysine-proline-valine) on fever in the rabbit,” conducted by Richards DB and Lipton JM, which was published in Peptides in 1984, the researchers investigated the impact of alpha-melanocyte-stimulating hormone (α-MSH) peptide fragment 11-13, with the amino acid sequence lysine-proline-valine (KPV), on fever in rabbits. The study aimed to assess the antipyretic (fever-reducing) properties of this specific α-MSH fragment. The findings of the study demonstrated that α-MSH 11-13 (KPV) had an antipyretic effect, suggesting that this peptide fragment could potentially reduce fever in rabbits. This research contributes to the understanding of the physiological effects of α-MSH and its potential role in fever regulation.
For in-depth study https://www.sciencedirect.com/science/article/pii/0196978184900275
Strand FL, Zuccarelli LA, Williams KA, et al. Melanotropins as growth factors. Ann N Y Acad Sci. 1993;680:29-50.
Melanotropins as growth factors
The study titled “Melanotropins as growth factors,” authored by Strand FL, Zuccarelli LA, Williams KA, et al., was published in the Annals of the New York Academy of Sciences in 1993. This research focused on the potential role of melanotropins, including alpha-melanocyte-stimulating hormone (α-MSH), as growth factors. The study aimed to investigate whether melanotropins could influence growth and development processes in various biological systems. The findings and insights from this study contribute to the understanding of the broader physiological effects of melanotropins beyond their well-known roles in pigmentation and other functions.
For in-depth study https://europepmc.org/article/med/8390155
Gispen WH. The potential of melanotropins in the treatment of nervous system diseases. Ann N Y Acad Sci. 1993;680:401-11.
The potential of melanotropins in the treatment of nervous system diseases
The article titled “The potential of melanotropins in the treatment of nervous system diseases” was authored by W.H. Gispen and was published in the Annals of the New York Academy of Sciences in 1993. This article explores the potential therapeutic applications of melanotropins, including alpha-melanocyte-stimulating hormone (α-MSH), in the treatment of various nervous system diseases. It discusses the neuroprotective and neurotrophic effects of melanotropins and their potential to modulate processes such as neuronal regeneration and plasticity. The article contributes to our understanding of the potential role of melanotropins in addressing neurological disorders.
For in-depth study https://dspace.library.uu.nl/bitstream/handle/1874/4117/3772.pdf?sequence=2
Gispen WH, Adan RA. Melanocortins and the treatment of nervous system disease. Potential relevance to the skin?. Ann N Y Acad Sci. 1999;885:342-9.
Melanocortins and the treatment of nervous system disease
The article titled “The potential of melanotropins in the treatment of nervous system diseases” was authored by W.H. Gispen and was published in the Annals of the New York Academy of Sciences in 1993. This article explores the potential therapeutic applications of melanotropins, including alpha-melanocyte-stimulating hormone (α-MSH), in the treatment of various nervous system diseases. It discusses the neuroprotective and neurotrophic effects of melanotropins and their potential to modulate processes such as neuronal regeneration and plasticity. The article contributes to our understanding of the potential role of melanotropins in addressing neurological disorders.
For in-depth study https://dspace.library.uu.nl/bitstream/handle/1874/4117/3772.pdf?sequence=2
Sharma HS. Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury: an experimental study in the rat using pharmacological and morphological approaches. Ann N Y Acad Sci. 2005;1053:407-21.
Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury: an experimental study in the rat using pharmacological and morphological approaches
The article titled “Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury: an experimental study in the rat using pharmacological and morphological approaches” was authored by Hari Shanker Sharma and was published in the Annals of the New York Academy of Sciences in 2005. This study investigates the potential neuroprotective effects of neurotrophins and melanocortins in the context of spinal cord injury. It employs both pharmacological and morphological approaches to assess the impact of these substances on spinal cord injury in rats. The research aims to understand their therapeutic potential in mitigating the damage caused by spinal cord injuries and promoting neuroprotection and regeneration. This study contributes valuable insights into potential treatments for spinal cord injuries.
For in-depth study https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.2005.tb00050.x
Bar PR, Mandys V, Turecek R, Gispen WH. Alpha-melanocyte-stimulating hormone has protective properties against the toxic effect of cisplatin on cultured dorsal root ganglia. Ann N Y Acad Sci. 1993;680:649-51.
Alpha-melanocyte-stimulating hormone has protective properties against the toxic effect of cisplatin on cultured dorsal root ganglia
The article titled “Alpha-melanocyte-stimulating hormone has protective properties against the toxic effect of cisplatin on cultured dorsal root ganglia” was authored by P.R. Bar, V. Mandys, R. Turecek, and W.H. Gispen. It was published in the Annals of the New York Academy of Sciences in 1993. This study explores the potential protective properties of alpha-melanocyte-stimulating hormone (α-MSH) against the toxic effects of cisplatin on cultured dorsal root ganglia. Cisplatin is a chemotherapeutic agent known for its neurotoxic side effects, and this research investigates whether α-MSH can mitigate or counteract these toxic effects in nerve cells. The study’s findings provide insights into the potential neuroprotective role of α-MSH in the context of cisplatin-induced neurotoxicity.
For in-depth study https://dspace.library.uu.nl/bitstream/handle/1874/3572/3779.pdf?sequence=2
Windebank AJ, Smith AG, Russell JW. The effect of nerve growth factor, ciliaryneurotrophic factor, and ACTH analogs on cisplatin neurotoxicity in vitro. Neurology. 1994;44(3 Pt 1):488-94.
The effect of nerve growth factor, ciliaryneurotrophic factor, and ACTH analogs on cisplatin neurotoxicity in vitro
The article titled “The effect of nerve growth factor, ciliary neurotrophic factor, and ACTH analogs on cisplatin neurotoxicity in vitro” was authored by A.J. Windebank, A.G. Smith, and J.W. Russell. It was published in Neurology in 1994. This study investigates the impact of nerve growth factor (NGF), ciliary neurotrophic factor (CNTF), and analogs of adrenocorticotropic hormone (ACTH) on the neurotoxicity induced by cisplatin in vitro. Cisplatin is known to have neurotoxic side effects, and this research explores whether these neurotrophic factors and ACTH analogs can mitigate or alleviate the neurotoxic effects of cisplatin. The study’s findings provide insights into potential strategies for reducing cisplatin-induced neurotoxicity and preserving nerve function.
For in-depth study https://www.neurology.org/doi/abs/10.1212/WNL.44.3_Part_1.488
Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides. 2006;27(11):2846-57.
Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase
The article titled “Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase” was authored by B. Chai, J.Y. Li, W. Zhang, E. Newman, J. Ammori, and M.W. Mulholland. It was published in Peptides in 2006. This study focuses on the melanocortin-4 receptor (MC4R) and its role in inhibiting apoptosis (programmed cell death) in immortalized hypothalamic neurons. The researchers investigated the mechanism by which MC4R activation through mitogen-activated protein kinase (MAPK) signaling pathways prevents apoptosis in these neurons. Understanding how MC4R regulates apoptosis in hypothalamic neurons can provide insights into its role in hypothalamic functions, including appetite regulation and energy homeostasis.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0196978106002403
Caruso C, Durand D, Schiöth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148(10):4918-26.
Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes
The article titled “Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes” was authored by C. Caruso, D. Durand, H.B. Schiöth, R. Rey, A. Seilicovich, and M. Lasaga. It was published in Endocrinology in 2007. This study explores the role of melanocortin 4 receptors (MC4R) in modulating the inflammatory response and preventing apoptosis in astrocytes when exposed to lipopolysaccharide (LPS) and interferon-gamma (IFN-γ). The authors investigate how MC4R activation can mitigate the detrimental effects of neuroinflammation in astrocytes, potentially contributing to neuroprotection and maintaining central nervous system homeostasis. Understanding these mechanisms may have implications for various neurological conditions and inflammatory responses in the brain.
For in-depth study https://academic.oup.com/endo/article-abstract/148/10/4918/2501708
Bohm M, Wolff I, Scholzen TE, et al. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280(7):5795-802.
alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage
The article titled “α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage” was authored by M. Böhm, I. Wolff, T.E. Scholzen, and others. It was published in the Journal of Biological Chemistry in 2005. This study explores the protective effects of α-melanocyte-stimulating hormone (α-MSH) against ultraviolet (UV) radiation-induced apoptosis and DNA damage. The authors investigate how α-MSH can help mitigate the harmful effects of UV radiation on skin cells, which may have implications for sunburn, skin cancer prevention, and skin health. Understanding these protective mechanisms is crucial for addressing the impact of UV radiation on skin and developing strategies for skin protection.
For in-depth study https://www.jbc.org/article/S0021-9258(19)63084-X/abstract
Kadekaro AL, Kavanagh R, Kanto H, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65(10):4292-9.
alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes
The article titled “α-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes” was authored by A.L. Kadekaro, R. Kavanagh, H. Kanto, and others. It was published in Cancer Research in 2005. This study investigates the effects of α-melanocortin and endothelin-1 on human melanocytes, particularly their role in activating antiapoptotic pathways and reducing DNA damage. Understanding these mechanisms is essential for gaining insights into the protection of melanocytes from various forms of cellular stress, which can have implications for skin health and melanoma prevention.
For in-depth study https://aacrjournals.org/cancerres/article-abstract/65/10/4292/518009
Abdel-malek ZA, Kadekaro AL, Kavanagh RJ, et al. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006;20(9):1561-3.
Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity
The article titled “Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity” was authored by Z.A. Abdel-malek, A.L. Kadekaro, R.J. Kavanagh, and others. It was published in the FASEB Journal in 2006. This study presents a melanoma prevention strategy centered around tetrapeptide alpha-MSH analogs. These analogs are shown to protect human melanocytes from DNA damage and cytotoxicity caused by UV radiation, which is a significant risk factor for melanoma development. Understanding the protective effects of these analogs may offer potential avenues for melanoma prevention and skin health.
For in-depth study https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fj.05-5655fje
Hill RP, Wheeler P, Macneil S, Haycock JW. Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells. Peptides. 2005;26(7):1150-8.
Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells
The article titled “Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells” was authored by R.P. Hill, P. Wheeler, S. Macneil, and J.W. Haycock. It was published in the journal Peptides in 2005. This study explores the cytoprotective effects of alpha-melanocyte stimulating hormone (alpha-MSH) in human dermal fibroblast cells. The research investigates how alpha-MSH may contribute to cellular protection and its potential applications in skin health and wound healing. Understanding the cytoprotective properties of alpha-MSH is important for its potential therapeutic applications in various skin-related conditions.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0196978105000343
Kokot A , Sindrilaru A , Schiller S , Sunderkötter C , Kerkhoff C , Scharffetter-Kochanek K , Luger TA , Böhm M 2008 α-Melanocyte-stimulating hormone is a powerful agent in the bleomycin model of collagen synthesis and fibrosis. ExpDermatol 17:286.
Spatial and Temporal Localization of the Melanocortin 1 Receptor and Its Ligand α–Melanocyte-Stimulating Hormone during Cutaneous Wound Repair
The study by Kokot et al., published in Experimental Dermatology in 2008, demonstrates that α-Melanocyte-stimulating hormone (α-MSH) significantly suppresses collagen synthesis and reduces fibrosis in a bleomycin-induced mouse model. This research highlights the potential of α-MSH as a powerful agent for treating conditions characterized by excessive collagen deposition and fibrosis, such as scleroderma. The study provides evidence that α-MSH could be a novel therapeutic strategy for fibrotic diseases, offering a new approach to managing and potentially reversing the pathological accumulation of collagen in affected tissues
For in-depth study https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3201153/
Yamamoto T, Nishioka K. Cellular and molecular mechanisms of bleomycin-induced murine scleroderma: current update and future perspective. ExpDermatol. 2005;14(2):81-95.
Cellular and molecular mechanisms of bleomycin‐induced murine scleroderma: current update and future perspective
The study by Yamamoto and Nishioka, published in Experimental Dermatology in 2005, reviews the cellular and molecular mechanisms underlying bleomycin-induced scleroderma in mice, providing insights into the pathogenesis of this fibrotic condition. Scleroderma is characterized by immunologic abnormalities, vascular injury, and increased accumulation of matrix proteins in the skin. The review discusses the complex interactions among endothelial cells, lymphocytes, and activated fibroblasts that lead to the overproduction of the extracellular matrix. This research offers a valuable model for understanding scleroderma’s etiology and developing targeted therapies.
For in-depth study https://www.semanticscholar.org/paper/Cellular-and-molecular-mechanisms-of-murine-current-Yamamoto-Nishioka/b5063e7c47cbdf1d88039634e7991b2bf53b4eca
Bohm M, Raghunath M, Sunderkötter C, et al. Collagen metabolism is a novel target of the neuropeptide alpha-melanocyte-stimulating hormone. J Biol Chem. 2004;279(8):6959-66.
Collagen metabolism is a novel target of the neuropeptide α-melanocyte-stimulating hormone
The research conducted by Böhm, Raghunath, Sunderkötter, and colleagues, published in the Journal of Biological Chemistry in 2004, identifies collagen metabolism as a novel target for the neuropeptide α-Melanocyte-stimulating hormone (α-MSH). This study revealed that α-MSH has the ability to modulate collagen synthesis and deposition, suggesting its potential as a new class of modulators for the treatment of fibrotic disorders. By suppressing collagen synthesis, α-MSH could provide a significant therapeutic advantage in managing diseases characterized by excessive fibrosis, offering a new direction for future treatments of these conditions.
For in-depth study https://www.semanticscholar.org/paper/Collagen-Metabolism-Is-a-Novel-Target-of-the-B%C3%B6hm-Raghunath/3ff58a2350de6adde62295f7e0e6f6f38af8590b
Lee TH, Jawan B, Chou WY, et al. Alpha-melanocyte-stimulating hormone gene therapy reverses carbon tetrachloride induced liver fibrosis in mice. J Gene Med. 2006;8(6):764-72.
α‐Melanocyte‐stimulating hormone gene therapy reverses carbon tetrachloride induced liver fibrosis in mice
The study by Lee TH, Jawan B, Chou WY, et al., published in the Journal of Gene Medicine in 2006, demonstrates that α-Melanocyte-stimulating hormone (α-MSH) gene therapy can reverse established liver fibrosis induced by carbon tetrachloride (CCl4) in mice. This groundbreaking work not only showed that such gene therapy could reverse fibrosis but also prevented the upregulation of fibrogenic and pro-inflammatory gene responses following CCl4 administration. This research underscores the therapeutic potential of α-MSH in treating liver fibrosis, offering a promising avenue for the development of gene therapy approaches in managing chronic liver diseases characterized by fibrosis.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/16508911/
Edwards PM, Van der zee CE, Verhaagen J, Schotman P, Jennekens FG, Gispen WH. Evidence that the neurotrophic actions of alpha-MSH may derive from its ability to mimick the actions of a peptide formed in degenerating nerve stumps. J Neurol Sci. 1984;64(3):333-40.
Evidence that the neurotrophic actions of alpha-MSH may derive from its ability to mimick the actions of a peptide formed in degenerating nerve stumps
The 1984 study by Edwards, Van der Zee, Verhaagen, Schotman, Jennekens, and Gispen, published in the Journal of Neurological Sciences, provides evidence that the neurotrophic actions of α-Melanocyte-stimulating hormone (α-MSH) might be due to its ability to mimic the effects of a peptide that forms in degenerating nerve stumps. This research suggests that α-MSH could play a significant role in nerve regeneration and repair by promoting the growth and survival of neurons in conditions of nerve damage. The study indicates that α-MSH and possibly other related peptides could be beneficial in treating neurodegenerative diseases or injuries by enhancing the intrinsic regenerative capacities of the nervous system.
For in-depth study https://www.semanticscholar.org/paper/Evidence-that-the-neurotrophic-actions-of-%CE%B1-MSH-may-Edwards-Zee/b0d14632706399cc7c4bade6c497ec484081499f
Vadoudseyedi J, Liénard D, Lespagnard L, Ghanem G, Van wijck R, Lejeune F. Local administration of alpha-MSH exerts a trophic effect on the 200-kDa neurofilament in sciatic rat nerve. Ann N Y Acad Sci. 1993;680:655-9.
Local Administration of α‐MSH Exerts a Trophic Effect on the 200‐kDa Neurofilament in Sciatic Rat Nerve
The 1993 study by Vadoudseyedi, Liénard, Lespagnard, Ghanem, Van Wijck, and Lejeune, published in the Annals of the New York Academy of Sciences, investigated the effects of local administration of α-Melanocyte-stimulating hormone (α-MSH) on the 200-kDa neurofilament in the sciatic nerve of rats. Their findings indicated that α-MSH has a trophic effect on neurofilaments, an essential component of the neuronal cytoskeleton, suggesting that α-MSH could play a role in nerve regeneration and repair. This study adds to the body of evidence that α-MSH could be beneficial in the treatment of neurological disorders or injuries through its positive effects on neural structures.
For in-depth study https://nyaspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1749-6632.1993.tb19766.x
Van de meent H, Hamers FP, Lankhorst AJ, Joosten EA, Gispen WH. Beneficial effects of the melanocortin alpha-melanocyte-stimulating hormone on clinical and neurophysiological recovery after experimental spinal cord injury. Neurosurgery. 1997;40(1):122-30.
Beneficial effects of the melanocortin alpha-melanocyte-stimulating hormone on clinical and neurophysiological recovery after experimental spinal cord injury
The 1997 study by Van de Meent, Hamers, Lankhorst, Joosten, and Gispen, published in Neurosurgery, explored the therapeutic effects of the melanocortin α-Melanocyte-stimulating hormone (α-MSH) on the recovery process after experimental spinal cord injury in animals. Their findings highlighted that α-MSH significantly improved both clinical and neurophysiological outcomes following spinal cord injury. Specifically, the treatment with α-MSH resulted in improved motor performance, as evidenced by Tarlov scores, and enhanced neurophysiological measures, such as thoracolumbar height and amplitude of rubrospinal motor evoked potentials. These results suggest that α-MSH has a potent neuroprotective effect, potentially offering a novel therapeutic approach to enhance recovery after spinal cord injuries
For in-depth study https://www.semanticscholar.org/paper/Beneficial-effects-of-the-melanocortin-hormone-on-Meent-Hamers/b713eb8fe09df8d81b8d07d94439333734d5b03e
Lankhorst AJ, Duis SE, Terlaak MP, Joosten EA, Hamers FP, Gispen WH. Functional recovery after central infusion of alpha-melanocyte-stimulating hormone in rats with spinal cord contusion injury. J Neurotrauma. 1999;16(4):323-31.
Functional recovery after central infusion of alpha-melanocyte-stimulating hormone in rats with spinal cord contusion injury
The 1999 study by Lankhorst, Duis, Ter Laak, Joosten, Hamers, and Gispen, published in Journal of Neurotrauma, investigated the impact of central infusion of α-Melanocyte-stimulating hormone (α-MSH) on functional recovery in rats with spinal cord contusion injuries. The findings demonstrated that α-MSH significantly enhanced functional recovery, as evidenced by improvements in motor function and behavior. This research suggests that α-MSH has potent neuroprotective and regenerative effects on the injured spinal cord, offering a promising therapeutic strategy for enhancing recovery after spinal cord injuries.
For in-depth study https://www.eurekaselect.com/article/103010
Joosten EA, Majewska B, Houweling DA, Bär PR, Gispen WH. Alpha-melanocyte stimulating hormone promotes regrowth of injured axons in the adult rat spinal cord. J Neurotrauma. 1999;16(6):543-53.
Alpha-melanocyte stimulating hormone promotes regrowth of injured axons in the adult rat spinal cord
The study conducted by Joosten, Majewska, Houweling, Bär, and Gispen, published in Journal of Neurotrauma in 1999, explores the regenerative capabilities of α-Melanocyte stimulating hormone (α-MSH) in the adult rat spinal cord after injury. This research demonstrated that α-MSH promotes the regrowth of injured axons, highlighting its potential neurotrophic effects. By facilitating the recovery of damaged neural pathways, α-MSH could offer a promising therapeutic strategy for enhancing axonal regeneration and functional recovery following spinal cord injuries.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/10391370/
Sharma HS, Skottner A, Lundstedt T, Flärdh M, Wiklund L. Neuroprotective effects of melanocortins in experimental spinal cord injury. An experimental study in the rat using topical application of compounds with varying affinity to melanocortin receptors. J Neural Transm (Vienna). 2006;113(4):463-76.
Neuroprotective effects of melanocortins in experimental spinal cord injury
The 2006 study by Sharma, Skottner, Lundstedt, Flärdh, and Wiklund, published in Journal of Neural Transmission, investigates the neuroprotective effects of melanocortins in experimental spinal cord injury. This study used topical application of compounds with varying affinity to melanocortin receptors in a rat model. The research demonstrated that these compounds, particularly those with high affinity to melanocortin receptors, can significantly influence the pathophysiological outcome of spinal cord injury, suggesting their potential as therapeutic agents for neuroprotection and recovery after spinal cord trauma.
For in-depth study https://link.springer.com/content/pdf/10.1007/s00702-005-0404-3.pdf?pdf=preview
Huh SK, Lipton JM, Batjer HH. The protective effects of alpha-melanocyte stimulating hormone on canine brain stem ischemia. Neurosurgery. 1997;40(1):132-9.
The protective effects of α-melanocyte stimulating hormone on canine brain stem ischemia
The study by Huh, Lipton, and Batjer, published in Neurosurgery in 1997, explored the protective effects of α-Melanocyte stimulating hormone (α-MSH) on canine brain stem ischemia. The research demonstrated that α-MSH could significantly improve the recovery of brainstem auditory evoked potentials (BAEPs) in dogs subjected to ischemia and reperfusion injury. This improvement suggests that α-MSH has neuroprotective effects in the context of ischemic brain damage, possibly due to its antagonistic action on cytokine-induced damage. These findings indicate that α-MSH could be a valuable therapeutic agent in treating brain stem ischemia and related conditions.
For in-depth study https://journals.lww.com/neurosurgery/Fulltext/1997/01000/The_Protective_Effects_of__alpha__Melanocyte.30.aspx
Huang Q, Tatro JB. Alpha-melanocyte stimulating hormone suppresses intracerebral tumor necrosis factor-alpha and interleukin-1beta gene expression following transient cerebral ischemia in mice. NeurosciLett. 2002;334(3):186-90.
Alpha-melanocyte stimulating hormone suppresses intracerebral tumor necrosis factor-alpha and interleukin-1beta gene expression following transient cerebral ischemia in mice
The study by Huang and Tatro, published in Neuroscience Letters in 2002, investigated the effects of α-Melanocyte stimulating hormone (α-MSH) on the gene expression of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta) following transient cerebral ischemia in mice. Their findings revealed that α-MSH significantly suppresses the activation of these inflammatory cytokines in the brain after ischemic injury. This suppression suggests that α-MSH could be a potential therapeutic agent for reducing inflammation and protecting neural tissue in the context of cerebral ischemia.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/12453626/
Spulber S, Moldovan M, Oprica M, et al. Alpha-MSH decreases core and brain temperature during global cerebral ischemia in rats. Neuroreport. 2005;16(1):69-72.
Alpha-MSH decreases core and brain temperature during global cerebral ischemia in rats
The 2005 study by Spulber, Moldovan, Oprica, and others, published in NeuroReport, investigates the effects of α-Melanocyte-stimulating hormone (α-MSH) on core and brain temperature during global cerebral ischemia in rats. This research found that α-MSH decreases both core and brain temperature, which may contribute to its neuroprotective effects during cerebral ischemia. Lowering the temperature can reduce the metabolic rate and excitotoxic damage, potentially offering a therapeutic approach to mitigate brain injury following ischemic events.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/15618893/
Forslinaronsson S, Spulber S, Popescu LM, et al. alpha-Melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides. 2006;40(1):65-75.
α-Melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia
The 2006 study by Forslin Aronsson, Spulber, Popescu, and colleagues, published in Neuropeptides, aimed to investigate the neuroprotective effects of α-Melanocyte-stimulating hormone (α-MSH) following global cerebral ischemia and reperfusion in rats. This study adds to the growing body of evidence that α-MSH, a tridecapeptide derived from proopiomelanocortin (POMC), can mitigate neurodegeneration induced by ischemic events. By exploring α-MSH’s potential in reducing the damage following global cerebral ischemia, this research highlights the therapeutic relevance of α-MSH in managing and potentially treating ischemia-induced neurodegenerative conditions.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/16414116/
Giuliani D, Leone S, Mioni C, et al. Broad therapeutic treatment window of [Nle(4), D-Phe(7)]alpha-melanocyte-stimulating hormone for long-lasting protection against ischemic stroke, in Mongolian gerbils. Eur J Pharmacol. 2006;538(1-3):48-56.
Broad therapeutic treatment window of [Nle(4), D-Phe(7)]alpha-melanocyte-stimulating hormone for long-lasting protection against ischemic stroke, in Mongolian gerbils
The 2006 study by Giuliani, Leone, Mioni, and colleagues, published in European Journal of Pharmacology, investigated the neuroprotective effects of the melanocortin analog [Nle(4), D-Phe(7)]alpha-melanocyte-stimulating hormone (NDP-α-MSH) in Mongolian gerbils subjected to ischemic stroke. The research aimed to identify the therapeutic treatment window of melanocortins and determine their long-lasting protection against brain ischemia damage. Treatment with NDP-α-MSH, starting within 3-18 hours after the ischemic episode and administered every 12 hours for 11 days, significantly reduced hippocampal damage and improved functional recovery. This study highlights the broad therapeutic potential of melanocortins in treating ischemic stroke, providing insight into their role in chronic neuroprotection and recovery enhancement.
Giuliani D, Ottani A, Mioni C, et al. Neuroprotection in focal cerebral ischemia owing to delayed treatment with melanocortins. Eur J Pharmacol. 2007;570(1-3):57-65.
Neuroprotection in focal cerebral ischemia owing to delayed treatment with melanocortins
The 2007 study by Giuliani, Ottani, Mioni, and colleagues, published in the European Journal of Pharmacology, explores the neuroprotective properties of melanocortins in focal cerebral ischemia, highlighting the effectiveness of delayed treatment with these compounds. This research demonstrates that melanocortins, through their action on MC4 receptors, can confer protection in models of ischemic stroke, suggesting a broad therapeutic window. The findings support the potential of MC4 receptor agonists as neuroprotective agents in various experimental models of ischemic stroke, indicating their versatility in treating severe focal cerebral ischemia.
For in-depth study https://pubmed.ncbi.nlm.nih.gov/17588564/
Giuliani D, Mioni C, Altavilla D, et al. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology. 2006;147(3):1126-35.
Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia
The paper you’ve mentioned, authored by Giuliani D, Mioni C, Altavilla D, et al., titled “Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia,” published in Endocrinology in March 2006, explores the neuroprotective effects of melanocortin 4 receptor (MC4R) agonists in the context of cerebral ischemia. Cerebral ischemia refers to a condition where there is insufficient blood flow to the brain to meet metabolic demand, leading to potential brain damage or the death of brain cells.
This study is significant because it suggests that both early and delayed interventions using MC4R-stimulating melanocortins can offer protective benefits against the damage caused by cerebral ischemia. The findings imply that activating the MC4R could be a viable therapeutic strategy for mitigating the effects of stroke and other forms of brain ischemia, potentially extending the treatment window and improving outcomes for patients suffering from these conditions.
For in-depth study https://academic.oup.com/endo/article-abstract/147/3/1126/2500570
Vecsernyes M, Juhasz B, Der P, et al. The administration of alpha-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur J Pharmacol. 2003;470(3):177-83.
The administration of α-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium
The study by Vecsernyes M, Juhasz B, Der P, and colleagues, titled “The administration of alpha-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium,” published in the European Journal of Pharmacology in 2003, investigates the cardioprotective effects of alpha-melanocyte-stimulating hormone (α-MSH) in the context of ischemia/reperfusion (I/R) injury in the myocardium (heart muscle).
Ischemia/reperfusion injury is a critical concern in cardiovascular diseases, especially during events such as heart attacks and procedures like coronary artery bypass surgery. It refers to the damage caused when blood supply returns to the tissue after a period of ischemia or lack of oxygen. The sudden return of blood can cause inflammation and oxidative damage through the overproduction of free radicals and other mechanisms.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0014299903017801
Mioni C, Giuliani D , Cainazzo MM, Leone S, Bazzani C, Grieco P, Novellino E, Tomasi A, Bertolini A, Guarini S 2004 Further evidence that melanocortins prevent myocardial reperfusion injury by activating melanocortin MC3 receptors. Eur J Pharmacol 477:227–234.
Guarini S 2004 Further evidence that melanocortins prevent myocardial reperfusion injury by activating melanocortin MC3 receptors
The study by Mioni C, Giuliani D, Cainazzo MM, Leone S, Bazzani C, Grieco P, Novellino E, Tomasi A, Bertolini A, and Guarini S, titled “Further evidence that melanocortins prevent myocardial reperfusion injury by activating melanocortin MC3 receptors,” published in the European Journal of Pharmacology in 2004, focuses on the cardioprotective effects of melanocortins through the activation of melanocortin MC3 receptors in the context of myocardial reperfusion injury.
Myocardial reperfusion injury occurs when the blood supply to the heart is restored after an ischemic event, such as a heart attack. While reperfusion is crucial for salvaging heart tissue, the process itself can lead to further damage through mechanisms like oxidative stress, inflammation, and apoptosis (cell death).
Melanocortins are peptides derived from the precursor molecule proopiomelanocortin (POMC) and are known to interact with melanocortin receptors (MC1 through MC5), which are involved in a wide variety of physiological processes, including inflammatory responses. Previous research has suggested that melanocortins have anti-inflammatory and potentially cardioprotective effects.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0014299903021848
Bertolini A, Guarini S, Rompianesi E, Ferrari W. Alpha-MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock. Eur J Pharmacol. 1986;130(1-2):19-26.
α-MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock
The study by Bertolini A, Guarini S, Rompianesi E, Ferrari W, titled “Alpha-MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock,” published in the European Journal of Pharmacology in 1986, investigates the effects of alpha-melanocyte-stimulating hormone (α-MSH) and other adrenocorticotropic hormone (ACTH) fragments on cardiovascular function and survival rates in models of experimental hemorrhagic shock.
Hemorrhagic shock is a form of hypovolemic shock resulting from significant blood loss, leading to inadequate tissue perfusion, oxygen delivery, and subsequent organ failure. It represents a critical condition requiring immediate intervention to restore circulatory volume and support the cardiovascular system to prevent mortality.
For in-depth study https://www.sciencedirect.com/science/article/pii/0014299986901792
Guarini S, Schiöth HB, Mioni C, et al. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. NaunynSchmiedebergs Arch Pharmacol. 2002;366(2):177-82.
MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias
The study by Guarini S, Schiöth HB, Mioni C, et al., titled “MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias,” published in Naunyn-Schmiedeberg’s Archives of Pharmacology in 2002, focuses on the role of melanocortin 3 (MC3) receptors in mediating the cardioprotective effects of melanocortins against arrhythmias induced by myocardial ischemia/reperfusion (I/R) injury.
Myocardial I/R injury is a critical condition that can occur after the restoration of blood flow to previously ischemic heart tissue, such as after a heart attack or during cardiac surgery. This condition can lead to arrhythmias, which are irregular heartbeats that can be life-threatening if not properly managed.
For in-depth study https://link.springer.com/article/10.1007/s00210-002-0572-8
Yamaoka-tojo M, Tojo T, Shioi T, Masuda T, Inomata T, Izumi T. Central neurotranspeptide, alpha-melanocyte-stimulating hormone (alpha-MSH) is upregulated in patients with congestive heart failure. Intern Med. 2006;45(7):429-34.
Central neurotranspeptide, alpha-melanocyte-stimulating hormone (alpha-MSH) is upregulated in patients with congestive heart failure
The study by Yamaoka-tojo M, Tojo T, Shioi T, Masuda T, Inomata T, Izumi T, titled “Central neurotranspeptide, alpha-melanocyte-stimulating hormone (alpha-MSH) is upregulated in patients with congestive heart failure,” published in Internal Medicine in 2006, explores the relationship between levels of the central neurotransmitter alpha-melanocyte-stimulating hormone (α-MSH) and congestive heart failure (CHF).
Congestive heart failure is a condition where the heart’s ability to pump blood is inadequate to meet the body’s needs, leading to symptoms such as shortness of breath, fatigue, and fluid retention. It is a complex syndrome that involves various neurohormonal mechanisms contributing to its pathophysiology and progression.
α-MSH is a peptide hormone known for its roles in regulating skin pigmentation, inflammation, energy homeostasis, and appetite. Recent research has also suggested its involvement in cardiovascular regulation and the body’s response to heart failure.
For in-depth study https://www.jstage.jst.go.jp/article/internalmedicine/45/7/45_7_429/_article/-char/ja/
Chiao H, Kohda Y, Mcleroy P, Craig L, Housini I, Star RA. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99(6):1165-72
Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats
The study by Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA, titled “Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats,” published in the Journal of Clinical Investigation in 1997, investigates the protective effects of alpha-melanocyte-stimulating hormone (α-MSH) against renal injury induced by ischemia in rodent models.
Renal ischemia, which is a reduction in blood flow to the kidneys, can lead to acute kidney injury (AKI), characterized by a rapid decline in renal function. AKI is a significant clinical problem associated with high morbidity and mortality rates, especially when it is a consequence of ischemia-reperfusion (I/R) injury, a common occurrence in kidney transplantation, shock, and sepsis.
α-MSH is a peptide hormone known for its anti-inflammatory and immunomodulatory effects. This study explores its potential in protecting the kidneys from damage following ischemia. The findings of the research indicate that α-MSH administration significantly reduces the extent of renal injury in mice and rats subjected to ischemic conditions. The protective effects of α-MSH were observed through various measures of renal function and injury, including reductions in serum creatinine levels, diminished histological signs of damage, and decreased mortality rates.
For in-depth study https://www.jci.org/articles/view/119272
Jo SK, Yun SY, Chang KH, et al. alpha-MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant. 2001;16(8):1583-91.
α‐MSH decreases apoptosis in ischaemic acute renal failure in rats: Possible mechanism of this beneficial effect
The study by Jo SK, Yun SY, Chang KH, et al., titled “alpha-MSH decreases apoptosis in ischemic acute renal failure in rats: possible mechanism of this beneficial effect,” published in Nephrology Dialysis Transplantation in 2001, investigates the role of alpha-melanocyte-stimulating hormone (α-MSH) in reducing apoptosis (programmed cell death) in the context of ischemic acute renal failure (ARF) in rats.
Acute renal failure (ARF), now more commonly referred to as acute kidney injury (AKI), is a rapid loss of kidney function that can result from various causes, including ischemia, which is a reduction in blood flow to the kidneys. Ischemia can lead to significant kidney damage, partly through the induction of apoptosis in renal cells.
For in-depth study https://academic.oup.com/ndt/article-abstract/16/8/1583/1826555
Kwon TH, Frøkiaer J, Fernández-llama P, Knepper MA, Nielsen S. Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by alpha-MSH. Am J Physiol. 1999;277(3):F413-27.
Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by alpha-MSH
The study by Kwon TH, Frøkiaer J, Fernández-Llama P, Knepper MA, Nielsen S, titled “Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: prevention by alpha-MSH,” published in the American Journal of Physiology in 1999, delves into the effects of alpha-melanocyte-stimulating hormone (α-MSH) on the expression of aquaporins (AQPs) in the context of acute renal failure (ARF) induced by bilateral renal ischemia in rats.
Aquaporins are a family of water channel proteins that play crucial roles in the regulation of water balance in various tissues, including the kidneys. They are essential for the kidney’s ability to concentrate urine and maintain fluid balance.
In the study, the researchers observed a reduction in the abundance of several aquaporins, specifically AQP1, AQP2, AQP3, and AQP4, in the kidneys of rats with bilateral ischemia-induced ARF. This decrease in aquaporin expression contributes to the impaired kidney function associated with ARF, affecting the renal handling of water and leading to difficulties in urine concentration and fluid regulation.
For in-depth study https://journals.physiology.org/doi/abs/10.1152/ajprenal.1999.277.3.F413
Gong H, Wang W, Kwon TH, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66(2):683-95
EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney
The study by Gong H, Wang W, Kwon TH, et al., titled “EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney,” published in Kidney International in 2004, explores the protective effects of erythropoietin (EPO) and alpha-melanocyte-stimulating hormone (α-MSH) against the down-regulation of aquaporins (AQPs) and sodium transporters in the rat kidney following ischemia/reperfusion (I/R) injury.
Ischemia/reperfusion injury in the kidneys can lead to acute kidney injury (AKI), characterized by a rapid decline in renal function. This condition often results in alterations in the expression of various proteins essential for kidney function, including AQPs, which are vital for water transport, and sodium transporters, which are crucial for sodium balance and hence, indirectly, for water balance as well.
For in-depth study https://www.sciencedirect.com/science/article/pii/S0085253815500987
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

