GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Dihexa
- Key Takeaways
- What is Dihexa?
- How Dihexa Works
- Chemical Structure of Dihexa
- Research on Dihexa
- Dihexa Side Effects
- What is Dihexa Peptide?
- Dihexa How to Use
- Dihexa Uses
- Dihexa Dosage
- Dihexa Acetate
- Dihexa Capsules
- Dihexa Bodybuilding
- Dihexa Supplement
- Dihexa Nootropic (Improves Cognitive Functions)
- What is Brain Derived Neurotrophic Factor?
- Dihexa and Hepatocyte Growth Factor
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of Dihexa
- Key Takeaways
- What is Dihexa?
- How Dihexa Works
- Chemical Structure of Dihexa
- Research on Dihexa
- Dihexa Side Effects
- What is Dihexa Peptide?
- Dihexa How to Use
- Dihexa Uses
- Dihexa Dosage
- Dihexa Acetate
- Dihexa Capsules
- Dihexa Bodybuilding
- Dihexa Supplement
- Dihexa Nootropic (Improves Cognitive Functions)
- What is Brain Derived Neurotrophic Factor?
- Dihexa and Hepatocyte Growth Factor
- FAQ
- Reference
Overall Health Benefits of Dihexa
Dihexa benefits include treating Alzheimer’s and Parkinson’s diseases, improving cognitive function, and enhancing stroke recovery. Additionally, it accelerates recovery from spinal cord injuries and nerve damage while also protecting against hearing loss.
- Treats Alzheimer’s disease [1-8]
- Combats Parkinson’s disease [9-14]
- Improves cognitive function [15-25]
- Improves stroke recovery [26-29]
- Accelerates recovery from spinal cord injury and nerve damage [30-34]
- Protects against hearing loss [35]
Key Takeaways
- Cognitive Enhancement: Dihexa is a peptide known for its potential to enhance cognitive function, particularly in areas such as memory retention and learning ability.
- Mechanism of Action: It works by promoting neurogenesis and increasing the density of synapses in the brain, which are crucial for cognitive processes.
- Research Focus: Dihexa has garnered attention in neuroscience research for its possible therapeutic applications in conditions involving cognitive decline, such as Alzheimer’s disease.
- Potential Side Effects: While generally well-tolerated in animal studies, human trials are limited, and potential side effects and long-term safety considerations require further investigation.
- Future Directions: Ongoing research aims to expand understanding of Dihexa’s mechanisms and explore its clinical potential, highlighting its role as a promising candidate in neuroprotective therapies.
What is Dihexa?
Dihexa, also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amideor PNB-0408, is a relatively new drug for the treatment of Alzheimer’s disease (AD) and cognitive impairment. Unlike other drugs for AD, this potent nootropic does not only impede the progression of the disease but it actually repairs the damage in the synapse (junction) between neurons. Because of its regenerative properties, most medical professionals prescribe Dihexa for the treatment of a wide array of medical conditions that affect cognitive function.
How Dihexa Works
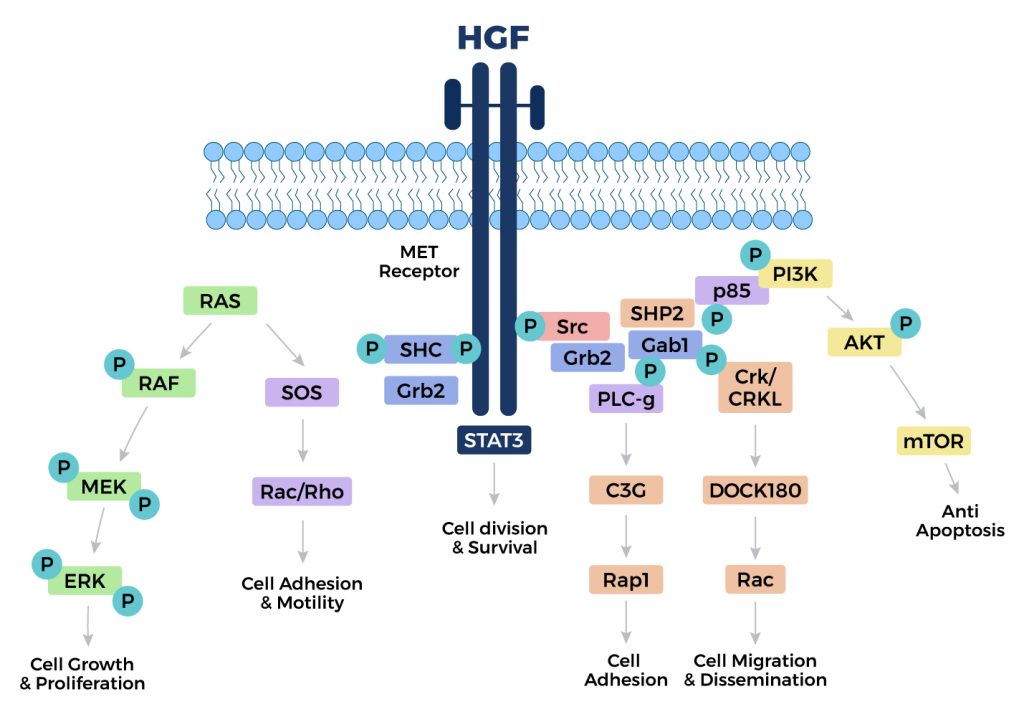
By binding to hepatocyte growth factor (also known as HGF, c-Met or tyrosine-protein kinase Met), Dihexa increases HGF’s activity while lowering harmful chemical reactions in the body. This in turn doubles the capacity of the available growth factors to promote signaling cascades necessary for cell development and regeneration.
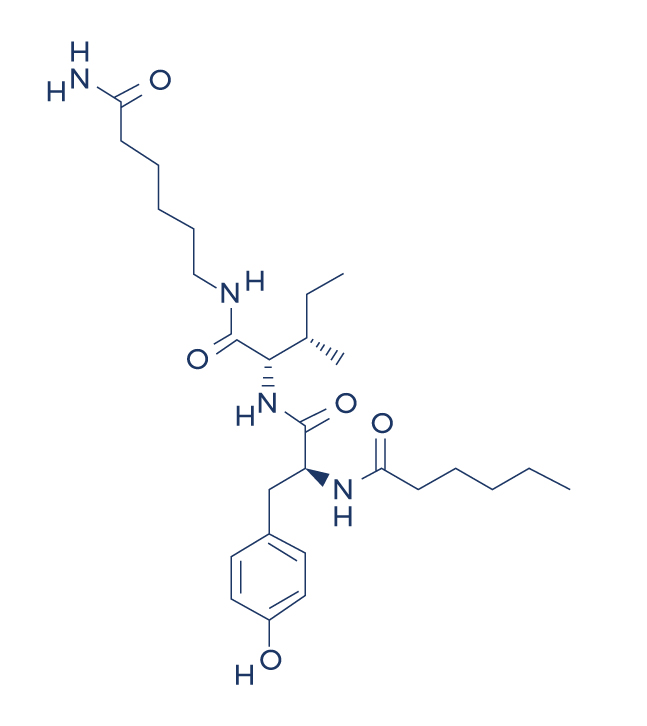
Chemical Structure of Dihexa
Research on Dihexa
A. Treats Alzheimer’s Disease
There is increasing evidence that Dihexa may help treat not only the symptoms of AD but the root cause of the disease itself. Studies show that Dihexa exerts its beneficial effects on AD through various important mechanisms:
- Dihexa activates the c-Met receptor which in turn stimulates mitogenesis (cell division), motogenesis (promotion of cellular motility), morphogenesis (structural development), neurogenesis (growth and development of nervous tissue), production of stem cells, and protection of a wide range of cells against injury. [1-2]
- Dihexa induces the development of dendritic spines in neurons of the brain. [3]
- Activation of HGF by Dihexa augments synaptic connectivity, protects neurons from underlying death inducers, and promotes renewal of lost neurons. [4]
- Dihexa may actually prevent and improve symptoms of AD by decreasing the production of amyloid beta peptide, which are abnormal protein structures that cause the disease. [5]
- In aged rat models, Dihexa administration improves synaptogenic activity. [6-7]
- In rats with reduced blood flow to the brain, administration of Angiotensin IV such as Dihexa suppresses inflammation. [8]
B. Combats Parkinson’s Disease
People with Parkinson’s disease (PD), a neurodegenerative disorder that destroys dopamine-producing nerve cells in the brain, can also benefit from Dihexa supplementation. Studies show that this powerful nootropic can help combat PD through various mechanisms:
- Dihexa can help treat PD symptoms by augmenting synaptic connectivity via the formation of new functional synapses. [9]
- Activation of the HGF/c-Met system by Dihexa stimulates protection and restoration of neurons in the brain. [10]
- In patients with PD, HGF promoted the survival and migration of immature neurons in the brain. [11]
- In a PD lesion model, Dihexa administration improved behavioral deficits. [12]
- Gene transfer of human hepatocyte growth factor in a rodent model of PD prevented neuronal death, suggesting that Dihexa activation of HGF can be a potential novel therapy for PD. [13]
- Dihexa along with Angiotensin IV can prevent the progression of PD by increasing the production of dopamine. [14]
C. Improves Cognitive Function
According to studies, this powerful nootropic also has memory-enhancing properties that can benefit people with memory problems associated with aging and neurodegenerative disorders:
- Dihexa shows promise in treating memory and motor dysfunctions by enhancing synaptic connectivity through the formation of new functional synapses. [15]
- In rats, oral Dihexa administration at a dose of 2 mg/kg per day improved memory retention and performance in the Morris water maze test. [16]
- Administration of Angiotensin IV-related peptides such as Dihexa in rats reversed scopolamine-induced deficits in Morris water maze performance. [17]
- Dihexa and Angiotensin IV-related peptides are involved in spatial memory processing, and that activation of their binding sites can help combat spatial memory disruption. [18-19]
- In rats with Alzheimer’s-like mental impairment, Dihexa supplementation improved memory retention by building new brain cell connections. [20]
- In rats, HGF activation by Dihexa prevented learning and memory dysfunction after sustained cerebral ischemia (decreased blood flow to the brain) by protecting against injury to brain neurons. [21]
- Dihexa administration in rats improved performance in a series of tests related to learning and memory. [22]
- Dihexa exerts its cognitive-enhancing effects by increasing the production of acetylcholine and/or dopamine. [23]
- In a mouse model of cognitive impairment, oral administration with Dihexa restored spatial learning and cognitive functions in the Morris water maze test. [24]
- In animal models of cognitive deficit, Dihexa improved performance on spatial working memory and passive avoidance tasks. [25]
D. Improves Stroke Recovery
There’s also a great deal of evidence supporting the ability of Dihexa to accelerate recovery from stroke:
- In a mouse model of stroke, activation of HGF induced long-term neuroprotection and stroke recovery. [26]
- Pharmacological doses of Angiotensin IV-related peptides such as Dihexa are protective against acute cerebral ischemia. [27]
- Dihexa can help prevent stroke by increasing blood flow to the brain. [28]
- In rats with stroke caused by cerebral artery occlusion, Dihexa administration prevented programmed cell death of brain cells. [29]
E. Accelerates Recovery from Spinal Cord Injury and Nerve Damage
Numerous studies also found that Dihexa has the ability to treat the most debilitating nervous system injuries – spinal cord injury and nerve damage:
- By activating HGF, Dihexa may retard the progression of spinal cord injury caused by amyotrophic lateral sclerosis (ALS). [30]
- Patients with ALS especially those in advanced disease stage have disruption of the neuronal HGF-c-Met system, suggesting that Dihexa may play a part in preventing cellular degeneration and programmed cell death of spinal motor neurons. [31-32]
- In an animal model of spinal cord injury, Dihexa administration accelerated functional recovery. [33]
- In a rat with damage to the sciatic nerve (the largest nerve in the body located in the spinal cord), Dihexa (2-4 mg/kg body weight) administration via hydrogel and injection promoted limb function recovery. [34]
F. Protects against Hearing Loss
Evidence found that Dihexa can help protect against hearing loss caused by toxins:
- In the larval zebrafish, the administration of Dihexa at a concentration of 1 μM provided optimal protection against hearing loss caused by the toxic medications neomycin and gentamicin. [35]
Dihexa Side Effects
Dihexa side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on Dihexa. However, the issue wasn’t’ confirmed to be caused by the treatment and could have been a coincidence and not related to the use of Dihexa. Despite this, it was listed as a side effect associated with Dihexa even these associated side effects are very uncommon.
Side effects associated with Dihexa may include the following:
- Anxiety
- Change of taste
- Insomnia
- Irritability
- Mood swings
- Nausea
What is Dihexa Peptide?
Dihexa peptide, scientifically known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide, is a synthetic compound that has gained attention for its potential neuroprotective and cognitive enhancing properties. Developed from angiotensin IV, Dihexa is notable for its ability to cross the blood-brain barrier, a critical feature for drugs targeting neurological conditions. Its primary mechanism of action involves enhancing neurogenesis and increasing the density of synapses in the brain, which are essential for cognitive processes like learning and memory retention. Research into Dihexa peptide therapy continues to explore its efficacy and safety in treating cognitive decline and neurodegenerative disorders.
Research on Dihexa has primarily focused on its effects in animal models, where it has demonstrated promising results in improving memory and cognitive function, particularly enhancing conversational skills. Studies suggest that Dihexa not only enhances existing synaptic connections but also promotes the growth of new neurons, indicating potential applications in treating neurodegenerative diseases and age-related cognitive decline. Despite its early promise, the translation of these findings to human trials is still in its infancy, necessitating further research to understand its safety profile, optimal dosage, and long-term effects on human conversational skills and cognitive health.
In conclusion, Dihexa represents a cutting-edge development in the field of neurology and cognitive enhancement. Its ability to penetrate the brain and influence synaptic plasticity makes it a compelling candidate for future therapeutic interventions aimed at enhancing cognitive function and potentially treating neurological disorders characterized by damaged and lost neurons synaptic dysfunction. As research progresses, Dihexa holds promise for contributing to advancements in neuroprotective therapies and our understanding of brain health, particularly in conditions where damaged and lost neurons are significant factors.
Dihexa How to Use
Using Dihexa typically involves careful consideration of dosage and administration methods. As a peptide with potential cognitive benefits, Dihexa is often administered via injection or oral ingestion, though the latter is less common due to issues with bioavailability. For those opting for injections, precise measurement and administration by a healthcare professional are crucial to ensure safety and effectiveness, particularly when considering angiotensin IV. Due to the peptide’s potency and targeted effects on brain function, adherence to recommended dosage guidelines is essential to minimize risks and maximize potential benefits, especially with angiotensin IV in mind.
Before using Dihexa, consulting with a healthcare provider is advisable to determine the appropriate dosage based on individual health factors and desired outcomes. This ensures personalized guidance and supervision throughout the course of treatment. Monitoring for any adverse reactions or unexpected effects is also important during usage, as clinical studies continue to explore its long-term safety profile and optimal therapeutic applications, including the potential role of angiotensin IV. Overall, responsible use of Dihexa involves informed decision-making, adherence to recommended protocols, and ongoing communication with healthcare professionals to achieve desired cognitive enhancements effectively and safely, incorporating the potential benefits of angiotensin IV where applicable.
Dihexa Uses
Dihexa, a potent peptide with neuroregenerative properties, is primarily researched for its potential uses in enhancing cognitive function and treating neurological disorders. Studies suggest that Dihexa promotes the growth of new neurons and strengthens synaptic connections in the brain, which are essential for learning and memory. This mechanism of action has sparked interest in its application for conditions involving cognitive decline, such as Alzheimer’s disease and other forms of dementia. Researchers hope that Dihexa could potentially slow down or even reverse cognitive impairment by supporting brain cell growth and function. The peptide’s ability to mimic the effects of angiotensin IV further underscores its potential in neuroprotection and cognitive enhancement.
Beyond cognitive enhancement, Dihexa is also being explored for its therapeutic benefits in neurological conditions like stroke and traumatic brain injury (TBI). Preclinical studies have indicated that Dihexa may aid in neurorepair processes after such injuries by fostering neuronal regeneration and improving neurological outcomes. This aspect of angiotensin IV’s research underscores its potential as a neuroprotective agent, offering hope for novel treatments that could mitigate the long-term effects of brain trauma and improve recovery rates.
While promising, the clinical application of peptide dihexa is still in early stages, with most studies conducted on animal models. Human trials, including research involving angiotensin IV, are essential to further validate its efficacy and safety profile. The ongoing research on peptide dihexa not only aims to elucidate its therapeutic potential but also seeks to address concerns regarding optimal dosage, long-term effects, and potential side effects in human subjects, including those involving angiotensin IV. These efforts are crucial steps towards potentially harnessing peptide dihexa’s benefits for improving brain health and treating neurological disorders in clinical settings.
Dihexa Dosage
Determining the appropriate dosage of Dihexa, hailed as a potential neurogenic wonder drug, is crucial due to its potent effects on cognitive function and neurogenesis. Currently, there is limited clinical data on human dosing, as most studies have been conducted on animals. However, preliminary research suggests that doses used in animal models range from microgram to milligram levels per kilogram of body weight. This wide range underscores the need for cautious extrapolation to human dosing.
In human trials, initial studies have focused on establishing safety profiles and assessing potential cognitive benefits, particularly in relation to nerve growth factor. Researchers typically start with low doses to evaluate tolerance and then incrementally increase to find the optimal therapeutic range, considering nerve growth factor. It’s important to note that individual responses to Dihexa may vary due to factors such as age, health status, and genetic predispositions, emphasizing the need for personalized dosing strategies that consider nerve growth factor.
Moving forward, ongoing clinical trials aim to refine dosing guidelines and explore the long-term effects of Dihexa on brain disorders. These efforts are critical in determining not only the effective therapeutic dose but also the potential risks and benefits associated with prolonged use in treating brain disorders. As research progresses, clearer recommendations for safe and effective dosing in humans are expected to emerge, aiding in the responsible application of Dihexa in clinical settings for brain disorders, particularly in enhancing functional synaptic connections.
Dihexa Acetate
Dihexa acetate, a derivative of angiotensin IV ile 6 aminohexanoic amide, has emerged as a notable compound in the realm of cognitive enhancement and neuroprotection research. This peptide ile 6 aminohexanoic amide is known for its ability to cross the blood-brain barrier efficiently, which enhances its effectiveness in the central nervous system. Studies have shown that Dihexa acetate promotes the growth of new neurons and synapses, potentially improving cognitive functions such as memory and learning. This property ile 6 aminohexanoic amide has sparked interest in its therapeutic potential for conditions associated with cognitive decline, including Alzheimer’s disease.
Research into Dihexa acetate has primarily focused on its mechanisms of action and safety profile. Preclinical studies have demonstrated promising results in animal models, highlighting its neuroprotective effects and suggesting a role in enhancing synaptic plasticity, which could potentially support creative thinking. However, human trials are limited, and while early findings are encouraging, further research is needed to determine its efficacy and safety in clinical settings, particularly concerning its impact on creative thinking. Understanding its long-term effects and potential interactions with other treatments remains a crucial area of exploration before widespread clinical application can be considered.
In conclusion, Dihexa acetate represents a promising avenue in neuroscience research, offering potential benefits for cognitive enhancement and neuroprotection. As studies continue to unfold, its ability to influence synaptic growth and cognitive function could pave the way for novel therapeutic approaches in treating neurological disorders and age-related cognitive decline. Understanding its impact on blood flow metab and broader neural mechanisms will be crucial for assessing its full therapeutic potential in clinical settings.
Dihexa Capsules
Dihexa capsules are a formulation designed to deliver the peptide compound Dihexa, known for its potential cognitive enhancement properties, in a convenient oral form. These capsules are intended to provide a controlled and consistent dosage of Dihexa, optimizing its absorption and bioavailability for users interested in cognitive enhancement or neuroprotective effects. The formulation typically ensures that Dihexa reaches systemic circulation effectively, where it can exert its effects on brain function and potentially influence developmental code.
Users of Dihexa capsules often seek to improve cognitive abilities such as memory retention, learning capacity, and overall mental acuity, including spatial memory. The convenience of capsules allows for easy integration into daily routines, making it accessible for individuals looking to support brain health without the need for injections or other delivery methods. However, as with any supplement or pharmaceutical, it’s essential for users to consult healthcare professionals to ensure proper usage and to monitor for any potential side effects or interactions with existing medications, especially concerning spatial memory.
The development of Dihexa capsules underscores ongoing advancements in neurology and cognitive enhancement research, catering to a growing interest in optimizing brain function and potentially mitigating cognitive decline. While research into Dihexa’s efficacy and safety continues, these capsules represent a promising avenue in the quest to unlock the full potential of brain health supplementation, particularly in enhancing spatial memory.
Dihexa Bodybuilding
Dihexa peptide, while primarily studied for its potential cognitive benefits, has attracted interest within bodybuilding communities due to its purported ability to enhance muscle growth and recovery. The dihexa peptide is believed to stimulate angiogenesis, the formation of new blood vessels, which could potentially improve nutrient delivery to muscles during exercise and aid in recovery post-workout. This mechanism suggests dihexa peptide might have indirect benefits for muscle hypertrophy and endurance, though specific studies directly linking dihexa peptide to enhanced athletic performance are limited.
Despite its theoretical advantages, dihexa peptide’s use in bodybuilding remains speculative and unproven. The dihexa peptide’s safety profile and long-term effects on muscle development have not been thoroughly researched in the context of athletic performance. As with any experimental substance, caution is warranted, and athletes should prioritize evidence-based approaches to training and supplementation.
In conclusion, while Dihexa peptide holds promise in neuroscience and potential cognitive enhancement, its application in bodybuilding is largely speculative and lacks robust scientific validation. Athletes and bodybuilders should prioritize established methods of training, nutrition, and supplementation until more comprehensive research can verify its safety and efficacy for athletic performance.
Dihexa Supplement
Dihexa is a novel peptide supplement that has gained attention for its potential cognitive benefits. It is derived from angiotensin IV, a fragment of a protein involved in blood pressure regulation, and has shown promise in enhancing synaptic plasticity. Research suggests that Dihexa may promote the growth of new neurons and increase the density of synapses in the brain, which are crucial for memory formation and learning. These properties have led to interest in its potential applications for cognitive enhancement, particularly in conditions involving cognitive decline.
However, the use of Dihexa as a supplement is still in its early stages, with most studies conducted on animal models rather than humans. While preliminary findings are promising, the long-term safety and efficacy of Dihexa in humans have yet to be fully established through rigorous clinical trials. As with any novel compound, caution is advised, especially outside of clinical supervision.
As research into Dihexa continues, scientists are working to better understand its mechanisms of action and potential therapeutic uses. The supplement market has seen growing interest in nootropics and cognitive enhancers, with Dihexa positioned as a potentially groundbreaking addition. Future studies will be critical in determining its true benefits and limitations for human cognitive health.
Dihexa Nootropic (Improves Cognitive Functions)
Dihexa, classified as a nootropic, has sparked interest for its potential cognitive benefits. This peptide compound stands out for its ability to enhance memory and learning capabilities. Research suggests that Dihexa works by stimulating neurogenesis—the growth of new neurons—and increasing synaptic density in the brain, thereby promoting cognitive function and potentially benefiting conditions like Alzheimer’s disease and Parkinson’s diseases. These mechanisms are crucial for improving cognitive function, making Dihexa a focal point in the field of cognitive enhancement.
Studies on Dihexa have primarily focused on its effects in animal models, where it has shown promise in reversing cognitive decline associated with conditions like Alzheimer’s disease and Parkinson’s diseases. This has led to optimism about its potential therapeutic applications for neurodegenerative disorders in humans, possibly through mechanisms involving hepatocyte growth factor. However, human trials are still in early stages, and more comprehensive research is needed to validate its efficacy and safety in clinical settings.
While Dihexa holds promise, caution is warranted due to its potent nature and the need for further understanding of its long-term effects, particularly concerning hepatocyte growth factor and its potential application in treating conditions like Parkinson’s diseases. As with many novel compounds, rigorous evaluation through clinical trials will be essential to fully elucidate its benefits and establish appropriate usage guidelines.
What is Brain Derived Neurotrophic Factor?
Brain Derived Neurotrophic Factor (BDNF) is a protein that plays a critical role in the growth, maintenance, and survival of neurons in the brain. It is a member of the neurotrophin family of growth factors, which are essential for brain plasticity, the ability of the brain to change and adapt over time. BDNF supports the development of new neurons and synapses, which are crucial for learning and memory. Its presence is most notable in regions of the brain associated with cognitive function, such as the hippocampus, cortex, and basal forebrain.
In addition to its role in normal brain function, BDNF is also involved in the response to brain injuries and diseases. Higher levels of BDNF are linked to improved mental health, while lower levels are associated with conditions such as depression, schizophrenia, and neurodegenerative diseases like Alzheimer’s. Therapeutic strategies aimed at increasing BDNF levels are being explored for their potential to enhance brain repair mechanisms and cognitive function in these conditions.
Dihexa and Hepatocyte Growth Factor
Dihexa is a small molecule compound designed to enhance cognitive function and has shown potential in treating neurodegenerative diseases. One of the primary ways Dihexa exerts its effects is by mimicking and enhancing the activity of Hepatocyte Growth Factor (HGF), a protein known for its regenerative properties. HGF plays a critical role in cellular growth, motility, and morphogenesis, making it essential for tissue repair and regeneration. By binding to the c-Met receptor, Dihexa promotes neurogenesis and synaptogenesis, which are crucial for maintaining and improving cognitive functions.
FAQ
Does dihexa need to be refrigerated?
Dihexa does not typically need to be refrigerated. It is generally stable at room temperature, but storing it in a cool, dry place away from direct sunlight is recommended to maintain its integrity and efficacy in supporting brain derived neurotrophic factor.
What is dihexa used for?
Dihexa is primarily used for its potential cognitive enhancing effects, including improvements in memory, learning ability, and potentially aiding in neuroprotection against cognitive decline. Dihexa is believed to achieve these benefits by stimulating the production of brain derived neurotrophic factor, a key protein that supports the growth, maintenance, and survival of neurons in the brain. This mechanism is crucial for enhancing cognitive functions and promoting overall brain health..
What is the formula for Dihexa?
The chemical formula for Dihexa is C30H53N9O9. Dihexa is known for its potential to enhance cognitive function by promoting neurogenesis and increasing synaptic density in the brain, especially in addressing age-related deficits. These mechanisms are crucial for supporting brain-derived neurotrophic factor, which plays a key role in neuronal growth, synaptic plasticity, and cognitive processes, particularly in combating age-related deficits.
What peptides increase IQ?
Peptides such as Noopept and Semax have been studied for their potential to enhance cognitive functions, including aspects of intelligence. Research suggests that these peptides may influence neurotransmitter systems and promote neuronal growth factors like hepatocyte growth factor. These effects could potentially improve memory, learning, and overall cognitive performance. Understanding the mechanisms by which these peptides interact with hepatocyte growth factor is crucial for developing targeted therapies for cognitive enhancement. Further investigation into the relationship between these peptides and hepatocyte growth factor may uncover novel strategies for treating cognitive decline and neurodegenerative disorders.
What is the mechanism of Dihexa?
Dihexa works by stimulating neurogenesis (formation of new neurons) and increasing synaptic density in the brain, which are critical for cognitive functions like memory and learning. These mechanisms are particularly relevant in addressing age-related deficits in cognitive function, making Dihexa a promising candidate for enhancing cognitive abilities and potentially mitigating age-related deficits in cognitive function.
What peptide helps memory?
Peptides like Dihexa and Semax are known for their potential to improve memory function through mechanisms involving neural growth factors such as hepatocyte growth factor. These peptides have shown promise in mitigating age-related deficits in memory and cognitive function, making them a focal point in research on neurodegenerative diseases.
What is the use of Semax peptide?
Semax is used as a nootropic agent to enhance cognitive function, improve memory, and potentially as a neuroprotective agent against age-related deficits. Research suggests Semax may influence various neurotransmitter systems and promote the secretion of hepatocyte growth factor, which could contribute to its neuroprotective effects against age-related deficits.
What are the benefits of Dihexa?
The benefits of Dihexa, derived from angiotensin IV, include enhanced memory retention, improved learning ability, potential neuroprotection against cognitive decline, and overall cognitive enhancement. Research suggests that Dihexa achieves these effects by stimulating neurogenesis, increasing synaptic density, and promoting the secretion of hepatocyte growth factor, which plays a crucial role in brain repair and plasticity. These mechanisms underscore its potential therapeutic value in conditions related to cognitive impairment derived from angiotensin IV. Further exploration of Dihexa’s interaction with hepatocyte growth factor may elucidate additional pathways for enhancing cognitive function and mitigating neurodegenerative processes derived from angiotensin IV.
What is dihexa made of?
Dihexa is made of amino acids and is a synthetic peptide known for its potential cognitive benefits. This compound has garnered attention for its ability to enhance memory retention and learning capabilities. Research suggests that Dihexa works by stimulating neurogenesis—the growth of new neurons—and increasing synaptic density in the brain. These mechanisms are crucial for improving cognitive function, including mental stamina. Studies have primarily focused on its effects in animal models, where it has shown promise in reversing cognitive decline associated with conditions like Alzheimer’s disease. Human trials are limited, but preliminary findings indicate potential benefits for mental stamina and overall cognitive enhancement.
What is dihexa acetate?
Dihexa acetate is a form of Dihexa where it is combined with acetic acid to form a salt, which can affect its solubility and stability. This modification is being explored for potential therapeutic applications in Alzheimer’s disease, aiming to enhance its bioavailability and efficacy in protecting hippocampal neurons against neurodegeneration associated with Alzheimer’s disease.
What does dihexa do?
Dihexa promotes neurogenesis, increases synaptic density, and enhances cognitive functions such as memory and learning, potentially offering therapeutic benefits for conditions like Alzheimer’s disease. Research also suggests Dihexa’s potential in neurodegenerative disorders such as amyotrophic lateral sclerosis by supporting neuronal health and function through its mechanisms of action in the central nervous system.
What is Dihexa good for?
Dihexa is good for improving cognitive functions, memory retention, and potentially aiding in neuroprotection against Alzheimer’s disease cognitive decline
How does Dihexa interact with brain receptors?
Dihexa interacts with brain receptors involved in neurogenesis and synaptic plasticity, which are crucial for cognitive functions and brain hepatocyte growth factor. These interactions suggest potential benefits for cognitive enhancement through brain hepatocyte growth factor. Understanding how Dihexa influences these processes is key to exploring its therapeutic potential in enhancing brain hepatocyte growth factor. Further research is needed to elucidate the specific mechanisms by which Dihexa affects brain hepatocyte growth factor and cognitive function.
Are there any known side effects of taking Dihexa?
Known side effects of Dihexa are not extensively documented due to limited human trials, but potential risks may include unknown long-term effects and interactions, particularly concerning cerebral blood flow.
Can Dihexa be used alongside other nootropics or supplements?
Dihexa’s compatibility with other nootropics or supplements is not well-established and should be approached cautiously without sufficient clinical guidance, especially regarding its effects on cerebral blood flow.
What clinical trials have been conducted on Dihexa?
Clinical trials on Dihexa are limited, primarily focusing on its safety profile and preliminary efficacy in cognitive enhancement, but future studies may explore its potential to facilitate cerebral blood flow.
How quickly can effects from Dihexa be noticed?
The onset of effects from Dihexa can vary and may depend on individual response, with some anecdotal reports suggesting noticeable effects within weeks of use, potentially due to its ability to cross the blood brain barrier efficiently.
Is Dihexa effective for age-related cognitive decline?
Dihexa shows promise in potentially mitigating age-related cognitive decline by promoting brain health and cognitive function, but further research is needed, especially at washington state university.
What is the recommended dosage of Dihexa?
There is no established recommended dosage for Dihexa due to limited clinical data. Dosage recommendations, if any, should be based on consultation with healthcare professionals. Further studies, potentially involving clinical trials at Washington State University, are needed to determine safe and effective dosing protocols.
How does Dihexa influence neural growth factors?
Dihexa influences neural growth factors by promoting neurogenesis and increasing synaptic density, which are critical for brain plasticity and cognitive functions, particularly in addressing Parkinson’s disease. Research conducted at Washington State University suggests that these effects could potentially lead to advancements in treating neurodegenerative diseases like Parkinson’s disease. Understanding these mechanisms is crucial for further exploring Dihexa’s therapeutic potential in Parkinson’s disease. Ongoing studies at Washington State University aim to elucidate how Dihexa interacts with brain receptors and its long-term effects on neural health, particularly in the context of Parkinson’s disease.
Are there any contraindications for using Dihexa?
Contraindications for Dihexa use, especially involving hexanoic tyr ile 6, are not well-established due to limited clinical data, but caution is advised for individuals with underlying medical conditions or unknown interactions.
How does Dihexa affect long-term brain health?
Dihexa’s potential impact on long-term brain health, including aspects of mental clarity, is not fully understood, and further research is needed to determine its effects over extended periods, especially in conditions like Parkinson’s disease.
Can Dihexa improve concentration and focus?
Dihexa’s effects on concentration and focus are theoretically linked to its cognitive enhancement properties, though specific studies are needed to confirm this benefit. Its potential to improve mental clarity is of particular interest in understanding its role in enhancing cognitive functions related to mental clarity.
What populations should avoid using Dihexa?
Dihexa is typically administered orally, though other methods such as sublingual or intranasal delivery may also be explored for efficacy and absorption, particularly in enhancing heart health and other neurodegenerative diseases.
How is Dihexa administered?
Dihexa is typically administered orally, though other methods such as sublingual or intranasal delivery may also be explored for efficacy and absorption.
Is Dihexa available over the counter or is a prescription required?
Dihexa is not approved for medical use and is not available for purchase over the counter. It would require clinical trials and regulatory approval for prescription use, especially in the treatment of neurodegenerative diseases, where it shows potential for nerve regeneration and improved treatment outcomes.
What are the storage conditions for Dihexa?
Dihexa should be stored in a cool, dry place away from direct sunlight to maintain its stability. Refrigeration is generally not required for Dihexa storage, which is beneficial for its accessibility and convenience, especially in the context of managing neurodegenerative disease. Proper storage conditions ensure that Dihexa remains effective and ready for use without compromising its efficacy in interacting with the receptor system.
Has Dihexa been approved by health authorities like the FDA?
Dihexa has not been approved by regulatory authorities such as the FDA for medical use or as a dietary supplement, particularly in the treatment of neurodegenerative disease. Therefore, its usage in clinical settings for neurodegenerative disease is not supported by current regulatory approvals. Research on Dihexa suggests it may enhance cognitive function by promoting neurogenesis and fostering the formation of new functional synaptic connections. Therefore, its usage in clinical settings for neurodegenerative disease is not supported by current regulatory approvals.
What research is currently being conducted on Dihexa?
Current research on Dihexa focuses on expanding its understanding in neurobiology, potential therapeutic applications, and safety profiles through preclinical and clinical studies, particularly in its effects on neural precursor cell proliferation. Studies aim to elucidate how Dihexa enhances problem solving skills and cognitive function, paving the way for novel treatments in neurodegenerative diseases
Can Dihexa be combined with physical therapy for neurological conditions?
The combination of Dihexa with physical therapy for neurological conditions is speculative and would require rigorous study to assess safety and efficacy, especially when considering the potential synergistic effects to improve cognitive functions of tyr ile 6 aminohexanoic.
What are the ethical considerations in using Dihexa?
Ethical considerations in using Dihexa include informed consent in research, ensuring participant safety, and addressing potential societal implications of cognitive enhancement, especially in conditions like Parkinson’s disease. It is crucial to establish clear guidelines to protect vulnerable populations affected by Parkinson’s disease and to ensure that the benefits and risks of Dihexa are ethically balanced in research involving therapeutic treatment of Parkinson’s disease.
How does Dihexa compare to traditional cognitive enhancers?
Dihexa differs from traditional cognitive enhancers by targeting specific neurobiological mechanisms like neurogenesis and synaptic density rather than conventional neurotransmitter modulation. Studies have shown that Dihexa can cross the blood-brain barrier, leading to elevated concentrations in serum and cerebrospinal fluid. This dual presence in serum and cerebrospinal fluid underscores its potential to affect neuroplasticity directly within the brain, distinguishing it from compounds that primarily act peripherally. This unique ability makes Dihexa a promising candidate for therapeutic treatment in conditions involving cognitive decline and neurodegeneration.
Reference
Wright JW, Harding JW. The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer’s Disease. J Alzheimers Dis. 2015;45(4):985-1000.
A New Target for the Treatment of Alzheimer’s Disease
The 2015 study by Wright and Harding explores the brain hepatocyte growth factor (HGF)/c-Met receptor system as a potential target for Alzheimer’s disease treatment. This research delves into the role of the HGF/c-Met system in neural growth, repair, and neurogenesis. It suggests that modulating this system could offer new therapeutic strategies for Alzheimer’s, a condition currently lacking effective treatments. This study contributes to the growing field of Alzheimer’s research, particularly in identifying novel therapeutic targets.
For more details https://content.iospress.com/articles/journal-of-alzheimers-disease/jad142814
Alene T. McCoy; Caroline C. Benoist; John W. Wright; Leen H. Kawas; JyoteBule-Ghogare; Mingyan Zhu; Suzanne M. Appleyard; Gary A. Wayman; Joseph W. Harding (January 2013). “Evaluation of metabolically stabilized angiotensin IV analogs as pro-cognitive/anti-dementia agents”. The Journal of Pharmacology and Experimental Therapeutics. 344 (1): 141–154. doi: 10.1124/jpet.112.199497. PMC 3533412. PMID 23055539.
Evaluation of metabolically stabilized angiotensin IV analogs as pro-cognitive/anti-dementia agents
The 2013 study by McCoy et al. evaluated the effectiveness of metabolically stabilized angiotensin IV analogs as potential therapeutic agents for cognitive enhancement and dementia treatment. This research focused on understanding the role of angiotensin IV in brain functions related to memory and learning, and its potential in addressing cognitive impairments associated with diseases like Alzheimer’s. The study contributes to the field of neuropharmacology by exploring new avenues for treating cognitive disorders.
For more details https://jpet.aspetjournals.org/content/344/1/141.short
Benoist CC, Kawas LH, Zhu M, et al. The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system. J PharmacolExpTher. 2014;351(2):390–402. doi:10.1124/jpet.114.218735
The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system
The 2014 study by Benoist et al. investigated the role of angiotensin IV-derived peptides in cognitive enhancement, focusing on their procognitive and synaptogenic effects. This research emphasized the importance of the hepatocyte growth factor/c-met system in mediating these effects. The findings contribute to understanding the molecular mechanisms involved in memory and learning, potentially offering new therapeutic targets for cognitive disorders.
For more details https://jpet.aspetjournals.org/content/351/2/390.short
Nagahara and Tuszynski (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209-19
Potential therapeutic uses of BDNF in neurological and psychiatric disorders
The 2011 study by Nagahara and Tuszynski reviewed the potential therapeutic applications of Brain-Derived Neurotrophic Factor (BDNF) in treating neurological and psychiatric disorders. This research highlighted the significance of BDNF in brain function, including its role in neuroprotection, synaptic plasticity, and cognitive enhancement. The study also discussed the challenges in delivering BDNF as a therapeutic agent and proposed strategies for overcoming these challenges. The findings underscore the potential of BDNF in developing new treatments for various brain disorders.
For more details https://www.nature.com/articles/nrd3366
Calissano P, Matrone C, Amadoro G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. DevNeurobiol. 2010;70(5):372-83.
Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease
The 2010 study by Calissano, Matrone, and Amadoro focused on the role of nerve growth factor (NGF) as a neurotrophin related to Alzheimer’s disease. This research discussed NGF’s biological functions, particularly in the context of neurodegenerative diseases. The study highlighted NGF’s potential as a therapeutic agent for Alzheimer’s disease, emphasizing its significance in neuronal survival, maintenance, and regeneration.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1002/dneu.20759
McCoy AT, Benoist CC, Wright JW, et al. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J PharmacolExpTher. 2013;344(1):141–154. doi:10.1124/jpet.112.199497
Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents
The 2013 study by McCoy et al. evaluated metabolically stabilized angiotensin IV analogs for their potential as procognitive and antidementia agents. This research explored the efficacy of these analogs in enhancing cognitive functions and their possible applications in treating dementia-related disorders. The study contributes to the understanding of angiotensin IV’s role in cognitive processes and its therapeutic potential in neurodegenerative diseases.
For more details https://jpet.aspetjournals.org/content/344/1/141.short
Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. ProgNeurobiol. 2015;125:26-46.
The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases
The 2015 study by Wright, Kawas, and Harding discussed the development of small molecule angiotensin IV analogs for treating Alzheimer’s and Parkinson’s diseases. This research focused on the potential of these analogs in neurodegenerative disease therapy, exploring their role in neuroprotection, cognitive enhancement, and modulation of brain function. The study contributes significantly to the ongoing search for effective treatments for these challenging neurological conditions.
For more details https://www.sciencedirect.com/science/article/pii/S0301008214001245
Wang QG, Xue X, Yang Y, Gong PY, Jiang T, Zhang YD. Angiotensin IV suppresses inflammation in the brains of rats with chronic cerebral hypoperfusion. J Renin Angiotensin Aldosterone Syst. 2018;19(3):1470320318799587. doi:10.1177/1470320318799587
Angiotensin IV suppresses inflammation in the brains of rats with chronic cerebral hypoperfusion
The 2018 study by Wang et al. investigated the effects of angiotensin IV on inflammation in rat brains with chronic cerebral hypoperfusion. This research explored the anti-inflammatory properties of angiotensin IV, particularly in the context of brain conditions characterized by reduced blood flow. The study’s findings contribute to understanding the potential therapeutic uses of angiotensin IV in neurological conditions with inflammatory components.
For more details https://journals.sagepub.com/doi/abs/10.1177/1470320318799587
Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. ProgNeurobiol. 2015;125:26-46.
The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases
The 2015 study by Wright, Kawas, and Harding discussed the development of small molecule angiotensin IV analogs for treating Alzheimer’s and Parkinson’s diseases. This research focused on the potential of these analogs in neurodegenerative disease therapy, exploring their role in neuroprotection, cognitive enhancement, and modulation of brain function. The study contributes significantly to the ongoing search for effective treatments for these challenging neurological conditions.
You can read the abstract of this article at https://www.sciencedirect.com/science/article/pii/S0301008214001245.
Koike H, Ishida A, Shimamura M, et al. Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model: a model of gene therapy for Parkinson’s disease. Gene Ther. 2006;13(23):1639-44.
Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model
The 2006 study by Koike et al. investigated the use of gene therapy for preventing Parkinson’s disease. Specifically, it focused on the in vivo transfer of human hepatocyte growth factor in a rodent model. The study aimed to explore the potential of this approach in preventing the onset of Parkinson’s disease, offering insights into new therapeutic strategies. This research is significant in the field of neurodegenerative disease treatment, particularly in the use of gene therapy for Parkinson’s disease.
For more details https://www.nature.com/articles/3302810
Salehi Z, Rajaei F. Expression of hepatocyte growth factor in the serum and cerebrospinal fluid of patients with Parkinson’s disease. J ClinNeurosci. 2010;17(12):1553-6.
Expression of hepatocyte growth factor in the serum and cerebrospinal fluid of patients with Parkinson’s disease
The 2010 study by Salehi and Rajaei explored the expression of hepatocyte growth factor (HGF) in the serum and cerebrospinal fluid of patients with Parkinson’s disease. This research aimed to understand the potential relationship between HGF levels and Parkinson’s disease, contributing to the broader understanding of the disease’s pathophysiology and potential biomarkers.
For more details https://www.sciencedirect.com/science/article/pii/S0967586810003942
Friedman LG, Price K, Lane RF, et al. Meeting report on the Alzheimer’s Drug Discovery Foundation 14th International Conference on Alzheimer’s Drug Discovery. Alzheimers Res Ther. 2014;6(2):22. Published 2014 Apr 28. doi:10.1186/alzrt252.
Meeting report on the Alzheimer’s drug discovery foundation 14th international conference on Alzheimer’s drug discovery
The meeting report on the Alzheimer’s Drug Discovery Foundation 14th International Conference on Alzheimer’s Drug Discovery, published in 2014 in “Alzheimer’s Research & Therapy,” provides insights into the latest developments in Alzheimer’s research and drug discovery. The conference highlighted various innovative therapeutic strategies and findings from recent research aimed at tackling Alzheimer’s disease. This report is a valuable resource for understanding the current state and future directions in Alzheimer’s disease research and treatment.
For more details https://link.springer.com/article/10.1186/alzrt252
Koike H, Ishida A, Shimamura M, et al. Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model: a model of gene therapy for Parkinson’s disease. Gene Ther. 2006;13(23):1639-44
Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model
The 2006 study by Koike et al. investigated the potential of gene therapy in preventing the onset of Parkinson’s disease. The research utilized in vivo gene transfer of human hepatocyte growth factor in a rodent model to explore its efficacy in precluding Parkinson’s disease development. This innovative approach contributes to the understanding of gene therapy as a potential intervention for Parkinson’s disease.
For more details https://www.nature.com/articles/3302810
Gard PR. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008;9Suppl 2:S15.
Cognitive-enhancing effects of angiotensin IV
The 2008 study by Gard PR in BMC Neuroscience reviewed the cognitive-enhancing effects of angiotensin IV. The research focused on the role of angiotensin IV in memory and cognitive functions, discussing its potential as a therapeutic agent for cognitive impairments. The study highlights the importance of angiotensin IV in neurobiological research, particularly in understanding and treating cognitive disorders.
For more details https://bmcneurosci.biomedcentral.com/articles/10.1186/1471-2202-9-S2-S15
Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. ProgNeurobiol. 2015;125:26-46.
The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases
The 2015 study by Wright, Kawas, and Harding discussed the development of small molecule angiotensin IV analogs for treating Alzheimer’s and Parkinson’s diseases. This research focused on the potential of these analogs in neurodegenerative disease therapy, exploring their role in neuroprotection, cognitive enhancement, and modulation of brain function. The study contributes significantly to the ongoing search for effective treatments for these challenging neurological conditions.
You can read the abstract of this article at https://www.sciencedirect.com/science/article/pii/S0301008214001245.
Benoist CC, Kawas LH, Zhu M, et al. The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system. J PharmacolExpTher. 2014;351(2):390–402. doi:10.1124/jpet.114.218735.
The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system
The 2014 study by Benoist et al. investigated the role of angiotensin IV-derived peptides in cognitive enhancement, focusing on their procognitive and synaptogenic effects. This research emphasized the importance of the hepatocyte growth factor/c-met system in mediating these effects. The findings contribute to understanding the molecular mechanisms involved in memory and learning, potentially offering new therapeutic targets for cognitive disorders.
For more details https://jpet.aspetjournals.org/content/351/2/390.short
Benoist CC, Wright JW, Zhu M, Appleyard SM, Wayman GA, Harding JW. Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs. J PharmacolExpTher. 2011;339(1):35-44.
Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs
The 2011 study by Benoist et al. in the Journal of Pharmacology and Experimental Therapeutics explored the effects of C-terminal truncated Nle1-angiotensin IV analogs on hippocampal synaptogenesis and spatial memory. The research investigated how these analogs facilitate cognitive functions, particularly focusing on their impact in the hippocampus, a brain region crucial for memory formation. This study contributes to understanding potential treatments for cognitive impairments related to memory.
For more details https://jpet.aspetjournals.org/content/339/1/35.short
Pederson ES, Krishnan R, Harding JW, Wright JW. A role for the angiotensin AT4 receptor subtype in overcoming scopolamine-induced spatial memory deficits. RegulPept. 2001;102(2-3):147-56
A role for the angiotensin AT4 receptor subtype in overcoming scopolamine-induced spatial memory deficits
The 2001 study by Pederson, Krishnan, Harding, and Wright investigated the role of the angiotensin AT4 receptor subtype in counteracting memory deficits caused by scopolamine, a substance known to impair spatial memory. The research aimed to understand how the AT4 receptor could be targeted to improve cognitive functions, especially in conditions where memory is adversely affected. This study contributes to the field of memory research and pharmacological interventions for memory impairments.
For more details https://www.sciencedirect.com/science/article/pii/S0167011501003123
Albiston AL, Fernando RN, Yeatman HR, et al. Gene knockout of insulin-regulated aminopeptidase: loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory. Neurobiol Learn Mem. 2010;93(1):19-30
Gene knockout of insulin-regulated aminopeptidase: loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory
The 2010 study by Albiston et al. in “Neurobiology of Learning and Memory” investigated the effects of knocking out the gene for insulin-regulated aminopeptidase. This gene knockout resulted in the loss of the specific binding site for angiotensin IV. The study found an age-related deficit in spatial memory, highlighting the significance of this binding site in memory functions. This research provides insight into the molecular mechanisms underlying memory and cognitive aging.
For more details https://www.sciencedirect.com/science/article/pii/S1074742709001488
Available from https://www.sciencedaily.com/releases/2012/10/121011090653.htm.
Date I, Takagi N, Takagi K, et al. Hepatocyte growth factor improved learning and memory dysfunction of microsphere-embolized rats. J Neurosci Res. 2004;78(3):442-53.
Hepatocyte growth factor improved learning and memory dysfunction of microsphere‐embolized rats
The 2004 study by Date et al. in the Journal of Neuroscience Research explored the effects of hepatocyte growth factor (HGF) on learning and memory dysfunction in rats subjected to microsphere embolization. The study aimed to understand the therapeutic potential of HGF in addressing cognitive impairments associated with brain ischemia. This research contributes to the knowledge of neuroprotective strategies and cognitive rehabilitation following cerebral injuries.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1002/jnr.20263
Braszko JJ, Kupryszewski G, Witczuk B, Wiśniewski K. Angiotensin II-(3-8)-hexapeptide affects motor activity, performance of passive avoidance and a conditioned avoidance response in rats. Neuroscience. 1988;27(3):777-83
Angiotensin II-(3-8)-hexapeptide affects motor activity, performance of passive avoidance and a conditioned avoidance response in rats
The 1988 study by Braszko et al. examined the effects of Angiotensin II-(3-8)-hexapeptide on motor activity and memory performance in rats. The research focused on how this peptide influences passive avoidance behavior and conditioned avoidance responses, providing insights into the neuromodulatory role of angiotensin fragments. This study contributes to the understanding of the complex interactions between neuropeptides and brain function.
For more details https://www.sciencedirect.com/science/article/pii/0306452288901820
Gard PR. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008;9Suppl 2:S15.
Cognitive-enhancing effects of angiotensin IV
The 2008 study by Gard in BMC Neuroscience reviewed the cognitive-enhancing effects of angiotensin IV. This research focused on understanding how angiotensin IV could potentially improve cognitive functions, offering insights into its therapeutic applications for cognitive impairments. The study contributes to the field of neuropharmacology and the exploration of new treatments for cognitive disorders.
For more details https://bmcneurosci.biomedcentral.com/articles/10.1186/1471-2202-9-S2-S15
Sun, X., Deng, Y., Fu, X., Wang, S., Duan, R., & Zhang, Y. (2021). AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway. Brain sciences, 11(11), 1487. https://doi.org/10.3390/brainsci11111487
AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway.
The 2021 study by Sun et al. focused on the effects of the Angiotensin IV analog Dihexa in treating cognitive impairment and restoring memory in APP/PS1 mice, a model for Alzheimer’s disease. The research demonstrated that Dihexa could enhance cognitive functions and recover memory, likely through the activation of the PI3K/AKT signaling pathway. This study provides insights into potential therapeutic approaches for Alzheimer’s disease using AngIV analogs.
For more details https://doi.org/10.3390/brainsci11111487
Ho, J. K., & Nation, D. A. (2018). Cognitive benefits of angiotensin IV and angiotensin-(1-7): A systematic review of experimental studies. Neuroscience and biobehavioral reviews, 92, 209–225. https://doi.org/10.1016/j.neubiorev.2018.05.005.
Cognitive benefits of angiotensin IV and angiotensin-(1-7): A systematic review of experimental studies
The 2018 systematic review by Ho and Nation examined the cognitive benefits of angiotensin IV and angiotensin-(1-7) based on experimental studies. The review synthesized evidence from various studies to assess the potential of these peptides in enhancing cognitive functions. The findings contribute to the understanding of the role of angiotensin peptides in cognitive processes and their potential therapeutic applications in cognitive disorders.
For more details https://doi.org/10.1016/j.neubiorev.2018.05.005
Doeppner TR, Kaltwasser B, Elali A, Zechariah A, Hermann DM, Bähr M. Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J Cereb Blood Flow Metab. 2011;31(5):1251-62.
Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation
The 2011 study by Doeppner et al. investigated the long-term neuroprotective and recovery effects of acute hepatocyte growth factor (HGF) treatment in stroke models. The study revealed that HGF treatment leads to neuroprotection and enhances stroke recovery by mechanisms that involve the proliferation and differentiation of neural precursor cells. These findings contribute to understanding potential therapeutic approaches for stroke recovery.
For more details https://journals.sagepub.com/doi/abs/10.1038/jcbfm.2010.211
Faure S, Chapot R, Tallet D, Javellaud J, Achard JM, Oudart N. Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors. J PhysiolPharmacol. 2006;57(3):329-42.
Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors
The 2006 study by Faure et al. explored the cerebroprotective effects of angiotensin IV in ischemic stroke in rats, mediated by AT(4) receptors. This research aimed to understand how angiotensin IV could be utilized for stroke therapy, focusing on its interaction with AT(4) receptors. The findings provide insights into potential therapeutic approaches for ischemic stroke using angiotensin IV.
For more details https://www.jpp.krakow.pl/journal/archive/09_06/articles/02_article.html
Kramár EA, Harding JW, Wright JW. Angiotensin II- and IV-induced changes in cerebral blood flow. Roles of AT1, AT2, and AT4 receptor subtypes. RegulPept. 1997;68(2):131-8.
Angiotensin II- and IV-induced changes in cerebral blood flow
The 1997 study by Kramár, Harding, and Wright investigated the effects of Angiotensin II and IV on cerebral blood flow, examining the roles of AT1, AT2, and AT4 receptor subtypes. This research provided insights into how these angiotensin receptors influence cerebral blood flow, which is crucial in understanding cerebrovascular regulation and potential therapeutic targets for cerebral disorders.
For more details https://www.sciencedirect.com/science/article/pii/S0167011596021167
Shang J, Deguchi K, Yamashita T, et al. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res. 2010;88(10):2197-206
Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats
The 2010 study by Shang et al. in the Journal of Neuroscience Research explored the effects of glial cell line-derived neurotrophic factor (GDNF) and hepatocyte growth factor (HGF) following transient middle cerebral artery occlusion in rats. The study focused on the antiapoptotic and antiautophagic effects of these growth factors, providing insights into potential therapeutic strategies for stroke and related cerebral injuries.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1002/jnr.22373
Kadoyama K, Funakoshi H, Ohya W, Nakamura T. Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci Res. 2007;59(4):446-56.
Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS
The 2007 study by Kadoyama et al. investigated the effects of hepatocyte growth factor (HGF) on gliosis and motor neuron degeneration in the brainstem motor nuclei of a transgenic mouse model of Amyotrophic Lateral Sclerosis (ALS). The research aimed to explore the potential therapeutic benefits of HGF in ALS, focusing on its neuroprotective properties. This study contributes to the understanding of ALS pathology and potential treatment strategies.
For more details https://www.sciencedirect.com/science/article/pii/S0168010207017646
Kato S, Funakoshi H, Nakamura T, et al. Expression of hepatocyte growth factor and c-Met in the anterior horn cells of the spinal cord in the patients with amyotrophic lateral sclerosis (ALS): immunohistochemical studies on sporadic ALS and familial ALS with superoxide dismutase 1 gene mutation. ActaNeuropathol. 2003;106(2):112-20.
Expression of hepatocyte growth factor and c-Met in the anterior horn cells of the spinal cord in the patients with amyotrophic lateral sclerosis (ALS)
The 2003 study by Kato et al. examined the expression of hepatocyte growth factor (HGF) and its receptor c-Met in the anterior horn cells of the spinal cord in patients with Amyotrophic Lateral Sclerosis (ALS). This study included cases of sporadic ALS as well as familial ALS with superoxide dismutase 1 gene mutation. The research provided insights into the molecular pathology of ALS and potential targets for therapy.
For more details https://link.springer.com/article/10.1007/s00401-003-0708-z
Ebens A, Brose K, Leonardo ED, et al. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17(6):1157-72.
Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons
The 1996 study by Ebens et al. in the journal Neuron investigated the roles of hepatocyte growth factor/scatter factor (HGF/SF) in spinal cord development. The research found that HGF/SF acts as an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. This study provides significant insights into the molecular mechanisms guiding the development of motor neuron pathways and their potential therapeutic implications.
For more details https://www.cell.com/fulltext/S0896-6273(00)80247-0
Kitamura K, Fujiyoshi K, Yamane J, et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS ONE. 2011;6(11):e27706.
Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury
The 2011 study by Kitamura et al. explored the effects of human hepatocyte growth factor (HGF) on recovery after spinal cord injury in primates. The research demonstrated that HGF promoted significant functional recovery, highlighting its potential as a therapeutic agent for spinal cord injuries. This study is important in understanding treatments that can aid in neuroregeneration and recovery post-injury.
For more details https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0027706
Weiss, J. B., Phillips, C. J., Malin, E. W., Gorantla, V. S., Harding, J. W., & Salgar, S. K. (2021). Stem cell, Granulocyte-Colony Stimulating Factor and/or Dihexa to promote limb function recovery in a rat sciatic nerve damage-repair model: Experimental animal studies. Annals of medicine and surgery (2012), 71, 102917. https://doi.org/10.1016/j.amsu.2021.102917.
Granulocyte-Colony Stimulating Factor and/or Dihexa to promote limb function recovery in a rat sciatic nerve damage-repair model: Experimental animal studies
The 2021 study by Weiss et al. investigated the efficacy of stem cells, Granulocyte-Colony Stimulating Factor (G-CSF), and Dihexa in promoting limb function recovery in a rat model of sciatic nerve damage and repair. This experimental animal study aimed to understand the potential therapeutic effects of these treatments on nerve regeneration and functional recovery. The findings contribute to the development of new strategies for treating nerve injuries.
For more details https://doi.org/10.1016/j.amsu.2021.102917
Uribe, P. M., Kawas, L. H., Harding, J. W., & Coffin, A. B. (2015). Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure. Frontiers in cellular neuroscience, 9, 3. https://doi.org/10.3389/fncel.2015.00003.
Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure
The 2015 study by Uribe et al. investigated the protective effects of a hepatocyte growth factor mimetic on lateral line hair cells in response to aminoglycoside exposure. This study focused on understanding how this mimetic can safeguard sensory cells from the damaging effects of aminoglycosides, which are known for their ototoxic properties. The research contributes to potential protective strategies against aminoglycoside-induced hair cell damage.
For more details https://www.frontiersin.org/articles/10.3389/fncel.2015.00003/full
Other Peptides
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.