GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Book a Free Consultation
Potential Benefits of BPC 157
BPC 157 offers a wide range of therapeutic benefits, including accelerating wound healing and soft tissue injury recovery, improving bone and joint health, enhancing digestive function, and normalizing blood pressure, potassium, calcium, and magnesium levels. It also strengthens the immune system, protects against NSAID-related side effects, reverses alcohol intoxication, and positively impacts mood, behavior, and cognitive health. Additionally, BPC 157 exhibits potential anti-cancer properties, making it a versatile peptide with various health-promoting effects.
- Accelerates wound healing [1-12]
- Accelerates healing of soft tissue injuries [13-28]
- Improves bone and joint health [29-32]
- Improves digestive health [33-45]
- Normalizes blood pressure [46-49]
- Corrects potassium imbalance [50-55]
- Corrects calcium imbalance [56]
- Corrects magnesium imbalance [57]
- Strengthens the immune system [58-63]
- Protects against NSAID toxicity and related adverse side effects [64-68]
- Reverses alcohol intoxication [69-72]
- Improves mood and behavior [73-77]
- Improves cognitive health [78-82]
- Exerts anti-cancer properties [83-86]
Key Takeaways of BPC 157
- BPC-157 is a synthetic peptide derived from a protein found in the stomach.
- It’s often referred to as a “stable gastric pentadecapeptide” due to its stability in human gastric juice.
- BPC-157 has shown potential benefits in laboratory and preclinical studies, including promoting tissue repair and healing.
- Potential applications of BPC-157 include wound healing, anti-inflammatory effects, gastrointestinal health, and bone healing.
- If you are considering using BPC-157, it is important to talk to your doctor first to assess the risks and benefits of this treatment and provide you with guidance on how to use it safely.
What is BPC 157?
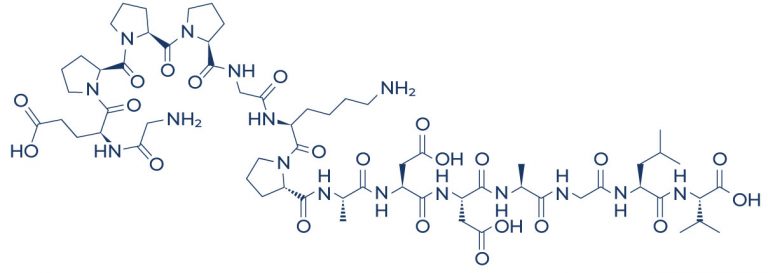
BPC 157 peptides, also known as Body Protecting Compound 157, Body Protection Compound-157, or BPC-157, consist of a chain of 15 amino acids and originate from human gastric juices. A peptide is simply a compound consisting of two or more amino acids. BPC 157 is commonly known as a “stable gastric pentadecapeptide” because of its ability to maintain stability even within the environment of human gastric juice.
Your body already produces BPC-157 in very small amounts, which serves to signal certain body processes to happen and protect the digestive system. Researchers believe that if you get the super concentrated version of BPC-157 into your system, it has an extremely high level of regenerative effects.
How BPC-157 works?
BPC-157 works by stimulating the formation of new blood vessels. This process is called angiogenesis and is important in promoting healing and faster cell regeneration. Angiogenesis is an integral part of the wound-healing process and the organization of the blood vessel network.
Chemical Structure of BPC-157
Research on BPC 157 Benefits
A. Speeds Up the Wound Healing Process
Numerous studies show that BPC-157 may help accelerate the wound-healing process through multiple mechanisms:
- In mice with burn injuries, BPC-157 was able to treat skin burns by significantly improving collagen fiber formation and decreasing the number of inflammatory cells. [1-4]
- In wounded rats, BPC-157 administration enhanced the formation of granulation tissues (new tissues that form on an ulcer or the healing surface of a wound) and new blood vessels. [5-6]
- A study found that BPC-157 was able to treat skin burns by enhancing wound healing in a model of alkali burn-induced skin injury. [7]
- In rats with intestinal lesions, BPC-157 administration significantly reduced damage to blood vessels and occlusion. [8-9]
- In rats with celecoxib-induced gastrointestinal, liver, and brain lesions, BPC-157 reversed the damaging effect of the drug. [10]
- In broiler chicks with severe damage to the tissues of the heart, liver, and spleen, BPC-157 administration at 10 micrograms/kg reduced organ damage. [11]
- In honeybees with severe damage to midgut wall layers, supplementation with sugar syrup containing BPC-157 decreased damage to the outer muscular coat. [12]
Discover the future of wound healing with innovative peptides. Explore the power of Wound Healing Peptides in accelerating recovery and regeneration. Unleash the potential of cutting-edge research for effective wound healing solutions.
B. Accelerates Healing of Soft Tissue Injuries
Soft tissue injuries refer to damage to the muscles, tendons, and ligaments. They can be partial or complete tears and may require surgical repair. Interestingly, BPC-157 has shown promise in preclinical studies for promoting the healing of tendon-to-bone damage, potentially accelerating the recovery process in musculoskeletal injuries:
- In rats with Achilles tendon injury, BPC-157 significantly improved healing, thereby eliminating the need for surgical repair. [13-15]
- BPC-157 promoted tendon outgrowth, cell survival, and cell migration in the injured soft tissues of rats, resulting in improved soft tissue healing. [16-18]
- In rats with muscle crush injury, BPC-157 induced faster muscle healing and full function restoration. [19-21]
- In rat tendon cells, pretreatment with BPC-157 showed outcomes close to the noninjured ligament as evidenced by faster granulation tissue formation, better organization of collagen, and reduced inflammatory cells, suggesting improved ligament healing. [22]
- In rats that had a surgical operation of the colon, BPC-157 treatment accelerated the healing time. [23]
- In rats with eye injuries, administration of BPC-157 eye drops successfully closed perforating corneal incisions. [24-25]
- In rats with injury to the sciatic nerve (nerve in the spinal cord), BPC-157 administration reversed the death of nerve cells and cyst formation and protected against damage to nerve structures. [26]
- A study also found that BPC-157 has the capacity to protect against cancer cachexia, a condition characterized by progressive loss of muscle and fat. [27]
- In rats with vessel injury, BPC-157 administration counteracted direct vein injury, blood clots, and prolonged bleeding. [28]
C. Improves Bone and Joint Health
BPC-157 plays a crucial role in maintaining bone and joint health. Studies show that BPC-157 exerts this beneficial effect through the following:
- In rabbits, BPC-157 significantly improved the healing of segmental bone defects by increasing bone density. [29]
- In rats, BPC-157 administration counteracted knee osteoarthritis and reduced cartilage lesions and joint pain, resulting in improved leg length and mobility. [30-32]
D. Improves Digestive Health
Current research suggests that BPC-157 may also help improve digestive function and overall digestive health through the following:
- In rats, BPC-157 prevented the development of stomach ulcers by protecting the layers of the stomach against the direct cellular damaging effect of ethanol. [33-34]
- BPC-157 fully interacted with the dopamine system (group of nerve cells in the midbrain) of rats to prevent mechanisms involved in ulcer formation. [35]
- Continuous administration of BPC-157 in rats with chronic gastric ulcers accelerated the rebuilding of stomach tissues and the formation of granulation tissues. [36]
- BPC-157 administration in rats was able to treat stomach ulcer by reducing gastrointestinal tract lesions. [37-40]
- In rats with inflammatory bowel disease, BPC-157 administration resulted in faster healing of the damaged colon and improved tendon, bone, and ligament healing by boosting the levels of Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF), and Vascular Endothelial Growth Factor (VEGF). [41-45]
E. Normalizes Blood Pressure
Studies show that BPC-157 can help normalize blood pressure through the following mechanisms:
- In rats with abnormally low blood pressure, BPC-157 exerted hypertensive effects by increasing blood pressure near the normal range. [46-47]
- In rats with high blood pressure, BPC-157 decreased blood pressure by causing blood vessels to dilate. [48-49]
F. Corrects Potassium Imbalance
Normal blood levels of potassium are crucial for optimum heart health. Abnormally low or high potassium levels can both lead to heart failure. Studies show that BPC-157 can help protect against heart failure caused by potassium imbalance:
- In rats with hyperkalemia (potassium overdose), BPC-157 therapy protects against heart failure by completely restoring the normal heart rhythm and electrical activities of the heart. [50-53]
- In rats with hypokalemia (potassium deficiency), BPC-157 therapy protects against heart failure by counteracting abnormal heart rhythm. [54-55]
G. Corrects Calcium Imbalance
Hypercalcemia (excessive calcium levels) negatively affects almost every organ system in the body. Evidence suggests that BPC-157 can counteract life-threatening conditions associated with high calcium levels:
- In rats, BPC-157 administration protected against organ failure induced by hypercalcemia via the reduction of calcium deposits. [56]
H. Corrects Magnesium Imbalance
High levels of magnesium in the blood (hypermagnesemia) can lead to life-threatening conditions including heart problems, breathing difficulties, and coma. Evidence found that BPC-157 can help reverse this electrolyte imbalance:
- In rats, BPC-157 administration counteracted the initial event leading to hypermagnesemia and the life-threatening effects of magnesium overdose. [57]
I. Strengthens the Immune System
BPC-157 can help boost the immune system and protect against infection caused by viruses, bacteria, and other disease-causing microorganisms through the following important mechanisms:
- BPC-157 has been shown to have anti-inflammatory and regenerative effects on multiple target tissues and organs of rats. [58]
- In rats, BPC-157 increased the production of growth factors that fight infections such as vascular endothelial growth factors (VEGF), ultimately strengthening the immune system. [59-62]
- A cell study found that BPC-157’s inflammation reduction effects on the intestines are exerted through its antioxidant properties. [63]
K. Reverses Alcohol Intoxication
BPC-157 can help reverse the adverse effects of acute and chronic alcohol intoxication. Studies show that BPC-157 exerts this effect through the following:
- In mice, BPC-157 rapidly opposed the strongest disturbance presentations in acute alcohol intoxication such as loss of muscle reflex, no reaction to external stimuli, and low body temperature, as well as symptoms of alcohol withdrawal such as prominent seizures. [69]
- BPC-157 protected against both acute and chronic alcohol-induced lesions in the stomach, esophagus, and liver of mice. [70-72]
M. Improves Cognitive Health
An overwhelming body of research supports the benefits of BPC-157 on the brain:
- In rats with brain damage similar to Parkinson’s disease (PD), administration of BPC-157 appears to mitigate some of the damage. [78]
- In rodents with multiple sclerosis (MS), a chronic disease affecting the brain and spinal cord, oral BPC-157 administration decreased brain damage and clinical abnormalities. [79-80]
- In rats with traumatic brain injury, BPC-157 administration is associated with a reduction of unconsciousness and a lower prevalence of deaths. [81]
- In rats, BPC-157 counteracted ischemic/reperfusion injuries (tissue damage caused when blood supply returns after a period of insufficient blood circulation in the brain), resulting in improved memory, orientation, and motor capabilities. [82]
N. Exerts Anti-Cancer Properties
Therapeutic peptides such as BPC-157 are known to possess potent anti-cancer activity. Most animal studies assessing the therapeutic benefits of BPC-157 have shown that this therapeutic peptide helps fight cancer by:
- Inducing programmed cell death (apoptosis) of cancer cells [83-85]
- Assembling and forming pores that can disrupt the structures of cancer cells [86]
7. Boost Cell Health and Recovery with BPC 157 Peptide
The potential to boost cell health and recovery with BPC-157 is an area of ongoing research. While there are indications that BPC-157 may have positive effects on tissue repair and healing processes, its precise mechanisms and overall impact on cell health are not fully understood. Some potential ways in which BPC-157 might contribute to cell health and recovery include:
- Tissue Repair: BPC-157 has been studied for its ability to stimulate collagen production and angiogenesis, which could aid in the repair of damaged tissues, including muscles, tendons, ligaments, and the gastrointestinal tract.
- Anti-Inflammatory Effects: BPC-157 may exhibit anti-inflammatory properties, potentially helping to treat inflammatory conditions and support the healing process.
- Blood Flow and Oxygen Supply: By promoting angiogenesis, BPC-157 might enhance blood vessel formation, leading to improved blood flow and oxygen supply to tissues, which are essential for cell health and recovery.
- Cellular Protection: Some research suggests that BPC-157 might have antioxidant effects, which could help protect cells from oxidative stress and damage.
- Wound Healing: BPC-157’s potential to accelerate wound healing might extend to cellular repair processes, contributing to overall tissue regeneration.
BPC 157 Side Effects
BPC-157 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on BPC-157. However, the issue wasn’t’ confirmed to be caused by the treatment and could have been a coincidence and not related to the use of BPC-157. Despite this, it was listed as a side effect associated with BPC-157 even though these associated side effects are very uncommon.
Side effects associated with BPC-157 may include the following:
- Changes in blood pressure
- Changes in heart rhythm
- Dizziness
- Fatigue
- Hot flashes
- Nausea and vomiting
BPC 157 Dosage
The dosage of BPC-157 can vary depending on the individual and the condition being treated. In general, the prescriptions are based on body weight. However, a typical dosage range is 200-800 micrograms (mcg) per day. This can be taken as a single dose or divided into two or more doses.
For intramuscular injection of BPC-157, the dose is typically 16 units (16 mcg) per dose, twice a day. The injections can be given subcutaneously (under the skin) or intramuscularly (into the muscle).
For oral administration of BPC-157, the dose is typically 500 mcg per dose, twice a day. The capsules can be taken with or without food.weight
It is important to start with a low dose of BPC-157 and gradually increase it as tolerated. Some people may experience side effects such as nausea, headache, or fatigue at higher doses.
If you are considering using BPC-157, it is important to talk to your doctor first. They can help you assess the risks and benefits of this supplement and make sure that it is right for you.
Here are some additional dosage guidelines for BPC-157:
- For the treatment of acute injuries, a higher dose may be necessary.
- For the treatment of chronic conditions, a lower dose may be sufficient.
- The dosage may need to be adjusted depending on the individual’s response.
BPC 157 Before and After Results
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctorRead more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
FAQ
What does BPC 157 do for the body?
BPC-157 is a synthetic pentadecapeptide (a chain of 15 amino acids) that originates from a protein found in the stomach. It has been shown to have a variety of regenerative and healing effects in animal research. BPC-157 is thought to work by stimulating the production of growth factors and other molecules that promote tissue repair.
While a substantial portion of current research has focused on BPC-157’s capacity to assist in digestion, a growing body of evidence indicates that it might possess additional therapeutic attributes. Some of the potential benefits of BPC-157 include:
- Accelerated wound healing
- Faster healing of soft tissue injuries (muscle, tendon, nerve, or ligament damage)
- Reduced pain in damaged areas
- Increased growth hormone receptors
- Increased collagen production
- improved gut health
- Enhanced athletic and physical performance
Is BPC 157 a steroid?
No, BPC-157 is not a steroid. It is a naturally occurring peptide that originates from a protein found in the stomach and has been modified to improve its stability and bioavailability. Steroids are synthetic hormones that can have a number of side effects, including liver damage, heart disease, and mood swings. BPC-157 is not thought to have the same side effects as steroids.
Is BPC 157 legal in the USA?
Yes, BPC-157 is legal in the United States.
Does BPC 157 suppress testosterone?
There is no evidence that BPC-157 suppresses testosterone. In fact, some studies have shown that it can actually increase testosterone levels.
What does peptide BPC 157 do?
BPC-157 is a synthetic pentadecapeptide that is derived from proteins in gastric juices and has been shown to have a variety of regenerative and healing effects in animal models. It is thought to work by stimulating the production of growth factors and other molecules that promote tissue repair.
Does BPC 157 increase testosterone?
There is some evidence that BPC-157 can increase testosterone levels in men.
What is BPC 157 for long COVID?
There is some evidence that BPC-157 may be helpful for people with long COVID. A small study published in 2022 found that BPC-157 improved symptoms such as fatigue, shortness of breath, and cognitive dysfunction.
Is BPC 157 safe to take?
Derived from human gastric juices, BPC-157 boasts exceptional stability and is generally well-tolerated by the majority of individuals. BPC-157 is generally considered safe to take. However, there have been some reports of side effects, such as nausea, headache, and fatigue. It is important to start with a low dose and gradually increase it as tolerated.
How does BPC 157 affect the heart?
BPC-157 has been shown to have some beneficial effects on the heart. In animal studies, it has been shown to reduce inflammation and improve heart function.
Does BPC 157 cause weight gain?
There is no evidence that BPC 157 causes weight gain. In fact, some studies have shown that it can actually help to reduce Discover the transformative potential of with our cutting-edge solutions. Unlock a healthier and more vibrant you by exploring our range of peptide-based weight loss strategies. Join us on the journey towards achieving your weight loss goals – explore the power of peptides today!
What does BPC 157 peptide do?
BPC-157 is a synthetic pentadecapeptide that has been shown to have a variety of regenerative and healing effects in animal studies. It is thought to work by stimulating the production of growth factors and other molecules that promote tissue repair.
Is BPC 157 a steroid or peptide?
BPC-157 is a peptide, not a steroid.
Does BPC 157 interact with anything?
BPC 157 is a peptide that generally does not interact with other medications or substances in a significant way. However, if you are taking any specific medications or have underlying medical conditions, it’s always best to consult with a healthcare professional to ensure there are no potential interactions or adverse effects.
What do you need to know about BPC 157?
BPC 157 is commonly known as a “stable gastric pentadecapeptide” because of its ability to maintain stability even within the environment of human gastric juice. BPC 157 is a peptide that has shown potential in promoting healing and reducing inflammation in the body. It may be beneficial for various conditions, such as muscle and tendon injuries, bone damage, and gut problems. If considering using BPC 157, it’s essential to consult with a healthcare professional for guidance and to understand the proper dosage and potential risks.
How long does it take to see results with BPC 157?
the time it takes to see results with BPC 157 can vary depending on the individual and the specific condition being treated. Some people may notice improvements within a few days to a couple of weeks, while others might take longer. It’s important to be patient and consistent with BPC 157 use, as its effects may take time to become noticeable. Always follow the recommended dosing and consult a healthcare professional for personalized guidance.
Does BPC 157 have a systemic effect?
Yes, BPC 157 does have a systemic effect. This means that when it is introduced into the body, it can circulate throughout the bloodstream and affect various organs and tissues, not just the specific area of intramuscular injection.
How does BPC 157 affect the immune system?
BPC 157 can positively influence the immune system. It is a peptide that has been studied for its potential to reduce inflammation and promote tissue healing. By modulating the immune response, BPC 157 may help the body’s defense mechanisms work more effectively in repairing damaged tissues and supporting overall healing. As with any medication or dietary supplement, it’s essential to consult with a healthcare professional before using BPC 157 to ensure it is safe and appropriate for your specific needs.
Does BPC 157 increase performance?
BPC 157 is not specifically known as a performance-enhancing substance. It is a peptide that has been studied for its potential to promote tissue healing and reduce inflammation. While some athletes and bodybuilders may use BPC 157 in the hope of improving recovery and performance, its direct impact on athletic performance is not well-established. The primary benefits of BPC 157 are believed to be related to its ability to support the healing process in the body. As with any supplement, it’s important to use BPC 157 responsibly and consult with a healthcare professional before use.
It’s important for athletes to be aware of the current World Anti-Doping Agency (WADA) list and to consult with their medical professionals and relevant sporting authorities before using any substance, including compounds like BPC-157. Athletes must ensure they are in compliance with anti-doping regulations to avoid potential penalties or disqualification from competitions.
Does BPC 157 affect the liver?
BPC 157 is believed to have a protective effect on the liver. Clinical trials have shown that BPC 157 may help reduce liver damage caused by certain toxins and substances. It can promote the healing and regeneration of liver cells, which can be beneficial for overall liver health.
Does BPC 157 heal muscle tears?
Yes, BPC 157 has shown a potential to help heal muscle tears. Studies have indicated that BPC 157 can promote the repair and regeneration of injured muscle tissues, leading to faster recovery and reduced inflammation. It is believed to enhance the body’s natural healing processes, which can be beneficial for recovering from muscle injuries.
Should BPC 157 be cycled?
There is no definitive answer on whether BPC 157 should be cycled or not. Some people choose to use it in cycles, where they take it for a period and then take a break before using it again. Others may use it continuously without cycling. It’s essential to follow the dosing instructions and guidelines provided by a healthcare professional or follow the product’s instructions carefully. As with any supplement, it’s crucial to prioritize safety and discuss the best approach with a healthcare provider.
Does BPC 157 repair nerve damage?
Yes, BPC 157 has shown potential in promoting the repair and regeneration of nerve damage. Some research suggests that BPC 157 may have neuroprotective effects and can help improve nerve healing and function. However, more studies are needed to fully understand its effectiveness in treating nerve injuries. As always, it’s essential to consult with a healthcare professional before using any supplement for specific medical conditions.
Can BPC 157 heal cartilage?
Yes, there is some evidence to suggest that BPC 157 may have the ability to promote the healing and repair of cartilage. It has been studied for its potential in treating joint and cartilage injuries. BPC 157’s regenerative properties may aid in the repair and restoration of damaged cartilage tissue.
Does BPC 157 affect the brain?
BPC 157 may have some positive effects on the brain. Studies have shown that BPC 157 has the potential to protect brain cells and promote brain health. It may help with conditions like traumatic brain injury and cognitive impairment. BPC 157’s regenerative properties could aid in repairing and supporting brain tissue.
Which is better BPC 157 and tb500?
BPC 157 and TB-500 are both peptides that have potential healing properties, but they work differently in the body.
BPC 157 is known for its regenerative effects on various tissues, such as muscles, tendons, and ligaments. It may promote healing and repair of injuries, reduce inflammation, and support overall tissue health.
TB-500, on the other hand, is known for its ability to promote the growth of new blood vessels and improve blood flow. It may also support tissue repair and reduce inflammation.
Does BPC 157 promote muscle growth?
BPC 157 is not specifically known for promoting significant muscle growth like traditional muscle-building supplements or steroids. Instead, it is more recognized for its potential to support tissue healing and repair, including muscles, tendons, and ligaments. While it may indirectly contribute to muscle health by aiding in recovery from injuries and reducing inflammation, its primary focus is on overall tissue health rather than direct muscle growth.
Does BPC 157 increase blood flow?
Yes, BPC 157 has been suggested to have the potential to increase blood flow. It may support improved circulation by promoting the formation of new blood vessels, a process known as angiogenesis. This can help in providing more oxygen and nutrients to damaged tissues, aiding in their repair and recovery.
Is BPC 157 good for heart?
BPC 157 shows potential benefits for the heart. Some studies suggest that it may have protective effects on the heart and cardiovascular system. It may help improve heart function, reduce inflammation in the heart tissues, and support the recovery of damaged blood vessels.
Does BPC increase growth hormone?
Yes, BPC-157 has been shown to increase the production of growth hormones. This can be beneficial for various processes in the body, including tissue repair, muscle growth, and overall health.
Discover the potential of Growth Hormone-Boosting Peptides for enhanced vitality. Explore our range of peptides designed to naturally stimulate growth hormone production. Unlock the benefits of optimized growth hormone levels today!
Does BPC 157 affect blood sugar?
BPC-157 does not significantly affect blood sugar levels. It appears to have a neutral or minimal impact on blood sugar when used at appropriate doses. However, it’s essential to keep in mind that individual responses may vary, and it’s always best to consult with a healthcare professional if you have concerns about how BPC-157 might interact with your specific health condition or medications.
What is the mechanism of action of BPC 157?
The mechanism of action of BPC-157 involves promoting the body’s natural healing processes. It works by influencing various biological pathways, such as enhancing blood vessel formation, reducing inflammation, and supporting tissue repair and regeneration. BPC-157 seems to help repair damaged tissues and accelerate healing, making it beneficial for injuries and certain health conditions.
What is the half-life of BPC?
The half-life of BPC-157 is the time it takes for half of the peptide to be eliminated from the body. The half-life of BPC-157 is relatively short, typically ranging from 2 to 6 hours. This means that after a few hours, about half of the administered BPC-157 will be cleared from the body, and the remaining amount will continue to decrease over time. Due to its short half-life, BPC-157 is often administered multiple times throughout the day to maintain its therapeutic effects.
How long should you take peptides?
The duration of taking peptides depends on the specific peptide being used and the intended purpose. Some peptides may be used for short-term goals, while others may be used for longer periods. It is essential to follow the guidelines and recommendations provided by a healthcare professional or the product’s manufacturer.
Which brand of BPC 157 is best?
it is essential to choose a reputable and trusted brand when considering BPC 157 or any other supplement. Look for brands that have a good reputation, positive customer reviews, and have been independently tested for quality and purity. Additionally, consult with a healthcare professional or a qualified expert to get personalized recommendations based on your specific health needs.
Is BPC 157 harmful?
BPC 157 is generally considered safe when used as directed and in appropriate dosages. It is a naturally occurring peptide in the body and is believed to have healing properties. However, like any supplement or medication, it is essential to follow the recommended guidelines and consult with a healthcare professional before use, especially if you have any underlying health conditions or are taking other medications. They can help determine if BPC 157 is safe and suitable for your specific situation.
Does BPC-157 help with bone growth?
Yes, BPC-157 is believed to help with bone growth. It has been studied for its potential regenerative and healing properties, which may include promoting the repair and growth of bones.
Does BPC-157 cause inflammation?
BPC-157 is believed to have anti-inflammatory properties, which means it may help reduce inflammation in the body. This is one of the reasons why it has been studied for its potential healing effects.
Is BPC 157 anti aging?
BPC-157 is being explored for its potential role in anti-aging treatments, with researchers investigating its effects on tissue repair and regeneration. Some studies suggest that BPC-157 may promote tissue repair and regeneration, which could be beneficial for various age-related conditions.
Does BPC 157 lower blood pressure?
BPC-157 is not known to have a direct effect on lowering blood pressure. Its primary function is believed to promote tissue repair and healing, but it does not have a specific impact on blood pressure regulation. If you have concerns about your blood pressure, it’s important to work with your healthcare provider to find appropriate treatments and solutions tailored to your individual health needs.
Should you take BPC 157 with food?
it is generally recommended to take BPC-157 on an empty stomach. Taking it with food may interfere with its absorption and effectiveness. However, for specific dosing instructions and to ensure the best results, it’s essential to follow the guidance provided by your healthcare provider or the product’s instructions.
Does BPC 157 affect hormones?
BPC-157 is not known to directly affect hormones in the body. It primarily works to promote healing and tissue repair, particularly in the gastrointestinal system, muscles, and other tissues.
What is the truth about BPC 157?
BPC-157 is a peptide that has shown promising effects in promoting tissue healing and repair in various studies. It appears to have potential benefits for muscle, ligament, and joint healing, as well as for gastrointestinal health. There are a number of clinical trials currently underway to investigate the safety and efficacy of BPC-157 in human subjects. As with any supplement or peptide, it’s crucial to consult with a healthcare professional before using BPC-157, and always follow their guidance for safe and appropriate use.
How quickly does BPC 157 work?
The time it takes for BPC-157 to work may vary from person to person and depends on the specific condition being treated. Some individuals may start noticing improvements in a few days, while for others, it may take a few weeks. The healing process can be gradual, and consistent use of BPC-157 is important to give it a chance to work effectively. As always, it’s essential to follow the guidance of a healthcare professional and be patient with the healing process.
What are the benefits of BPC 157?
The benefits of BPC-157 include promoting tissue repair and healing, reducing inflammation, improving joint health, and supporting recovery from injuries. It can also potentially enhance bone and muscle healing, and some studies suggest it may have positive effects on the digestive system.
Does BPC 157 increase collagen production?
Yes, BPC-157 has been shown to increase the production of collagen, an essential protein that supports the structure and health of various tissues in the body, such as skin, tendons, ligaments, and muscles. BPC-157’s ability to stimulate collagen synthesis contributes to its role in promoting tissue repair and healing, making it beneficial for injuries and improving joint health.
Does BPC 157 heal muscles?
Yes, BPC-157 has the ability to promote the healing of muscles. It aids in tissue repair and supports the recovery of damaged muscle fibers. This can be particularly beneficial for athletes or individuals recovering from muscle injuries, as BPC-157’s regenerative properties can help speed up the healing process and reduce downtime after workouts or injuries.
Does BPC 157 repair nerve damage?
Current research suggests that BPC-157 may have a promoting effect on tissue healing and regeneration. It seems to support the body’s natural healing processes and may promote nerve tissue recovery.
What are the potential benefits and considerations of using BPC-157 in compounded medications?
Using BPC-157 in compounded medications involves incorporating the peptide BPC-157 into customized formulations created by compounding pharmacies. Compounding pharmacies specialize in preparing medications tailored to individual patient’s needs, often by altering the strength, dosage form, or combination of active ingredients.
When BPC-157 is used in compounded medications, it typically means that the peptide is combined with other ingredients to create a specific dosage form, such as creams, gels, sprays, or even oral capsules, that suits the patient’s preferences and medical requirements. This approach allows for more personalized treatment options beyond standard commercially available medications (e.g. targeting specific localized injuries, aiding in tissue repair, or addressing conditions involving inflammation and pain). The idea is to leverage the potential therapeutic effects of BPC-157 within a formulation that is convenient and effective for the individual patient.
Blog

Improving Bone and Joint Health: The Role of BPC-157
Introduction:
Maintaining strong and healthy bones and joints is crucial for our overall well-being and mobility. However, various factors such as aging, injury, or certain medical conditions can impact the health of our skeletal system. In recent years, a peptide called BPC-157 has gained attention for its potential role in improving bone and joint health. In this article, we will explore the benefits of BPC-157 and its impact on the skeletal system.
BPC-157 and Bone Healing:
BPC-157, short for Body Protective Compound-157, is a synthetic peptide derived from a naturally occurring protein in the stomach. It has demonstrated promising effects on bone healing and regeneration. Studies have shown that BPC-157 can accelerate the healing of fractures, enhance bone density, and promote the formation of new blood vessels, which are crucial for delivering nutrients and oxygen to the bones.
Joint Health and BPC-157:
In addition to its benefits for bones, BPC-157 has also shown potential in promoting joint health. It has been found to stimulate the production of collagen, a protein that forms the building blocks of connective tissues in joints. By increasing collagen synthesis, BPC-157 may help repair damaged cartilage and improve joint function. This can be particularly beneficial for individuals suffering from conditions such as osteoarthritis or sports-related joint injuries.
Anti-Inflammatory and Pain-Relieving Effects:
BPC-157 exhibits potent anti-inflammatory properties, which can be beneficial for individuals dealing with joint inflammation and pain. By modulating the inflammatory response, BPC-157 may help reduce swelling and alleviate discomfort associated with various joint conditions.
Safety and Future Research:
While the current research on BPC-157 is promising, it is important to note that further studies are needed to fully understand its mechanisms of action, optimal dosage, and potential side effects. As with any therapeutic intervention, it is crucial to consult with a healthcare professional before considering the use of BPC-157 or any other peptide-based therapy.
Conclusion:
BPC-157 shows great potential in improving bone and joint health. Its ability to enhance bone healing, promote collagen synthesis, and reduce inflammation makes it an intriguing candidate for individuals looking to support their skeletal system. However, it is important to remember that more research is needed before widespread clinical application. If you are considering using BPC-157 or any other peptide-based treatment, it is advisable to consult with a medical professional to determine the most appropriate course of action for your specific situation. With further research and advancements in medical science, BPC-157 may pave the way for new therapeutic options in the field of bone and joint health.

BPC-157 and Heart Health: Balancing Potassium and Calcium for Optimum Function
Introduction:
The heart is a vital organ responsible for pumping blood throughout the body, and maintaining its health is crucial for overall well-being. In recent years, a peptide called BPC-157 has emerged as a potential player in promoting heart health. This article delves into the role of BPC-157 in balancing potassium and calcium levels for optimal heart function.
The Heart’s Electrical System:
The heart relies on a precisely regulated electrical system to ensure its proper functioning. This system involves the coordinated movement of potassium and calcium ions across cell membranes, which helps generate electrical signals that regulate heartbeats. Any disruption in this delicate balance can lead to heart rhythm abnormalities, known as arrhythmias.
BPC-157 and Potassium:
BPC-157 has shown promise in maintaining the balance of potassium in the heart. Studies have indicated that BPC-157 can enhance the uptake of potassium ions by heart cells, promoting their optimal levels. This balance is crucial for maintaining stable heart rhythms and preventing arrhythmias that can lead to serious cardiac complications.
BPC-157 and Calcium:
In addition to potassium, BPC-157 also plays a role in regulating calcium levels in the heart. Calcium is essential for muscle contraction, including the contractions that occur during each heartbeat. BPC-157 has been found to modulate calcium channels, ensuring the appropriate influx and efflux of calcium ions in cardiac cells. This regulation helps maintain the balance of calcium, preventing abnormalities in heart muscle contraction and supporting overall cardiac function.
Promoting Heart Health with BPC-157:
By optimizing the balance of potassium and calcium in the heart, BPC-157 holds the potential to promote heart health and prevent arrhythmias. Maintaining stable heart rhythms reduces the risk of cardiovascular events and improves overall cardiac performance. However, it is important to note that further research is needed to fully understand the mechanisms of BPC-157’s action on the heart and its long-term effects.
Conclusion:
BPC-157 demonstrates promise in promoting heart health by balancing potassium and calcium levels, essential for maintaining stable heart rhythms. Its potential to optimize cardiac electrical signaling provides a potential avenue for preventing arrhythmias and supporting overall cardiac function. However, it is crucial to conduct further research to establish its safety, efficacy, and optimal usage. If you are concerned about your heart health, it is recommended to consult with a healthcare professional to determine the most appropriate course of action. With ongoing research, BPC-157 may hold new possibilities for maintaining a healthy heart and reducing the risk of cardiac complications.

Cancer Prevention and Treatment: BPC-157’s Potential as an Adjunct Therapy
Cancer has become one of the leading causes of death globally, affecting millions of people yearly. While we have made significant strides in cancer prevention and treatment research, there is still much progress to be made.
Recent medical research has shown that the use of BPC-157 in cancer prevention and treatment as an adjunct therapy could be a breakthrough. In this blog, we’ll explore what BPC-157 is and how it can be an essential addition to conventional cancer treatments.
What is BPC-157?
BPC-157 is a peptide derived from the Body Protection Compound, which is a protein found in the human gastric juice. It plays a vital role in protecting the gastrointestinal tract from damage or disease. The discovery of BPC-157 and its potential benefits in tissue healing has led to the exploration of its use in cancer prevention and treatment.
How does BPC-157 work?
BPC-157 works by accelerating the healing process of damaged tissues, including those damaged by cancer. It stimulates the production of new blood vessels, which supplies blood and nourishment to the affected tissues, allowing them to repair and regenerate. It also has anti-inflammatory properties that help reduce inflammation, which is a common side-effect of cancer treatments such as chemotherapy and radiation.
What are the potential benefits of using BPC-157 in cancer prevention and treatment?
Aside from its healing properties, BPC-157 can help prevent cancer by boosting the body’s immune system, thus making it more resilient against the development and spread of cancer cells. It can also reduce the occurrence of cancer in individuals with a family history of the disease, making it an essential addition to their health regimen.
When used in cancer treatment, BPC-157 can help mitigate the side-effects of chemotherapy and radiation. It can reduce inflammation and promote healing, which can help improve the quality of life of cancer patients.
Conclusion:
Cancer is a devastating disease that affects millions of people worldwide. While there have been significant advances in cancer prevention and treatment research, there is still much work to be done. The use of BPC-157 in cancer prevention and treatment as an adjunct therapy is a promising breakthrough. It has the potential to improve cancer patients’ quality of life by reducing the side-effects of conventional treatments such as chemotherapy and radiation.
It is worth noting that BPC-157 is not a stand-alone treatment for cancer. Instead, it is used as an adjunct therapy to complement conventional treatments. Further research is needed to fully understand the potential benefits of BPC-157 in cancer prevention and treatment. However, the initial research on its positive effects is a step in the right direction towards achieving our goal of finding effective cancer prevention and treatment methods.

Everything You Need to Know About BPC-157 and Its Benefits
Body Protective Compound (BPC)-157 is a highly promising peptide that has been extensively researched for its potential health benefits. Despite being a lesser-known compound, it is said to have great potential in the healing and regeneration of body tissue, enhancing muscle recovery, and repairing damage caused by various medical conditions. In this article, we’ll explore everything you need to know about BPC-157, including its benefits, usage, and potential drawbacks.
What is BPC-157?
BPC-157 is a 15-amino-acid peptide that occurs naturally in the stomach and is derived from the body’s own gastric juices. The peptide stimulates cell growth and, as such, plays a vital role in regeneration and repair of the body tissues. Researchers have studied the peptide extensively for its healing properties, which include improving the recovery process of damaged tissues. It is also believed to promote inflammation reduction, ease pain, and enhance healing.
What Are the Benefits of BPC-157?
The benefits of BPC-157 are widespread, and some of them may include:
Improved Muscle Recovery: BPC-157 supports the regeneration of tendons and muscles, helping to speed up the process of recovery from injuries.
Facilitates Body Regeneration: One of the primary benefits of BPC-157 is its ability to promote the regeneration of tissue, leading to the growth of healthy tissues.
Reduces Inflammation: The peptide is incredibly anti-inflammatory, and this can help reduce inflammation caused by an injury, enhancing the healing process.
Boosts Bone Health: Evidence suggests that BPC-157 can enhance bone mineral content and can help heal bone fractures.
Improves Gut Function: BPC-157 has been shown to possess protective properties and may help in fighting ulcers in the gut.
Conclusion:
BPC-157 is an exciting compound with significant potential to offer its users. Its ability to enhance the healing and regeneration of body tissues, reduce inflammation, and promote overall health has made it an increasingly popular supplement. However, its usage must be properly managed, and care must be taken to avoid any adverse effects. If you are considering using BPC-157, it is essential to consult your healthcare provider to determine if it is a good option for your unique medical needs.

BPC-157 – An Overview of the Benefits, Potential Risks, and Research from genemedics
BPC-157, a peptide gaining attention in the field of regenerative medicine, has sparked curiosity due to its potential therapeutic effects. In this blog, we will delve into the benefits, potential risks, and highlight research findings on BPC-157, drawing insights from Genemedics, a reputable source in the field.
Benefits of BPC-157:
Accelerated Healing: BPC-157 has shown promise in promoting tissue repair and accelerating the healing process. It aids in the regeneration of muscles, tendons, and ligaments, making it useful for athletes or individuals recovering from injuries.
Anti-Inflammatory Properties: Research suggests that BPC-157 possesses anti-inflammatory effects, aiding in the reduction of inflammation and alleviation of associated symptoms. This property may benefit those with conditions like arthritis or inflammatory bowel disease.
Gastrointestinal Health Support: BPC-157 has been observed to have positive effects on gut health. It protects the lining of the digestive tract, aids in the healing of ulcers, and promotes overall gastrointestinal well-being.
Joint and Bone Health: Preliminary studies indicate that BPC-157 may contribute to joint and bone health by stimulating cartilage regeneration and enhancing bone density. This could be advantageous for individuals with conditions such as osteoarthritis or osteoporosis.
Potential Risks:
Although BPC-157 appears to be well-tolerated, it is essential to approach its use with caution. As with any therapeutic agent, potential risks and side effects should be considered. Consulting with a healthcare professional is crucial to ensure proper guidance and monitoring.
Research Findings from Genemedics:
Genemedics, a reputable research institution, has conducted studies on BPC-157. Their research has provided valuable insights into its regenerative and healing properties, supporting its potential use in various clinical applications.
Conclusion:
BPC-157 shows promise in the field of regenerative medicine, but it is vital to note that further research is needed to fully understand its mechanisms of action, long-term effects, and optimal dosage. As with any new treatment, it is recommended to consult with a healthcare professional before considering BPC-157 supplementation or therapy.
Disclaimer: The information provided in this blog is for educational purposes only and should not be considered as medical advice. Always consult with a qualified healthcare professional before initiating any new treatment or supplementation.
Uncovering the Healing Power of BPC-157 Peptide Therapy
BPC-157, a promising peptide therapy, has gained attention for its potential healing properties. This blog aims to explore the therapeutic benefits, mechanisms of action, and emerging research surrounding BPC-157.
The Healing Potential of BPC-157:
BPC-157 has shown remarkable potential in promoting healing and tissue regeneration. It works by stimulating angiogenesis, collagen synthesis, and various cellular processes involved in tissue repair. This makes it beneficial for athletes, individuals recovering from injuries, and those seeking accelerated healing.
Musculoskeletal Recovery:
One notable aspect of BPC-157 is its ability to support musculoskeletal recovery. Research suggests it can enhance the healing of muscle, tendon, and ligament injuries. By promoting tissue regeneration and reducing inflammation, BPC-157 aids in restoring normal function and alleviating pain.
Gastrointestinal Support:
Another area where BPC-157 shines is gastrointestinal health. It has exhibited protective effects on the gut lining, reducing ulcer formation, and promoting healing. This makes it a potential therapy for conditions like inflammatory bowel disease and gastric ulcers.
Anti-Inflammatory Effects:
BPC-157 exerts anti-inflammatory properties by modulating cytokine production and reducing oxidative stress. These effects contribute to its ability to mitigate inflammation in various tissues, offering potential relief for conditions associated with chronic inflammation.
Research Insights:
Emerging research on BPC-157 continues to uncover its therapeutic potential. Studies have demonstrated its efficacy in both animal models and human trials, further validating its healing properties. Ongoing research aims to explore additional applications and optimize dosing protocols for various conditions.
Conclusion:
BPC-157 peptide therapy holds immense promise as a regenerative treatment option. Its ability to accelerate healing, support musculoskeletal recovery, and promote gastrointestinal health makes it a valuable tool in regenerative medicine. As with any therapy, it is essential to consult with a healthcare professional to determine the appropriate dosage and monitor its use.
References
- Mikus D, Sikiric P, Seiwerth S. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns: journal of the International Society for Burn Injuries. 2001; 27(8):817-27.
Pentadecapeptide BPC 157 Cream Improves Burn-wound Healing and Attenuates Burn-gastric Lesions in Mice
A partial sequence of the body protection compound (BPC), pentadecapeptide BPC-157 is made up of 15 amino acids and was identified and obtained from human stomach acid. It has been shown in experiments to hasten the healing of many different wounds, including the superior healing of fractured bones and tendon-to-bone repair. BPC-157 has also demonstrated evidence of protecting organs and helping avoid injuries such as stomach ulcers.
A study evaluated how the gastric pentadecapeptide BPC-157 affects mice with severe partial skin thickness burns. In this study, controlled burning was used to create 20% body-covering burns on the backs of the mice. BPC-157, silver sulfadiazine cream, or no medication was applied to the mice. BPC-157 was given topically or systemically right after the burn, once per day for 24 hours prior to sacrifice. On days 1, 2, 3, 7, 14, and 21 after the injury, the treated area was evaluated. In later tests, deeper burns were generated and various topically applied doses of BPC 157 cream were tested.
Results showed that compared to untreated controls, the mice treated with BPC-157 cream had improved burn healing throughout the experiment, including reduced edema (swelling), decreased inflammatory cell numbers, increased capillaries, advanced formation of dermal reticulin and collagen fibers, and an increased number of preserved follicles. Mice treated with BPC 157 cream exhibited a considerably higher re-epithelization ratio two weeks after the injury. Tensiometry results indicated that charred skin had less water and had enhanced breaking strength and relative elongation. In addition to reducing the quantity of inflammatory cells and water content in burned skin, systemically injected BPC 157 significantly raised the breaking strength and proportion elongation of burned skin during tensiometry. Moreover, stomach lesions were consistently observed in all thermally wounded mice that were not given either local treatment but these injuries were attenuated only by BPC 157 treatments whether administered systemically or locally.
According to the study, BPC 157 may promote burn healing at the site of the defect by regulating growth factors and affecting other regional variables.
Read the full article: https://pubmed.ncbi.nlm.nih.gov/11718984/ - Bilic M., Bumber Z., Blagaic A.B., Batelja L., Seiwerth S., Sikiric P. The stable gastric pentadecapeptide BPC 157, given locally, improves CO2 laser healing in mice. Burns. 2005;31(3):310–315.
The stable gastric pentadecapeptide BPC 157, given locally, improves CO2 laser healing in mice
In phase II clinical studies for the treatment of inflammatory bowel disease, the gastric pentadecapeptide BPC 157 has demonstrated the capacity to minimize the adverse effects of systemic corticosteroids on tissue regeneration in burned mice. Researchers have now looked at how BPC 157 affects CO(2) laser injuries, which frequently present unique healing complications. In this study, a layer of neutral cream containing either 1 microg, 1 ng, or 1 pg of BPC 157 (dissolved in saline) or just the cream was applied topically to the skin of male mice with CO(2) laser injuries once daily, starting 60 minutes after injury and ending 24 hours before the mice were sacrificed. BPC 157 consistently improved the healing of the injuries, as seen both macroscopically and microscopically.
The study revealed that the therapeutic advantages of BPC 157 could be accomplished with a simple method of application and that the peptide remained relatively stable without the need for a carrier. According to the findings, an ointment containing 1 microg of BPC 157 (dissolved in saline) per gram of neutral cream can speed up the healing of CO(2) laser wounds. Overall, these data imply that BPC 157 has therapeutic potential for treating diverse injuries and may offer a novel pathway for aiding healing. To prove its efficacy and safety in humans, additional research is needed.
Read the full article: https://pubmed.ncbi.nlm.nih.gov/15774286/ - Sikiric P., Seiwerth S., Mise S., Staresinic M., Bedekovic V., Zarkovic N., Borovic S., Gjurasin M., Boban-Blagaic A., Batelja L., Rucman R., Anic T. Corticosteroid-impairment of healing and gastric pentadecapeptide BPC-157 creams in burned mice. Burns. 2003;29(4):323–334.
Corticosteroid-impairment of healing and gastric pentadecapeptide BPC-157 creams in burned mice
The BPC-157 stable gastric pentadecapeptide’s effects on the recovery of deep partial skin thickness burns and burn-gastric lesions in thermally wounded (injury due to increased temperature) mice were examined in this work. The mice were given 6alpha-methylprednisolone or saline, and the wound was then treated with three different concentrations of BPC-157 cream. The findings demonstrated that BPC-157 treatment consistently enhanced burn healing, as measured by tensionmetry and under a microscope, and counteracted the negative effect of corticosteroids on wound healing. Moreover, BPC-157 enhanced the anti-ulcer effect shown in 6alpha-methylprednisolone-treated mice. This effect was also seen in burned non-corticosteroid-treated mice. In vitro evaluation of spleenic cells (the mice were sacrificed at day 21) revealed that the addition of BPC-157 restored cell reactivity to values recorded in control healthy mice. BPC-157 also reduced corticosteroid immunosuppression.
Overall, the research points to BPC-157 as a potential treatment for burn healing and a possible remedy for the detrimental effects of corticosteroids on wound healing. Moreover, BPC-157 may enhance immunological performance and have an anti-ulcer impact. According to the research, BPC-157 may hold promise as a treatment for inflammatory bowel disease and other illnesses that are linked to poor wound healing. However, additional clinical studies are required to verify these effects in people.
Read the full article: https://pubmed.ncbi.nlm.nih.gov/12781609/ - Mikus D, Sikiric P, Seiwerth S. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns: journal of the International Society for Burn Injuries. 2001; 27(8):817-27.
Pentadecapeptide BPC-157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice
The study looked at how the gastric pentadecapeptide BPC-157 affected burn healing in mouse models of burn injury. After inducing a controlled burn on the mice’s back, the drug was applied topically or systemically, and evaluations were done at various intervals. The findings demonstrated that BPC-157 cream-treated mice exhibited enhanced burn healing compared to untreated controls. The BPC-157 cream-treated mice also had less edema (swelling), lower inflammatory cell counts, expanded capillaries, and progressed skin reticulin and collagen fiber production.
In addition, BPC-157 treatment increased the breaking strength and relative length of burned skin while lowering its water content. All thermally damaged mice that were not given any local anesthetic developed stomach lesions, which were reliably diminished only by BPC-157 treatments.
By upregulating growth factors and affecting other local variables, BPC-157 appears to enhance burn healing in the wound area. Overall, the research points to the stable gastric pentadecapeptide BPC-157 as a potentially effective treatment for burn wounds or injuries.
Read the full article: https://pubmed.ncbi.nlm.nih.gov/11718984/ - Seiwerth S, Sikiric P, Grabarevic Z. BPC 157’s effect on healing. Journal of physiology, Paris. ; 91(3-5):173-8.
BPC 157’s effect on healing
The 15 amino acid agent BPC-157 was used in this study in order to evaluate its effects on various elements linked with the wound healing process. Previous studies in different experimental models have shown that BPC-157 can help protect the body organs against damage or injury.
There are key elements in the process of wound healing. These include granulation tissue formation, angiogenesis (formation of new blood vessels), and the production of an essential skin protein called collagen. In this study, the authors tested the effects of BPC-157 on different factors associated with wound healing such as granulation tissue formation, angiogenesis, collagen production, and tensile strength development in experimental rat models.
Results showed that BPC-157-treated animals had improved granulation tissue formation, angiogenesis, collagen production, and tensile strength development compared to untreated rat models. These results suggest that BPC-157 can enhance the wound-healing process by affecting key elements.
You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/9403790/ - Seveljević-Jaran D, Cuzić S, Dominis-Kramarić M. Accelerated healing of excisional skin wounds by PL 14736 in alloxan-hyperglycemic rats. Skin pharmacology and physiology. 2006; 19(5):266-74.
Accelerated healing of excisional skin wounds by PL 14736 in alloxan hyperglycemic rats
A synthetic peptide with anti-inflammatory and tissue-protective properties called PL 14736 was used in this study. The effects of topical treatment of PL 14736 in a gel formulation on full-thickness excisional wounds in rats—both healthy and hyperglycemic (with higher blood sugar levels) — were examined.
In this study, PL 14736 caused the hyperglycemic rats’ wound healing to increase in a dose-dependent manner compared to healthy rats. This effect was comparable to that seen with becaplermin, a common treatment for diabetic foot ulcers. By day 7, as established histologically, the positive effect was linked to an increase in the deposition of organized granulation tissue and mature collagen.
The study suggests that topical PL 14736 may be an alternative therapy for delayed wound healing, such as in patients with diabetic foot ulcers. The treatment can accelerate the wound healing process by facilitating granulation tissue formation and collagen maturation.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16785777/ - Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485–2499. doi: 10.2147/DDDT.S82030.
Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro
Chemical burns are a significant cause of admissions for burn treatment. Unfortunately, there is currently no optimal treatment available for this condition.
A study investigated the effects of using a compound called body protective compound (BPC-157) on wound healing in a rat model of alkali burn. Results showed that BPC-157 treatment accelerated wound closure, promoted better tissue formation and collagen deposition, and enhanced the proliferation and migration of human umbilical vein endothelial cells (HUVECs) in vitro. In addition, it was observed that BPC-157 improved signaling pathways such as extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Fos, c-Jun, and Egr-1, which are key molecules that play a role in cell growth, migration, and angiogenesis (formation of new blood vessels).
The therapeutic mechanism of BPC-157 may be associated with accelerated granulation tissue formation, reepithelialization, dermal remodeling, and collagen deposition through the extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling pathway. Overall, BPC-157 treatment may be a promising approach to accelerate wound healing in cases of alkali burn-induced skin injury.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4425239/ - Drmic D, Samara M, Vidovic T, et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24(48):5462-5476.
Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine
In this study, various substances were applied to rats that had had cecum (a pouch that is located at the first part of the large intestine) injuries, including BPC-157, L-NAME, L-arginine, and saline baths as controls. The effects of these substances on a number of indicators, including the levels of MDA and NO in cecum tissue, vascular reappearance, defect contraction, bleeding attenuation, and defect contraction, were tracked by the researchers. Also, they evaluated the degree of adhesions and lesions in the cecum at various times. The vessels spreading towards the defect at the cecum surface were also captured with a USB microcamera. The agents’ effects were assessed after 15 minutes, one day, and seven days.
Results showed that a saline bath after damage led to a notable decline in vessels, more bleeding, greater MDA levels, and lower NO levels. With normal MDA and NO levels, the BPC-157 bath enhanced vascular presentation, reduced bleeding, and reduced the defect. L-NAME decreased vascular presentation but had no effect on the defect or the rate of bleeding. Less vessel reduction, no change in the defect, and more bleeding persisted with L-arginine. Saline, L-NAME, L-arginine, and L-NAME + L-arginine all failed to close the lesions.
In conclusion, BPC-157 can help accelerate wound healing of perforated cecum lesions by enhancing blood vessel formation and reducing bleeding.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6319139/
- Amic F, Drmic D, Bilic Z, et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24(47):5366-5378.
Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine
The approach for the study involved ligating the superior anterior pancreaticoduodenal vein in male Wistar rats (SAPDV). The rats were then given different dosages of BPC-157, L-NAME, and L-arginine, either by bathing them at the site of the ligation or by injecting BPC-157 directly into their stomachs.
Researchers examined levels of nitric oxide (NO) and malondialdehyde (MDA) while observing how arteries and lesions grew in the duodenum (the first part of the small intestine). The researchers also compared the results of administering BPC-157 and L-NAME/L-arginine to rats with SAPDV-ligated controls.
Increased connections and branching, reduced mucosal lesions and serosal congestion, and normalized NO and MDA levels in duodenal tissues were all effects of BPC-157. Treatment with L-NAME and L-arginine alone decreased mucosal and serosal lesions in the duodenum but the effect deteriorated after 24 hours and had no impact on collateral vessels or branching. When compared to the L-NAME/L-arginine treatment, BPC-157 had a more apparent beneficial effect, and the two therapies seemed to have different effects. The original duodenal flow was saved by BPC-157, which quickly circumvented the obstruction. This had an impact on the NO system and decreased the production of free radicals.
In conclusion, BPC-157 can help treat blood vessel injuries by promoting the growth of new blood vessels, reducing lesions, and normalizing nitric oxide levels.
You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC6305534/. - Drmic D, Kolenc D, Ilic S, et al. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23(29):5304-5312.
Celecoxib-induced gastrointestinal, liver, and brain lesions in rats, counteraction by BPC–157 or L-arginine, aggravation by L-NAME
Celecoxib, a medication used to treat inflammation and discomfort, was tested in this study to find out how it affects rat liver, brain, and gastrointestinal lesions. The medications included celecoxib alone or in conjunction with L-arginine, stable gastric pentadecapeptide BPC-157, and NOS (nitric oxide system) blocking (using N(G)-nitro-L-arginine methyl ester). The liver enzyme serum (blood) levels and lesions were assessed 24 and 48 hours after treatment.
Results showed that high doses of celecoxib caused abnormalities in the NO system, which led to liver, brain, and stomach lesions as well as increased liver enzyme blood values. These lesions were reversed by L-arginine, whereas L-NAME aggravated stomach lesions but had no effect on liver or brain lesions. Celecoxib and L-NAME had deleterious effects that BPC-157 was able to counter, suggesting that the treatment could address both COX-2 inhibition and further NOS blockage.
These findings show an important involvement of the NO system in these phenomena and a potential role for BPC-157 as a therapeutic alternative for various organ lesions. In addition to reducing gastrointestinal, liver, and brain lesions, BPC-157 and L-arginine can reduce the post-surgical use of non-steroidal anti-inflammatory drugs (NSAIDs).
You can read the full article at:- https://www.ncbi.nlm.nih.gov/pubmed/29510201.
- Grabarevic Z, Tisljar M, Artukovic B, et al. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris. 1997;91(3-5):139-49.
The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks
In this study, the authors evaluated how different chemicals influence the development of tissue lesions and pulmonary hypertension syndrome in chicks. The effects of nitric oxide (NO) agonists, antagonists, and a new organoprotective pentadecapeptide named BPC-157 were specifically examined.
The chicks were given single doses of various drugs as part of experiments to study acute toxicity. After that, spleen, heart, liver, lungs, and pathohistological tests as well as hematological analysis were performed on the chicks. The authors also performed a chronic toxicity trial, giving the chicks daily treatments for five weeks with various chemicals.
Results demonstrated that the utilization of L-NAME, a (nitric oxide) NO antagonist, resulted in the development of pulmonary hypertension syndrome in the treated chicks as well as significant tissue damage in the myocardial, hepatic, and lymphoid cells. Nevertheless, pulmonary hypertension syndrome and tissue damage were minimized by giving BPC-157 and L-arginine at the same time. Hematological testing revealed that the L-NAME-treated groups of chicks had considerably lower hemoglobin and leukocyte counts. L-arginine, on the other hand, predominantly led to engorgement, edema, and hemorrhages in all organs. There was no organ or tissue damage caused by BPC-157.
In conclusion, BPC-157 can treat pulmonary hypertension syndrome and tissue damage without causing serious adverse events.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29682749/.- TlakGajger I, Ribarić J, SmodišŠkerl M, Vlainić J, Sikirić P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J Vet PharmacolTher. 2018;41(4):614-621. doi:10.1111/jvp.12509.
Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions
Nosema ceranae is a small, unicellular parasite that affects honeybees. The aim of this study is to assess whether the stable gastric pentadecapeptide BPC-157 can be used in honeybee therapy to control Nosema ceranae invasions.
The research revealed that Nosema ceranae invasions in honeybee colonies can be avoided by using the stable gastric pentadecapeptide BPC-157 as a treatment. In a sugar syrup, the BPC-157 supplement was mixed and given to honeybee colonies over the course of 21 days. The study discovered that the initial feeding of a sugar syrup supplemented with BPC-157 increased the strength of honeybee colonies. It was also observed that after initial feeding with the BPC-157 supplement, the number of Nosema ceranae spores per honeybee was rapidly reduced. In infected honeybees with Nosema ceranae infections, BPC-157 also reduced the damage to the layers of the midgut wall and the epithelial cells.
This study shows that oral therapy with BPC-157 supplement can significantly improve the health of honeybee colonies that are affected by Nosema ceranae.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29682749/.- Krivic A, Anic T, Seiwerth S, Huljev D, Sikiric P. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. Journal of orthopaedicresearch : official publication of the Orthopaedic Research Society. 2006; 24(5):982-9.
Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation
The stable gastric pentadecapeptide BPC-157 has no proven toxicity and can aid in the repair of a variety of tissues, including tendons and bones, without the need for a carrier. It is being tested as an anti-ulcer treatment for inflammatory bowel disease.
The Achilles tendon-to-bone connection, which normally does not heal on its own, was the study’s main emphasis. The peptide BPC-157 (10 microg, 10 ng, or 10 pg), 6alpha-methylprednisolone (1 mg), or 0.9% NaCl (5 mL) were given either alone or in combination after the Achilles tendons of rats were surgically severed. After BPC-157 treatment, increased Achilles functional index (AFI) values, better organization of collagen fibers, advanced vascular (blood vessel) appearance, and more collagen type I were observed. On the other hand, 6alpha-methylprednisolone made healing worse, although BPC-157 helped lessen these side effects.
This indicates that direct tendon-to-bone repair using BPC-157 may eventually take the place of the reconstructive surgical procedures currently used.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16583442/..- Staresinic M., Sebecic B., Patrlj L., Jadrijevic S., Suknaic S., Perovic D., Aralica G., Zarkovic N., Borovic S., Srdjak M., et al. Gastric pentadecapeptide BPC 157 accelerated healing of transected rat Achilles tendon and in vitro stimulates tenocytes growth. J. Orhtop. Res. 2003;21:976–983. doi: 10.1016/S0736-0266(03)00110-4.
Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth
Studies have demonstrated that the stable gastric pentadecapeptide BPC-157 is beneficial for encouraging the healing of transected Achilles tendons. Clinical trials are now being conducted to see whether this peptide can effectively treat inflammatory bowel disease.
BPC-157 was given to rats with transected Achilles tendons coupled with saline, and it was discovered that this dramatically accelerated recovery as compared to the control group. BPC-157 enhanced the load of failure, load of failure per area, Young’s modulus of elasticity, Achilles functional index (AFI) values, and superior formation of fibroblasts, reticulin, and collagen, according to biomechanical, microscopical, and macroscopical analyses. Contrary to TGF-beta, BPC 157 consistently reacted poorly to the growth inhibitor 4-hydroxynonenal (HNE).
These findings indicate that BPC-157 might be a successful therapy for Achilles tendon damage.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/14554208/..- Molloy T.J., Wang Y., Horner A., Skerry T.M., Murrell G.A. Microarray analysis of healing rat Achilles tendon: Evidence for glutamate signaling mechanisms and embryonic gene expression in healing tendon tissue. J. Orthop. Res. 2006;24:842–855. doi: 10.1002/jor.20093.
Microarray analysis of healing rat Achilles tendon: evidence for glutamate signaling mechanisms and embryonic gene expression in healing tendon tissue
The three phases of tendon healing are inflammation, proliferation, and remodeling. Tendon healing is a complicated process involving numerous sophisticated pathways. There is still a lot we don’t know about these processes despite major investigation.
A study conducted microarray studies on healing rat Achilles tendons at 1, 7, and 21 days after injury, which correspond to the three phases of healing, to look at the genetic components of tendon healing. An original temporal expression profile was found by the study, which revealed both well-known and novel pathways and genes involved in tendon healing. Within 24 hours after damage and 21 days later, there was a considerable upregulation of the inflammatory response and pro-proliferative genes. The most substantial increase in genetic activity appeared on day 7, especially in the expression of genes linked to the extracellular matrix and collagen.
Moreover, tendon cells exhibited indications of a glutamate-based signaling pathway, which is comparable to that found recently in bone cells. Tendon cells have not previously displayed this mechanism. The research also identified a number of genes that were elevated during the healing process and were mostly linked with proliferation and embryonic development. This shows that wound healing and the controlled course of growth and development in the embryo involve related pathways. The creation of new gene-based treatments to promote tendon healing may benefit from these findings.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16514666/- Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. Journal of applied physiology (Bethesda, Md. : 1985). 2011; 110(3):774-80.
The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration
A sequence of 15 amino acids termed as the pentadecapeptide BPC-157 is a component of the body protection compound (BPC), which was discovered and extracted from human stomach juice. Various tests revealed that it can speed up the recovery of a number of lesions, including those in rat Achilles tendons.
This particular research was aimed at investigating how BPC-157 improves tendon injury repair. The growth of tendon fibroblasts from tendon explants that were grown with or without BPC-157 was investigated by the researchers. The findings showed that BPC-157 greatly accelerated tendon explant outgrowth but had no direct impact on cell proliferation. Nevertheless, BPC-157 did encourage cell survival in H(2)O(2)-stressed conditions. The researchers also discovered that tendon fibroblasts’ in vitro migration and spread was dose-dependently enhanced by BPC-157. In addition, BPC- 157 stimulated the synthesis of F-actin and elevated FAK and paxillin protein expression and activation, with dose-dependent increases in both proteins’ phosphorylation levels.
In conclusion, BPC-157 activates the FAK-paxillin pathway, which promotes tendon fibroblast migration in vitro, cell survival under stress, and ex vivo growth of tendon fibroblasts from tendon explants.
You can read the full article at at https://journals.physiology.org/doi/full/10.1152/japplphysiol.00945.2010?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org- Staresinic M, Sebecic B, Patrlj L. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003; 21(6):976-83.
Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth
It has been investigated whether the stable gastric pentadecapeptide BPC-157 can speed up the healing of transected Achilles tendons. The peptide is presently undergoing clinical trials for inflammatory bowel disease after it was discovered to improve both internal and exterior wound healing.
BPC-157 has been demonstrated in rat experiments to positively affect the Achilles tendon’s recovery in terms of biomechanical, functional, microscopical, and macroscopical evaluations.
A study evaluated the effects of BPC-157 in rats with Achilles tendon injury. A group of rats received BPC-157 application to the injured area while another group received saline application 30 minutes after surgery. Achilles functional index (AFI) was assessed once a day. Results revealed that BPC-157 treatment was associated with full recovery of the Achilles tendon compared to saline treatment. BPC-157 increased AFI values, increased collagen, and reduced the size and depth of tendon defects. BPC-157 also re-established full tendon integrity.
In conclusion, BPC-157 can help achieve full recovery of the Achilles tendon in subjects with this kind of injury.
You can read the full article at at https://www.ncbi.nlm.nih.gov/pubmed/14554208- Gjurasin M, Miklic P, Zupancic B. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regulatory peptides. 2010; 160(1-3):33-41.
Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury
The research focused on the restoration of transected sciatic nerves in rats. The effects of giving stable stomach pentadecapeptide BPC 157 at a dose of 10 micrograms or 10 nanograms per kilogram of weight shortly after the damage were examined by the researchers. Following a 7 mm nerve segment excision, the peptide was administrated, intragastrically, locally at the anastomosis site, or directly into the non-anastomoized nerve tube. Using autotomy, microscopy/morphometry, electromyography (EMG), and walking recovery at weekly intervals for one to two months after the injury, the clinical success of nerve healing was assessed.
Results showed that rats treated with BPC-157 exhibited an improved neural fascicle presentation, homogeneous regeneration pattern, increased density and size of regenerative fibers, the existence of epineural and perineural regeneration, uniform target orientation of regenerative fibers, and a higher proportion of neural vs. connective tissue – all of which were associated with faster axonal regeneration. In addition, all fascicles in each nerve displayed enhanced blood vessel presentation, myelin sheet thickness, increased number of myelinated fibers per region and myelinated fibers as a portion of the nerve transected area. Electrophysiological testing showed increased motor action potentials. Functional recovery was demonstrated by the improved sciatic functional index (SFI) and the absence of autotomy.
Therefore, BPC-157 was found to significantly improve the healing of rat sciatic nerves. It does this by enhancing various parameters associated with nerve regeneration.
You can read the abstract of this article athttps://www.ncbi.nlm.nih.gov/pubmed/19903499.- Pevec D, Novinscak T, Brcic L. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Medical science monitor : international medical journal of experimental and clinical research. 2010; 16(3):BR81-88.
Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application
The research evaluated how a peptide known as BPC-157 can reduce the side effects of corticosteroid therapy and affect muscle recovery in rats with contusions. Whether used locally or systemically, BPC-157 enhanced healing in transected quadriceps muscle and muscular crush damage. Moreover, it mitigated the effects of corticosteroids on the tendon and bone repair.
Based on the study, BPC-157 may be a useful therapy for promoting muscle recovery even when corticosteroids are being used. After giving BPC-157 and/or corticosteroids to the muscles, their functional, macroscopical, and histological healing was evaluated. Controls with broken gastrocnemius muscle complex showed only a minor increase in the absence of treatment. Alpha-methylprednisolone actually slowed healing. Contrarily, when administered intraperitoneally or locally, BPC-157 induced quicker muscle healing and full function restoration and improved muscle healing despite systemic corticosteroid treatment. These effects were shown functionally, macroscopically, and histologically at all time points studied. Complete reversal of systemic corticosteroid-impaired muscle repair was achieved with BPC- 157.
You can read the full article at athttps://www.ncbi.nlm.nih.gov/pubmed/20190676- Novinscak T, Brcic L, Staresinic M. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surgery today. 2008; 38(8):716-25.
Gastric Pentadecapeptide BPC 157 As An Effective Therapy For Muscle Crush Injury In The Rat
It was shown that the stable gastric pentadecapeptide BPC-157 speeds up the recovery of transected quadriceps and Achilles tendons. This peptide may one day be used as a systemic or local peptide therapy for crush injuries to large muscles like the gastrocnemius muscle complex. The peptide is now being studied for inflammatory bowel disease and has no known toxicities. It does not need a carrier.
To test its effectiveness, BPC-157 was administered to rats with crush injury either intraperitoneally or locally as a cream immediately after injury and once a day for 14 days. Results showed that BPC-157 improved muscle healing both macroscopically (less bruising and swelling with no post-injury leg contracture) and microscopically. In addition, the treatment also improved the muscle function of the BPC-157-treated rats as well as their enzyme activity levels (creatine kinase, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase).
In conclusion, BPC-157 was found to accelerate post-injury muscle healing and restore full function when given locally or intraperitoneally, regardless of the interval tested.
https://www.ncbi.nlm.nih.gov/pubmed/18668315- Novinscak T, Brcic L, Staresinic M. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surgery today. 2008; 38(8):716-25.
Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157
In this study, the quadriceps muscle of rats was surgically injured. When the quadriceps muscle was totally severed in rats, one centimeter above the patella, there was a clear defect that cannot be compensated. The rat subjects were injected with BPC-157 once daily. The treatment was given immediately after the injury and the final 24 hours before the rats were sacrificed.
Results showed that there was an increase in the load of failure, walking recovery, and extensor postural thrust/motor function index. Microscopy/immunochemistry showed mostly muscle fibers connecting muscle segments, absent gap, significant desmin positivity for the ongoing regeneration of muscle, and larger myofibril diameters on both sides. Macroscopic presentation showed stumps connecting, atrophy markedly attenuated, and presentation close to normal noninjured muscle with no postsurgery leg contracture.
In conclusion, the administration of the systemic peptide BPC-157 in rats with complete transection of major muscle induced healing and was associated with the restoration of muscle function.
You can read the full article at at https://www.ncbi.nlm.nih.gov/pubmed/16609979- Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19(11):19066–19077. Published 2014 Nov 19. doi:10.3390/molecules191119066.
Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts
Tendon damage is a commonly occurring injury during sports activities, with most instances caused by overuse, aging, or accidents that result in the tearing of tendon fibers. Repairing a completely torn tendon can be a challenging process and typically requires surgery. Tendons consist of tendon fibroblasts, which are cells, and extracellular matrix, which is mainly composed of type I collagen, type III collagen, and glycoproteins.
The healing process of a ruptured tendon is categorized into three phases: inflammation, regeneration, and remodeling. During the regeneration phase, tendon fibroblasts move toward the affected area, multiply, and create various types of collagens and glycoproteins to form the extracellular matrix.
The goal of the current study was to clarify the probable mechanism by which BPC-157 influences tendon fibroblasts to enhance tendon repair. According to previous cDNA microarray analysis, tendon fibroblasts treated with BPC-157 had one of the most up-regulated expressions of the growth hormone receptor gene. So, the authors focused on BPC-157’s impact on this gene in the study. The research showed that BPC-157 has the ability to enhance tissue healing by increasing the expression of growth hormone receptors in tendon fibroblasts.
BPC-157 could be an alternative method to promote tissue healing by increasing the beneficial effects of growth hormone in terms of improved proliferation. Additionally, using BPC-157 to increase the expression of growth hormone receptors could potentially lead to a reduction in the amount of growth hormone needed, as well as decrease the cost of therapy. Overall, BPC-157 appears to have significant potential for promoting tendon healing and may be considered for clinical use in the future.
You can read the full article at at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6271067/- Zoricic I., Sikiric P., Seiwerth S. Pentadecapeptide BPC-157 beneficially influences the healing of colon-colon anastamoses in rats. In: Mozsik G., Nagy L., Par A., Rainsford K., editors. Cell Injury and Protection in the Gastrointestinal Tract. From Basic Science to Clinical Perspectives. Kluwer Academic Publishers; Dodrecht, The Netherlands: 1997. pp. 249–258.
Pentadecapeptide BPC 157 Beneficially Influences the Healing of Colon—Colon Anastomoses in Rats
In many ulcer models, the pentadecapeptide BPC-157, which is generated from the gastric juice peptide Body Protection Compound (BPC), has shown its capacity to safeguard the gastrointestinal tract and lessen inflammation. Both before and after treatment, it was shown to be more productive than various reference standards. BPC-157 was discovered to encourage mucosal healing in addition to its anti-inflammatory properties.
Further study was done to look into its potential for repairing colon-colon anastomoses. Male Albino Wistar rats weighing 250 g were utilized for the experiments. The bursting pressure was measured on days 2, 5, 7, and 10 following the surgical procedure. The rats were treated with either 10 µg or 10 ng/kg of BPC-157, administered immediately after a colon resection and colon-colon anastomosis, either locally or through intragastric (ig) or intraperitoneal (ip) routes. The rats were sacrificed after 2, 5, 7, or 10 days, or were given an additional dose of medication through ig or ip routes 24 hours after the first medication, and then sacrificed on postoperative day 2. The control group was given an equal amount of saline (5 ml/kg). A dose-dependent rise in bursting pressure and maximal intestinal wall tension compared to control values was visible after 2 days following a single BPC-157 injection. BPC showed a relative increase after two days when used frequently, even at the lower BPC-157 dosage.
Together, these results indicate that BPC-157 may have a positive impact on colon-colon anastomosis healing and increase bursting strength, especially in the early postoperative period, in accordance with its strong protective effect on the gastrointestinal tract, which was confirmed microscopically. It remains to be seen whether this will have clinical repercussions.
You can read the full article at at https://link.springer.com/chapter/10.1007/978-94-011-5392-8_25.- Konjevoda P., Nasic M., Curkovic T. Effects of BPC-157 on the healing of corneal lesions. In: Ohno S., Aoki K., Usui M., editors. Uveitis Today. Elsevier; Amsterdam, The Netherlands: 1998. pp. 311–314.
Effects of BPC-157 on the healing of corneal lesions
Based on its healing effects in various tissues, the authors hypothesized that the stable gastric pentadecapeptide BPC 157 has the ability to enhance the regeneration of corneal ulcerations in rats. The study was also performed to evaluate the effects of the treatment on corneal transparency.
A penetrant linear 2-mm incision in the corneal region at the 5 o’clock position was made in the rat subjects. BPC 157 eye drops were then administered immediately after injury induction and then every 8 hours up to 120 hours. The control group was given an equal volume of distilled water on the injured eye. Results showed that the administration of BPC 157 eye drops significantly accelerated the healing process, starting 24 h after the injury. In addition, The Seidel test and fluorescein test became negative. The Seidel test assesses for corneal defects while the fluorescein test determines the presence of a foreign body in the eye. The corneal injury completely healed at 72 hours in the BPC 157-treated group.
In conclusion, BPC 157 can help treat corneal injury and regain corneal transparency. The use of BPC 157 eye drops can successfully close perforating corneal incisions in rats.
You can read the full article at at https://www.sciencedirect.com/science/article/abs/pii/S0014483515001360?via%3Dihub.- Masnec S, Kokot A, Zlatar M, et al. Perforating corneal injury in rat and pentadecapeptide BPC 157. Exp Eye Res. 2015;136:9-15.
Perforating corneal injury in rat and pentadecapeptide BPC 157
The deterioration of collagenous stromal tissue, which was previously considered a simple physical breakdown known as corneal melting, is now recognized as corneal ulceration. The study hypothesized that the stable gastric pentadecapeptide BPC 157 could aid in healing corneal ulcerations in rats, allowing them to recover corneal transparency. BPC 157 has been found to have positive effects on various bodily systems, including the NO system, NSAID toxicity, gastrointestinal tract, wound healing, blood vessels, and bleeding. BPC 157 is an anti-ulcer peptide that maintains mucosal integrity and is stable in human gastric juice. It is typically administered alone, without a carrier, for systemic, peroral, or local application.
A study included rats that had poor spontaneous healing of corneal ulcerations. The Seidel test was positive, demonstrating corneal opacity and the fluorescein test was positive after 120 hours. The Seidel test assesses for corneal defects while the fluorescein test determines the presence of a foreign body in the eye. Aqueous cells, edema (swelling), fibrin, and granulation tissue were still present, and histology revealed an increase in granulocytes and mononuclear cells..
The use of BPC 157 eye drops was found to effectively heal corneal incisions in live rats. When combined with a specific protocol designed to speed up the healing process after removing the corneal epithelium, BPC 157 had quick positive effects on the epithelium. This included reducing the levels of granulocyte and mononuclear cells, resulting in fewer aqueous cells, granulation tissue, edema, and fibrin (a protein formed during blood clotting) formation.
Read the full article: https://www.sciencedirect.com/science/article/abs/pii/S0014483515001360?via%3Dihub.- Perovic D, Kolenc D, Bilic V, et al. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J Orthop Surg Res. 2019;14(1):199. Published 2019 Jul 2. doi:10.1186/s13018-019-1242-6.
Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats
In this study, the authors examined the healing properties of BPC 157, a stable gastric pentadecapeptide, in treating spinal cord injuries in rats. Although BPC 157 has been used in trials for treating inflammatory bowel disease and multiple sclerosis, its effects on spinal cord injury is not yet examined.
In animal models, BPC 157 has been found to possess anti-inflammatory properties and has been effective in promoting functional recovery and restoring somatosensory neurons in the sciatic nerve after transection (surgical incision), in healing brain injuries caused by concussive trauma, and in treating severe encephalopathies (a disease that affects brain structure or function). Furthermore, BPC 157 has been observed to have an impact on various molecular pathways.
A spinal cord damage surgery was performed in rat subjects followed by a single injection of BPC 157 treatment into the peritoneum. After laminectomy, the sacrocaudal spinal cord’s exposed dural sac was compressed for 60 seconds to cause the injury. After the injury, assessments were made at various intervals. The study found that injured rats treated with BPC 157 showed consistent clinical improvement and better motor function of the tail. BPC 157 also counteracted microscopic changes in the white and gray matter of the rats’ nerves and prevented the loss of motoneurons. The study also found a decreased number of large myelinated axons in the rats’ caudal nerves. EMG recordings showed a lower motor unit potential in the tail muscle.
Overall, the study suggests that BPC 157 may have therapeutic potential for treating nerve injuries. BPC 157 therapy was found to have a positive effect on all stages of the secondary injury phase after spinal cord injury, as it was able to counteract axonal and neuronal necrosis, demyelination, and cyst formation. The functional recovery observed suggests that BPC 157 can help prevent these types of damage.
You can read the full article at at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6604284/.- Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, et al. BPC157 as potential agent rescuing from cancer cachexia. Curr Pharm Des. 2018;24(18):1947–1956. doi: 10.2174/1381612824666180614082950.
BPC 157 as Potential Agent Rescuing from Cancer Cachexia
A metabolic syndrome called cancer cachexia can affect more than 50% of cancer patients who are nearing the end of their life. As a result of the loss of fat and muscle tissue, this condition causes significant weight reduction. Cachexia, which is responsible for up to 20% of cancer-related fatalities, is now untreatable. To create successful treatments, it is essential to comprehend the molecular mechanisms causing cachexia. The primary events that drive cachexia are related to the central nervous system or inflammation, leading to reduced food intake, muscle breakdown, and other metabolic disturbances. Nutritional support, anti-inflammatory agents, and exercise have been tried, but none have been effective. Combining therapies, such as using megestrol acetate and anti-inflammatory agents, along with novel therapeutics like BPC157, a cytoprotective agent found in gastric juices, may hold promise for treating cachexia. This review proposes using BPC157 to treat cancer cachexia and provides evidence for its effectiveness and mode of action before conducting clinical trials.
You can read the full article at at https://pubmed.ncbi.nlm.nih.gov/29898649/.- Vukojević J, Siroglavić M, Kašnik K, et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. VasculPharmacol. 2018;106:54-66. doi:10.1016/j.vph.2018.02.010.
Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157
The process of tying off the inferior vena cava (largest vein of the body) of rats, including the right ovarian vein, is often used to recreate the effects of Virchow’s triad (characterized by blood vessel damage, stasis of blood flow, and hypercoagulability). This involves causing damage to blood vessels, creating a blockage, and altering blood flow. However, it was discovered that treatment with BPC 157 was able to reduce or reverse all of these effects and the associated symptoms.
In this study, BPC 157 (10 g, 10 ng/kg) was used either as an early regimen or a delayed therapy. Microscopy, venography, hemorrhage, blood pressure, ECG, thermography, MDA and nitric oxide (NO) levels in plasma and ICV, and gene expression are all included in the assessment process. BPC 157 therapy was found to have multiple beneficial effects in rats subjected to inferior vena cava ligation, including reducing vein injury, preventing blood clot formation and low platelet count, and stopping prolonged bleeding. The treatment also facilitated the development of new blood vessels and redirected blood flow, which in turn alleviated venous hypertension, arterial hypotension, and tachycardia. Additionally, BPC 157 increased the amount of nitric oxide in the plasma while reducing harmful reactive oxygen species in the tissue. Gene expression changes in the veins indicated both increases and decreases in certain genes, while others remained unchanged.
These findings suggest that treating rats with BPC 157 can significantly reduce or completely prevent all the harmful effects of ligation of the inferior vena cava.
You can read the full article at at https://www.sciencedirect.com/science/article/abs/pii/S1537189117301945?via%3Dihub.- Sebecić B, Nikolić V, Sikirić P. Osteogenic effect of a gastric pentadecapeptide, BPC-157, on the healing of segmental bone defect in rabbits: a comparison with bone marrow and autologous cortical bone implantation. Bone. 1999; 24(3):195-202.
Osteogenic effect of a gastric pentadecapeptide, BPC-157, on the healing of segmental bone defect in rabbits: a comparison with bone marrow and autologous cortical bone implantation
Gastrectomy, or the removal of the stomach, often leads to an increased risk of osteoporosis, metabolic issues, and fractures. A new peptide called BPC-157, which has been shown to improve wound and fracture healing in rats and has angiogenic properties (stimulate the formation of new blood vessels), is studied in this research.
The researchers created a bone defect in rabbits that didn’t heal properly for six weeks and then administered BPC-157 in three different ways: locally into the bone defect, intramuscularly intermittently, or continuously. They compared this to the standard treatment of filling the bone defect with an autologous cortical graft and injecting the rabbits with their own bone marrow. Control rabbits were injected with saline. BPC-157 significantly improved the healing of the bone defect, as measured by radiographic assessment, microphotodensitometry, and quantitative histomorphometry, with similar results to the use of bone marrow or an autologous cortical graft. Additionally, a comparison of the proportion of animals with healed defects (complete bony continuity across the defect site) and unhealed defects (all controls) indicated that in addition to pentadecapeptide intramuscular application for 14 days (i.e., local application of bone marrow or autologous cortical graft), following other pentadecapeptide BPC-157 regimens (local application or intermittent intramuscular administration), the proportion of animals with healed defects was also increased.
The potential relevance of this pentadecapeptide BPC-157 effect (local or intramuscular effectiveness, lack of unwanted effects) could hopefully be a basis for methods of choice in the future management of healing impairment in humans.
athttps://www.sciencedirect.com/science/article/abs/pii/S875632829800180X?via%3Dihub.- Retrieved from http://www.fasebj.org/content/28/1_Supplement/844.11?related-urls=yes&legid=fasebj;28/1_Supplement/844.11.
- Retrieved from https://www.dovepress.com/role-of-nutrients-in-metabolic-syndrome-a-2017-update-peer-reviewed-fulltext-article-NDS.
Role of nutrients in metabolic syndrome: a 2017 update
Metabolic syndrome (MetS) and the chronic diseases associated with it, such as cardiovascular disease and type 2 diabetes, are major public health concerns worldwide, such as in the United States. Nutrition is one of the recommended preventive measures to manage these chronic diseases because good health is an investment in economic growth. However, it is not clear how nutrients can be beneficial in improving MetS. To address this question, a literature review was conducted on the emerging human data on single nutrients and MetS. The review covered macronutrients, micronutrients, polyphenols, and other compounds, as well as select lifestyle factors that may contribute to MetS. Observational studies have shown positive evidence supporting the beneficial role of numerous nutrients in MetS. While some clinical trials have confirmed these results, causality is not always clear or consistent across trials. Nutrition and health are complex and dynamic systems, so it is important to consider a holistic approach that integrates groups or classes of nutrients, lifestyle influencers, and population relevance when designing confirmatory trials to investigate nutrient(s) and MetS.
https://www.dovepress.com/role-of-nutrients-in-metabolic-syndrome-a-2017-update-peer-reviewed-fulltext-article-NDS- Retrieved from https://www.sciencedirect.com/science/article/pii/S0928425797894740.
RPentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats
The pentadecapeptide BPC 157, which is a crucial part of the gastric juice peptide known as BPC, has acute anti-inflammatory and analgesic actions in addition to protecting against gastrointestinal and liver damage. In order to learn more about its impact on gastrointestinal injuries brought on by non-steroidal anti-inflammatory drugs and chronic inflammatory disorders such as adjuvant arthritis, researchers conducted tests on rats. In the gastrointestinal studies, BPC 157 was administered to the rats at the same time as or 1 hour before the application of non-steroidal anti-inflammatory agents (NSAIAs) such as indomethacin, aspirin, and diclofenac. In the adjuvant arthritis studies, BPC 157 was given either as a single dose or in a once-daily regimen. The development of lesions was reduced in rats that received BPC 157 compared to those that did not. Even rats with established adjuvant arthritis experienced beneficial effects after taking BPC 157 for just 2 weeks and these effects persisted after 1 year of treatment.
Overall, the results suggest that BPC 157 has unique anti-inflammatory and protective effects on mucosal integrity.
You can read the full article at at https://www.sciencedirect.com/science/article/abs/pii/S0928425797894740.- Bódis B, Karádi O, Németh P, Dohoczky C, Kolega M, Mózsik G. Evidence for direct cellular protective effect of PL-10 substances (synthesized parts of body protection compound, BPC) and their specificity to gastric mucosal cells. Life sciences. 1997; 61(16):PL 243-8.
Evidence for direct cellular protective effect of PL-10 substances (synthesized parts of body protection compound, BPC) and their specificity to gastric mucosal cells
Four PL-10 compounds, which are synthesized components of BPC (a protein that protects the human body and is comprised of 14-15 amino acids), were the subject of the study. Both newly isolated rat gastric mucosal cells and a mouse myeloma cell line (Sp2/0-Ag14) were used in the study, which used an ethanol-induced cell damage paradigm. It was revealed that none of the four chemicals were hazardous to cells. Among them, two substances were found to be effective in protecting gastric mucosal cells from ethanol-induced cell damage. These two substances were PL 10.1.15AK-3 in 5 dose and PL 10.1.AK14-2 in a dose-dependent manner, with an ED50 of 50. However, this cytoprotective effect was not observed on mouse myeloma cells. These findings suggest that BPC’s in vivo protection could be due to its direct cellular protective effect on gastric mucosal cells.
You can read the full article at at https://pubmed.ncbi.nlm.nih.gov/9353174/.- Prkacin I., Aralica G., Perovic D., Separovic J., Gjurasin M., Lovric-Bencic M., Stancic-Rokotov D., Ziger T., Anic T., Sikiric P., et al. Chronic cytoprotection: Pentadecapeptide BPC 157, ranitidine and propranolol prevent, attenuate and reverse the gastric lesions appearance in chronic alcohol drinking rats. J. Physiol. Paris. 2001;95:295–301. doi: 10.1016/S0928-4257(01)00041-9.
Chronic cytoprotection: pentadecapeptide BPC 157, ranitidine and propranolol prevent, attenuate and reverse the gastric lesions appearance in chronic alcohol drinking rats
The effects of chronic alcohol administration on rats and the anti-ulcer effects of the gastric pentadecapeptide BPC 157 were investigated. The ulcerogenic effect of continuous alcohol treatment is less studied than the severe stomach damage that is initially generated by ethanol injection in rats. The emphasis was on how chronic alcohol-consuming rats’ stomach lesions were prevented or reversed by BPC 157, ranitidine, and propranolol.
A group of rats received BPC 157 once daily throughout 10 days preceding alcohol consumption, throughout the study, and throughout the last month of alcohol consumption. Gastric lesions were then assessed. The results showed that BPC 157, ranitidine, and propranolol can prevent, attenuate or reverse the appearance of gastric lesions in chronically alcohol-drinking rats. Additionally, these treatments have a beneficial effect on liver lesions and portal hypertension, with the exception of ranitidine.
This study suggests that BPC 157, ranitidine, and propranolol may be used for further therapy in treating gastric lesions induced by chronic alcohol consumption.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11595453/.- Jelovac N, Sikiric P, Rucman R. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. European journal of pharmacology. 1999; 379(1):19-31.
Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats
The pentadecapeptide BPC 157, which consists of the amino acid sequence Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val and has a molecular weight of 1419, is known for its protective effects in the gastrointestinal tract and other organs. Recent research has shown that it has a particular impact on dopamine systems. It can block the negative effects of acute amphetamine exposure in rats and prevent the development of haloperidol-induced supersensitivity to amphetamine in mice.
The focus of this study was to determine whether BPC 157 could alleviate the immediate effects of neuroleptics (antipsychotics), especially catalepsy (prolonged muscular rigidity and immobility). According to the study, significant catalepsy in mice given haloperidol or fluphenazine was significantly lessened when BPC 157 was also administered. Over the majority of the trial time, there were much fewer cataleptic mice and catalepsy appeared later in mice receiving lower doses of neuroleptics. Also, when mice were given haloperidol, fluphenazine, sulpiride, or clozapine, BPC 157 lessened their somatosensory disorientation. When given together with haloperidol, BPC 157 fully blocked the lesions that would have otherwise become visible 24 hours later in control rats.
Taken together, the findings suggest that BPC 157 has a significant impact on the dopamine system and can interfere with some steps involved in catalepsy and/or ulcer formation, both centrally and peripherally.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299999004860?via%3Dihub.- Xue XC, Wu YJ, Gao MT. Protective effects of pentadecapeptide BPC 157 on gastric ulcer in rats. World journal of gastroenterology. 2004; 10(7):1032-6.
Protective effects of pentadecapeptide BPC 157 on gastric ulcer in rats
To compare the results in the treatment of human gastric ulcers using various administration techniques and to investigate the protective effects of gastric pentadecapeptide BPC 157 on acute and chronic gastric ulcers in rats.In this study, rats were given either a single or continuous dose of the peptide BPC 157 via either their stomach or muscles. This was done before or after inducing acute or chronic gastric ulcers. The rats were then sacrificed to observe how BPC 157 protected against these ulcers.The study found that both intramuscular and intragastric administration of BPC 157 reduced ulcer size and improved healing in different models of induced ulcers in rats. The effect was better with intramuscular administration. Higher doses of BPC 157 showed significantly less lesion and a varied inhibition rate of ulcer formation, except in one model where the inhibition rate was lower. When compared to famotidine, BPC 157 had a better inhibition rate. Continuous application of BPC 157 in a chronic ulcer model accelerated rebuilding of glandular epithelium and formation of granulation tissue.The gastric pentadecapeptide BPC 157, which can be given intramuscularly or intravenously, seems to have the ability to treat acute stomach ulcers in rats and counteract the long-lasting effects of acetate challenge on chronic ulcers. BPC 157 works better when administered intravenously (im) than when administered intravenously (ig), and the effective dosage of the former is smaller than the latter.
Read the full article:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4717094/.
- Stancic-Rokotov D, Slobodnjak Z, Aralica J. Lung lesions and anti-ulcer agents beneficial effect: anti-ulcer agents pentadecapeptide BPC 157, ranitidine, omeprazole and atropine ameliorate lung lesion in rats. Journal of physiology, Paris. ; 95(1-6):303-8.
Lung lesions and anti-ulcer agents beneficial effect: anti-ulcer agents pentadecapeptide BPC 157, ranitidine, omeprazole and atropine ameliorate lung lesion in rats
Anti-ulcer agents have the potential to alleviate injuries outside of the gastrointestinal tract due to their ability to protect gastrectomized rats, indicating a direct cytoprotective effect. This suggests that they may be useful in treating lung and stomach lesions. Rats were subjected to an intratracheal HCl instillation to induce lung lesions, followed by an intragastric instillation of ethanol to induce gastric lesions. The subsequent ethanol-gastric lesion was observed to be considerably worsened in the lung-injured rats. However, this aggravation did not affect the severity of the lung lesions for at least an hour.An intraperitoneal injection of the gastric pentadecapeptide GEPPPGKPADDAGLV, ranitidine, atropine, and omeprazole was given to the rats as a part of the study. Even though they weren’t immediately noticeable after a single application, the lung lesions were significantly reduced when the preventive and therapeutic regimes were combined. In single application studies, co-treatment and pre-treatment with pentadecapeptide BPC 157 and atropine (but not ranitidine and omeprazole) consistently reduced the severity of the lung lesions, whereas when used therapeutically, atropine, pentadecapeptide BPC 157 at the highest dose, and omeprazole reversed the otherwise more severe lung lesions.Overall, the study suggests that anti-ulcer agents may have therapeutic potential in treating injuries outside of the gastrointestinal tract, particularly when administered prophylactically or in combination with other agents.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11595454/.- Sikiric P, Seiwerth S, Grabarevic Z. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life sciences. 1994; 54(5):PL63-8.
The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides
A new 15 amino acid peptide called BPC 157 was found to protect the stomach and duodenum while also having anti-inflammatory effects. It is a part of a larger peptide found in gastric juice. In experiments done on rats, BPC 157 was compared to several other treatments for ulcers in three different experimental models. BPC 157 was the only treatment that consistently showed effectiveness in all models. The other treatments, including bromocriptine, amantadine, famotidine, cimetidine, and somatostatin, were not effective in the restraint stress model. Glucagon, NPY, and secretin showed a dose-dependent protection in the cysteamine model and a partial positive effect in the ethanol model. However, CCK/26-30 was not effective. Studies using Monastral blue showed that BPC 157’s beneficial effects are related to strong endothelial protection.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7904712/.- Djakovic Z, Djakovic I, Cesarec V, et al. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World Journal of Gastroenterology. 2016;22(41):9127-9140. doi:10.3748/wjg.v22.i41.9127.
Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME
To treat rats with esophagogastric anastomosis, which is often fatal, in particular to restore sphincter function and anastomosis healing.We conducted research on rats with esophagogastric fistulas to examine into the possible advantages of employing stable gastric pentadecapeptide BPC 157 because we think that esophagogastric fistulas are a particular kind of handicap connected to the NO-system. It has been demonstrated that this peptide promotes the repair of intestinal anastomosis and fistulas, as well as esophagitis and gastric lesions, while also restoring sphincter function. It has also been studied for its efficacy in treating illnesses including ulcerative colitis and multiple sclerosis. BPC 157 is known to interact with the NO-system in particular. In our study, rats underwent esophagogastric anastomosis and were treated with different combinations of BPC 157, L-NAME, and L-arginine. We monitored the rats’ stomach damage, esophagitis, anastomosis strength, esophageal and pyloric sphincter pressure, weight loss, and mortality. We also assessed the immediate effects on blood vessels at the stomach surface after anastomosis creation.The study found that BPC 157 had a positive effect on the disease course and eliminated mortality when used in combination with L-NAME or L-arginine in rats undergoing esophagogastric anastomosis. BPC 157 counteracted the negative effects of L-NAME, improved gastric and esophageal lesions, and demonstrated an innate NO-system disability. BPC 157 had a distinctive effect on corresponding events, either counteracting worsening or improving conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5107594/.- Klicek R., Sever M., Radic B., Drmic D., Kocman I., Zoricic I., Vuksic T., Ivica M., Barisic I., Ilic S., et al. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: Role of the nitric oxide-system. J. Pharmacol. Sci. 2008;108:7–17. doi: 10.1254/jphs.FP0072161./PMC5107594/.
Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system
The study examined the therapeutic effects of a gastric pentadecapeptide known as BPC 157 on rats with persistent colocutaneous fistula and how it interacts to nitric oxide (NO). This peptide is safe to use in the treatment of inflammatory bowel disease and intestinal anastomosis therapy, according to earlier clinical trials. The researchers treated rats with BPC 157 by intraperitoneal injection or drinking water, either alone or in combination with other drugs, and we observed the development of their healing during a 28-day period. It was found that BPC 157 accelerated the healing of colonic and skin defects, leading to the closure of the fistula, as observed through macroscopic, microscopic, biomechanical, and functional analyses. However, the co-administration of N(G)-nitro-L-arginine methyl ester (L-NAME) aggravated the healing failure of the fistula, skin, and colon wounds. L-arginine was effective only in combination with L-NAME, but not on its own. The beneficial effects of BPC 157 were not affected by the blunting of NO-generation or the addition of NO substrate or other substances. Sulphasalazine had a moderate effect, while corticosteroid had an aggravating effect.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/18818478/.- Sikiric P., Petek M., Rucman R., Seiwerth S., Grabarević Z., Rotkvić I., Jagić V., Turković B., Mildner B., Duvnjak M., et al. The significance of the gastroprotective effect of body protection compound (BPC): Modulation by different procedures. Acta Physiol. Hung. 1992;80:89–98.
The significance of the gastroprotective effect of body protection compound (BPC): modulation by different procedures
To know how a body defends itself, researchers must study how specific organs affect observed events. Its capacity to defend the stomach has been investigated in the case of Body Protection Compound (BPC), a peptide found in gastric juice. Before subjecting rats to 48 hours of restraint stress, researchers removed or performed sham operations on certain organs, including the vagus nerve, ovaries, testes, spleen, adrenal medulla, and thyroid and parathyroid glands. The rats were then intraperitoneally administered either BPC or saline.The results showed that BPC had a strong protective effect on the stomach in baseline conditions, but this effect was abolished by ovariectomy and demedullation, decreased by thyroparathyroidectomy, and unaffected by vagotomy, splenectomy, or orchidectomy. These findings suggest that the beneficial effects of BPC are complex and specific to sex-related factors and that stomach protection plays a crucial role in organo-protection. These findings may also mediate the suggested “stomach stress organoprotective response” and may have implications for protecting other organs such as the intestines, kidneys, liver, pancreas, inflammation, diabetes mellitus, and delayed type of hypersensitivity.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1345210/.- Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr Pharm Des. 2018;24(18):1972–1989. doi: 10.2174/1381612824666180712110447.
BPC 157 and Standard Angiogenic Growth Factors. Gastrointestinal Tract Healing, Lessons from Tendon, Ligament, Muscle and Bone Healing
It is well known that angiogenic growth factors aid in the promotion of healing. However, the mechanisms underlying gastrointestinal ulceration and the pharmacological/pathophysiological roles of different angiogenic growth factors in wound healing are not fully understood. This research project focused on a particular peptide, stable gastric pentadecapeptide BPC 157, and compared its efficacy for promoting healing with basic peptidergic angiogenic growth factors such as EGF, FGF, and VEGF. The study also examined how healing in the gastrointestinal tract may be related to healing in other tissues such as bone, muscle, and tendons.The results showed that BPC 157 was consistently effective in promoting healing in various models of acute and chronic injuries to the esophagus, stomach, duodenum, and lower gastrointestinal tract, regardless of the method of administration. In contrast, the effectiveness of other growth factors varied depending on the injury type and the delivery system used. The study concludes that BPC 157 represents a unique pharmacological and pathophysiological role among various peptidergic growth factors in promoting healing across different tissues, including the gastrointestinal tract.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29998800/.- Sikiric P, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr Pharm Des. 2018;24(18):1990–2001. doi: 10.2174/1381612824666180608101119.
Novel Cytoprotective Mediator, Stable Gastric Pentadecapeptide BPC 157. Vascular Recruitment and Gastrointestinal Tract Healing
Several years ago, a new cytoprotective agent called stable gastric pentadecapeptide BPC 157 was discovered. This particular anti-ulcer peptide is native in human gastric juice and maintains the integrity of the GI-tract mucosa. It has been investigated in research studies for ulcerative colitis and multiple sclerosis, and it has been shown to cure lesions in different organs. Although the concept of stomach cytoprotection and cell protection and endothelium security within the stomach has been well elaborated, there has been little research on the protection of cells and endothelium outside of the stomach. But by focusing on these two areas, BPC 157 has demonstrated to have even more advantageous effects than typical cytoprotective drugs, offering cell and endothelium protection outside the stomach as well. BPC 157 has been found to lessen bleeding duration and thrombocytopenia, as well as inhibit the production of new thrombi and reverse those that have already formed. Additionally, it has been demonstrated to cause blood vessels to “run” toward defects after a perforated injury and towards bypassing defects after an obstruction, resuming blood flow and changing the course of the injured tissue. Accordingly, BPC 157 is a prototype cytoprotective agent that, depending on the kind of injury, perforated defect, or vessel obstruction, can regulate blood vessel function. Overall, BPC 157 represents the third and most important part of the cytoprotection concept. By protecting stomach cells, endothelium, and blood vessels, it can recover mucosal integrity and practically implement the cytoprotection concept in different organs.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29879879/.- Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19(1):126–132. doi: 10.2174/092986712803414015.
Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157
The consistent gastric pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419) is a promising new drug that is immune to human gastric acid and has been shown to be useful in both the upper and lower gastrointestinal tracts without causing any side effects. It has shown antiulcer effects, has been effective in treating inflammatory bowel disease (IBD) (PL 14736), has a favorable safety profile, and exhibits notable wound healing abilities. BPC 157 has been found to interact with the NO-system, which protects the endothelium and promotes angiogenesis, even in severely impaired conditions. This has been attributed to its ability to stimulate the expression of the early growth response 1 gene, which is responsible for generating cytokines and growth factors, as well as promoting early extracellular matrix formation (collagen). This is particularly important for countering the severe complications associated with advanced and poorly controlled IBD. Animal studies have shown that BPC 157 has significant potential for further IBD therapy, particularly in cases of advanced intestinal anastomosis healing, reversed short bowel syndrome, and fistula healing. Furthermore, the absence of toxic effects, negative limit test, failure to achieve LD1, and the lack of side effects in clinical trials may help to address the concerns that commonly arise with the long-term use of peptidergic agents.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22300085/.- Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: new insights. World J Gastroenterol. 2017;23(48):8465–8488. doi: 10.3748/wjg.v23.i48.8465.
Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights
The colon segment without blood supply was treated with different medications, including BPC 157, L-NAME, L-arginine (alone or combined), and saline. During the process of reperfusion, either BPC 157 or saline was used as medication. The presentation of blood vessels in ischemic colitis (IC-rats) or reperfusion (IC + RL-rats) was recorded using a USB microscope camera for the next 15 minutes. The level of oxidative stress was measured by MDA levels, which increased in both IC and IC + RL-rats, and NO levels, which decreased in IC-rats and increased in IC + RL-rats, in the colon tissue. Additionally, IC-rats that had additional colon obstruction (OB) were studied for 3 days (IC + OB-rats) and then received a BPC 157 bath. Most frequently, in a colon segment (25 mm, 2 ligations on the left colic artery and vein, 3 arcade vessels within the ligated segment), BPC 157 (10 g/kg) bath (1 mL/rat) increased vessel presentation, inside/outside arcade interconnections quickly reappeared, mucosal folds were preserved, and the pale areas were small and noticeably reduced. BPC 157 counteracted worsening effects induced by L-NAME (5 mg) and L-arginine (100 mg). MDA- and NO-levels were normal in BPC 157 treated IC-rats and IC + RL-rats. In addition, on day 10, BPC 157-treated IC + OB-rats provided almost completely spared mucosa with very small pale areas and no gross mucosal faults; the treated colon segment was of normal diameter, and only small adhesions were visible.BPC 157 is a crucial therapy that efficiently reinstates the blood flow to the region that suffered from ischemic injury and swiftly triggers alternate blood vessels. The process entails the participation of the NO system.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752708/.- Sikirić P, Seiwerth S, Grabarević Z. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. European journal of pharmacology. 1997; 332(1):23-33.
The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure
The study aimed to investigate the effects of pentadecapeptide BPC157 on gastric lesions and blood pressure maintenance in rats. The experiment involved administering BPC157, L-NAME (a competitive inhibitor of endothelium nitric oxide generation), and L-arginine (an NO precursor) to the rats. In the gastric lesions assay, BPC157 and L-arginine had an antiulcer effect, but L-NAME had no effect. The combination of L-NAME and L-arginine impaired the effect of BPC157 on gastric lesions. In blood pressure studies, BPC157 had a mimicking and preventive effect on blood pressure compared to L-arginine. However, the effect of BPC157 disappeared when administered after the L-NAME and L-arginine combination, which still led to a blood pressure increase. The study aimed to investigate the effects of pentadecapeptide BPC157 on gastric lesions and blood pressure maintenance in rats. The experiment involved administering BPC157, L-NAME (a competitive inhibitor of endothelium nitric oxide generation), and L-arginine (an NO precursor) to the rats. In the gastric lesions assay, BPC157 and L-arginine had an antiulcer effect, but L-NAME had no effect. The combination of L-NAME and L-arginine impaired the effect of BPC157 on gastric lesions. In blood pressure studies, BPC157 had a mimicking and preventive effect on blood pressure compared to L-arginine. However, the effect of BPC157 disappeared when administered after the L-NAME and L-arginine combination, which still led to a blood pressure increase.In vitro, BPC157 induced the generation of NO in gastric mucosa from rat stomach tissue homogenates, similar to L-arginine, but the BPC157 effect could not be inhibited by L-NAME, even at a higher dose. When BPC157 and L-arginine were combined, NO synthesis was blunted.Overall, the study suggests that BPC157 can interfere with the effects of NO on gastric mucosal integrity and blood pressure maintenance in a specific way, especially with L-arginine. The findings could have implications for the development of novel therapies for gastric lesions and blood pressure regulation.
Read the full article:https://www.mdpi.com/1420-3049/19/11/19066
- Sikiric P, Seiwerth S, Rucman R. Stress in Gastrointestinal Tract and Stable Gastric Pentadecapeptide BPC 157. Finally, do we have a Solution? Current pharmaceutical design. 2017; 23(27):4012-4028.
Stress in Gastrointestinal Tract and Stable Gastric Pentadecapeptide BPC 157. Finally, do we have a Solution?
Selye’s stress triad is a response to harmful agents that aims to restore the body’s balance. This response triggers the release of growth factors, particularly in the gastrointestinal tract, which are important for healing. One of these factors is the gastric pentadecapeptide BPC 157, which has a wide range of beneficial effects and acts as an integrative mediator that helps the body adapt to stress. Clinical trials have shown that BPC 157 is effective in healing gastrointestinal tract and extragastrointestinal tissues such as skin, tendon, ligament, muscle, bone, nerve, cornea, and brain. BPC 157 is stable in gastric juice and has beneficial effects on gastric and intestinal tract lesions and adaptation, wounds and fistulas healing, blood vessels, somatosensory neurons, and side effects of NSAIDs, pancreas, liver, brain lesions, and blood disturbances.BPC 157 interacts with the gut-brain axis and the NO-system to counteract complications of L-NAME and L-arginine applications. It also affects gene functions, which suggests its potential for further therapy. Overall, BPC 157 is an integrative mediator that helps the body adapt to stress, and its healing properties make it a promising therapy for various conditions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28228068/.- Sikiric P, Seiwerth S, Rucman R. Stable gastric pentadecapeptide BPC 157-NO-system relation. Current pharmaceutical design. 2014; 20(7):1126-35.
Stable gastric pentadecapeptide BPC 157-NO-system relation
This article discusses the potential healing effects of a peptide called BPC 157 in relation to the NO-system, which plays an important role in maintaining vascular integrity and controlling platelets. The article explains that BPC 157 has been shown to promote healing in a variety of injury models, reducing the risk of thrombosis or bleeding/thrombocytopenia. The peptide has also been found to be safe in high doses. The article suggests that BPC 157 may be effective at competing with L-arginine analogues and L-arginine, which are important in generating NO, a molecule that plays a role in many physiological processes. The article suggests that BPC 157 may be particularly effective in the gastric mucosa, where it may help protect against alcohol lesions and regulate blood pressure. Overall, the article suggests that BPC 157 has potential as a therapeutic agent in a range of conditions, including cardiovascular disturbances, healing failure, and alcohol intoxication. However, more research is needed to determine how BPC 157 can be practically used to enhance clinical performance.
https://www.ncbi.nlm.nih.gov/pubmed/23755725.- Prkacin I, Separovic J, Aralicia G. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. Journal of physiology, Paris. ; 95(1-6):315-24.
Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine
Liver damage and high blood pressure in the portal vein caused by chronic alcohol consumption can be treated with a stable gastric pentadecapeptide called BPC 157 and propranolol. This treatment can counteract liver lesions induced by 7.28 grams of alcohol per kilogram of body weight given in drinking water for three months in rats. The treatment is effective when administered either throughout the entire three-month period or during the last month only. Both prophylactic and therapeutic effects were observed. Control rats that consumed the same amount of alcohol consistently showed a significant increase in portal blood pressure values after two or three months of drinking. However, rats that received gastric pentadecapeptide BPC 157 or propranolol had reduced portal pressure levels compared to control rats. Liver weight, surface area and circumference of hepatocytes, and advanced steatosis (fat accumulation in the liver) were also decreased or eliminated in rats that received the treatment, and these improvements were related to reduced portal hypertension. In summary, BPC 157 and propranolol can prevent and reverse portal hypertension and liver damage caused by chronic alcohol consumption in rats. This treatment could be a promising therapy for liver diseases in humans caused by alcohol consumption.
https://www.ncbi.nlm.nih.gov/pubmed/11595456.- Barisic I, Balenovic D, Klicek R. Mortal hyperkalemia disturbances in rats are NO-system related. The life-saving effect of pentadecapeptide BPC 157. Regulatory peptides. 2013; 181:50-66.
Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157
In several contexts, including intraperitoneal, intragastric, and in vitro, the study showed that the stable gastric pentadecapeptide BPC 157 has the capacity to reverse the harmful effects of KCl overdose. The study discovered that BPC 157 could prevent the fatal side effects of KCl overdose, such as hyperkalemia, arrhythmias, muscle weakness, hypertension, and low pressure in the lower esophageal and pyloric sphincter when it was given alone or in conjunction with l-arginine. The study demonstrated a BPC 157-NO-system connection and emphasized its potential to save lives. According to the research, BPC 157 given 30 minutes before KCl may restore sinus rhythm, lessen QRS prolongation, and avoid asystolic pause. When given 10 minutes after KCl, BPC 157 began the rescue process within 5–10 minutes and finished it after an hour. Moreover, BPC 157 proved effective in treating additional hyperkalemia-related issues like muscle weakness, high blood pressure, and low sphincteric pressure.The study also found that intragastric BPC 157 application given 30 minutes before or 10 minutes after KCl was able to fully counteract severe stomach mucosal lesions, sphincter failure, and peaked T waves. In HEK293 cells, BPC 157 directly affected potassium conductance, counteracting the effect on membrane potential and depolarizations caused by hyperkalemic conditions. Overall, the study demonstrated the potential of BPC 157 as a life-saving treatment for KCl-overdose and related hyperkalemia-related disturbances.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23327997/.- Stambolija V, Stambolija TP, Holjevac JK. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. European journal of pharmacology. 2016; 781:83-91.
BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias
Pentadecapeptide BPC 157 has been shown to have beneficial effects in various medical scenarios. Specifically, it has been found to be effective in treating severe hyperkalemia and aiding in the recovery of skeletal muscle following an injury. Additionally, BPC 157 has been shown to reduce the paralytic effect caused by succinylcholine and prevent muscle damage and disability. In rats, BPC 157 was found to counteract hyperkalemia, arrhythmias, and a rise in serum enzyme levels caused by succinylcholine. The effects of BPC 157 were evaluated at various time points after succinylcholine administration, ranging from 3 minutes to 7 days. BPC 157 was administered in different ways, including intraperitoneally 30 minutes before or immediately after succinylcholine or per-orally in drinking water for 24 hours prior to succinylcholine administration. Regardless of the mode of administration, BPC 157 was effective in mitigating both local and systemic disturbances caused by succinylcholine.Succinylcholine-treated rats were saved by BPC 157, and this association between BPC 157, succinylcholine, and hyperkalemia was clearly demonstrated. This result is consistent with previous hyperkalemia problems and other supporting evidence. Therefore, the depolarizing neuromuscular blocker effects of succinylcholine were successfully antagonized.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299916302072?via%3Dihub.- Available from https://www.croris.hr/crosbi/publikacija/prilog-skup/562833.
The pentadecapeptide BPC 157 prevents and reverses cardiac arrythmias and sphincter pressure failure caused by hyperkalemia
Extended and severe high levels of potassium in the body can lead to reduced electrical activity in the heart, potentially resulting in cardiac arrest, as well as sustained pressure failure in the lower esophageal and pyloric sphincters of rats.
To investigate the impact of gastric pentadecapeptide BPC 157 on the effects of hyperkalemia on cardiac function and sphincter pressure, Wistar rats were given an injection of KCl solution and their ECG and serum values were continuously recorded. BPC 157 was administered either before or after the KCl solution to assess its effects on sphincter pressure and cardiac function. The control group showed significant sphincter pressure failure and progressive arrhythmias, along with peaked T waves, widened QRS-complex, and bradycardia leading to asystolic pauses and cardiac arrest.The sphincter pressure of the BPC 157 pre-treated animals, however, gradually increased to normal levels, and they had a constant sinus rhythm with just peak T waves. Despite partial arrhythmia improvement and sphincter pressure recovery, post-treated animals did not reverse prolonged hyperkalemia. Without treating hyperkalemia, BPC 157 can prevent and treat sphincter pressure failure and arrhythmias. The potential method by which BPC 157 may activate Na/K ATPase and regulate the impact of hyperkalemia on cell membrane potential in HEK-293 cells is currently being investigated.
You can read the abstract of this article at https://www.croris.hr/crosbi/publikacija/prilog-skup/562833.- Zivanovic-Posilovic G, Balenovic D, Barisic I. Stable gastric pentadecapeptide BPC 157 and bupivacaine. European journal of pharmacology. 2016; 793:56-65.
Stable gastric pentadecapeptide BPC 157 and bupivacaine
The negative effects of accidental bupivacaine toxicity are currently without a proven medicinal cure. Yet, there are strong arguments in favor of putting BPC 157 to the test as an in vivo and in vitro remedy for rat bupivacaine toxicity. They include the fact that BPC 157 has no hazardous side effects, that it has been shown to reduce arrhythmias and hyperkalemia in rats, and that it can prevent and treat bupivacaine cardiotoxicity, even in extreme cases. High doses of bupivacaine caused a variety of deadly cardiovascular side effects in rats, including bradycardia, ventricular ectopies, and asystole. Yet, even in situations where the QRS complex was significantly extended, the treatment of BPC 157 either before or after the injection of bupivacaine was successful in reversing these effects. When administered following the emergence of protracted QRS intervals, BPC 157 was effective in delaying the fatal outcome. In addition, BPC 157 was shown to inhibit the depolarization of cell membranes induced by bupivacaine in HEK293 cells, suggesting that it has the potential to act as an antidote for bupivacaine cardiotoxicity. Overall, the results suggest that BPC 157 may be a promising candidate for the treatment of bupivacaine toxicity.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299916306756?via%3Dihub.- Strinic D, Belosic Halle Z, Luetic K. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life sciences. 2017; 186:66-79.
BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats
Prokinetics and neuroleptics frequently cause a longer QTc interval. In this study, Wistar rats were given daily doses of dopamine neuroleptics or prokinetics, and stable stomach pentadecapeptide BPC 157 was discovered to decrease the lengthening of the QTc interval. Earlier, BPC 157 reduced catalepsy and stomach ulcers brought on by neuroleptics in rats and mice.In order to counteract the QTc interval prolongation caused by neuroleptic or prokinetic drugs, rats were administered a daily regimen of BPC 157 for seven days, immediately after each dose of haloperidol, fluphenazine, clozapine, quetiapine, sulpiride, or metoclopramide. The dosages of the neuroleptic/prokinetic drugs ranged from 0.5 to 25.0 mg/kg ip. Controls received saline. ECG readings were taken before and after drug administration at various intervals ranging from 1 to 30 minutes, as well as at 30 minutes, 60 minutes, and 24 hours after the first administration and subsequent administrations.Haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide have all been associated with a central effect in rats that is not present with domperidone: a prolonged QTc interval. The stomach pentadecapeptide BPC 157, which is stable, regularly counteracts the effects. Consequently, the QTc prolongation brought on by neuroleptics and prokinetics is quickly and effectively reversed by BPC 157.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28797793/.
- Retrieved from https://www.omicsonline.org/mortal-furosemide-hypokalemia-disturbances-in-rats-no-system-related-shorten-survival-by-l-name.-therapy-benefit-with-bpc-157-peptide-morethan-with-l-arginine-2155-9880.1000201.php?aid=6879.
Mortal Furosemide-Hypokalemia-Disturbances in Rats NO-System Related Shorten Survival by L-NAME. Therapy Benefit with BPC 157 Peptide More Than With L-Arginine
This study looked at the effects of furosemide (a diuretic) on rats and how different treatments can improve or worsen the associated hypokalemia (low potassium levels). The study focused on the NO-system (nitric oxide system) and its relation to the outcomes. The researchers analyzed various aspects of the rats’ cardiac and extra-cardiac manifestations, including electrocardiogram (ECG) readings, myoclonal activity, and lethality. The findings indicate that none of the NO-system-related medications, whether administered before or after furosemide, altered hypokalemia, but they did to some extent lessen the furosemide-forced diuresis. A NOS-blocker called L-NAME exacerbated mortality and extra- and intra-cardiac symptoms. L-arginine, a precursor of NO, and BPC 157, a peptide, on the other hand, shown some favorable benefits. The most successful therapy was BPC 157, which preserved sinus rhythm and avoided ventricular premature beats, ventricular tachycardia, and AV block. It also got rid of myoclonus in skeletal muscles.The study suggests that L-NAME and L-arginine are counteractive, while BPC 157 completely eliminates the negative effects of L-NAME without any additive benefit when combined with L-arginine. The findings provide potentially effective therapeutic interventions for acute hypokalemia.
You can read the full article at https://www.omicsonline.org/mortal-furosemide-hypokalemia-disturbances-in-rats-no-system-related-shorten-survival-by-l-name.-therapy-benefit-with-bpc-157-peptide-morethan-with-l-arginine-2155-9880.1000201.php?aid=6879.- Ilic S, Brcic I, Mester M. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2009; 60 Suppl 7:107-14.
Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats
In addition to other adverse effects like seizures, neuronal damage in the cerebral cortex and hippocampus, hepatomegaly, fatty liver, elevated serum levels of AST, ALT, and amylase, breakdown of liver glycogen leading to hypoglycemia, and calcification development, our research concentrated on the gastric ulcers induced by an overdose of insulin (250 IU/kg i.p.) in rats. The stable gastric pentadecapeptide BPC 157 (10 microg/kg) was given intraperitoneally or intragastrically as an antidote right away after the insulin overdose. Saline (5 ml/kg) in an equivalent volume was administered to the control group. 180 minutes later, the still-alive rats were assessed. It’s interesting to note that BPC 157 not only functioned as an anti-ulcer peptide but also blocked all of insulin’s harmful effects and avoided deadly results.The BPC 157 rats had higher blood glucose levels, less liver pathology, less damaged neurons in the brain, and only occasional small gastric lesions. The presentation of calcium deposits in the BPC 157 rats was mostly dot-like. Our findings suggest that BPC 157 may play a role in insulin control and can influence one or more processes after excessive insulin administration.
at a full article
https://www.ncbi.nlm.nih.gov/pubmed/20388953.- Medvidovic-Grubisic M, Stambolija V, Kolenc D. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017; 25(4):439-449.
Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen
A pentadecapeptide known as BPC 157 has been shown to have potential as a treatment for magnesium toxicity and its effects on cell depolarization in rats. The peptide was administered before a high-dose magnesium injection and may have an effect on the NO system. BPC 157 has previously been shown to counteract a range of conditions, including paralysis, arrhythmias, hyperkalaemia, extreme muscle weakness, parasympathetic and neuromuscular blockade, and injured muscle healing.In the study, magnesium sulfate was administered intraperitoneally to rats, causing hypermagnesemia, hyperkalaemia, brain lesions, and muscular paralysis. Then, several drugs, including BPC 157, L-NAME, and L-arginine alone or in combination, were administered to the rats. Moreover, HEK293 cells were employed to measure cell depolarization. The findings demonstrated that BPC 157 prevented hypermagnesemia and all disorders brought on by magnesium, including those made worse by L-NAME or L-arginine. Also prevented in the presence of BPC 157 was cell depolarization. L-NAME and L-arginine, on the other hand, exacerbated the clinical course and led to increased hypermagnesemia and developing hyperkalaemia. Overall, BPC 157 appears to counteract the initial event leading to hypermagnesemia and the life-threatening actions that follow a magnesium overdose. This study highlights the potential use of BPC 157 as a therapy for magnesium toxicity and its effects on cell depolarization.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28210905/.- Keremi B, Lohinai Z, Komora P. Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2009; 60 Suppl 7:115-22.
Antiinflammatory effect of BPC 157 on experimental periodontitis in rats
The study’s objective was to find out how a peptide named pentadecapeptide BPC 157 affected bone resorption and inflammation in rats with periodontitis. Across many tissues and organs, the peptide has previously shown anti-inflammatory and wound healing benefits. At the beginning, the scientists examined in rats the immediate impact of BPC 157 on gingival blood flow. The rats’ bottom left first molars were then wrapped in a silk ligature to simulate periodontitis. The following twelve days saw the rats given either BPC 157 or a placebo. The gingivomucosal tissues surrounding the molars on both sides were removed on the thirteenth day. The Evans blue plasma extravasation technique and histopathology were both used to gauge the degree of inflammation.The researchers also analyzed alveolar bone loss using microCT.The study found that BPC 157 had no effect on gingivomucosal blood flow. However, the silk ligature caused inflammation, alveolar bone destruction, and increased Evans blue extravasation in the gingivomucosal tissue. The administration of BPC 157 for 12 days significantly reduced plasma extravasation, histological alterations, and alveolar bone resorption. The results suggest that BPC 157 has potent anti-inflammatory effects on periodontal tissues in rats with ligature-induced periodontitis. The study demonstrates the potential of BPC 157 as a peptide candidate in the treatment of periodontal disease.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20388954/.- Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2009; 60 Suppl 7:191-6.
Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing
Angiogenesis is a complicated natural process that is regulated by numerous molecules, including those that encourage and prevent blood vessel formation. This procedure is vital to the healing process. One important component that affects angiogenesis is vascular endothelial growth factor (VEGF). Significant angiogenic and angiomodulatory activities of pentadecapeptide BPC 157 are known to support healing. In this study, we used in vitro and in vivo models to investigate the relationship between BPC 157’s angiogenic effects and the expression of VEGF.The results indicated that BPC 157 did not have a direct angiogenic effect on cell cultures. However, in vivo studies on animals with crushed muscle and transected muscle and tendon injuries showed that BPC 157 adequately modulated angiogenesis, resulting in more effective healing. Immunohistochemical analysis of muscle and tendon healing using VEGF, CD34, and FVIII antibodies showed that BPC 157 stimulated angiogenesis by up-regulating VEGF expression. Therefore, the angiogenic potential of BPC 157 seems to be closely related to the healing process in vivo, and it has a positive effect on angiogenesis by regulating VEGF expression.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20388964/.- Cox HD, Miller GD, Eichner D. Detection and in vitro metabolism of the confiscated peptides BPC 157 and MGF R23H. Drug testing and analysis. 2017; 9(10):1490-1498.
Detection and in vitro metabolism of the confiscated peptides BPC 157 and MGF R23H
Recently, authorities discovered new compounds in seized vials – a peptide called Body Protecting Compound 157 (BPC 157) and a variation of Mechano-Growth Factor (MGF) known as MGF R23H. BPC 157, which contains the amino acid sequence GEPPPGKPADDAGLV, is being studied for its potential to aid in the healing and recovery of various tissues. In laboratory tests, MGF R23H demonstrated good stability in plasma and is expected to be detectable in urine. BPC 157, on the other hand, forms a stable metabolite that can also be detected in urine. To detect BPC 157 in urine, a weak cation exchange solid phase extraction method was established and validated. The method is capable of detecting BPC 157 at levels as low as 0.1 ng/mL, has a precision of less than 20%, and is highly linear with an r2 value of 0.998. Moreover, BPC 157 remains stable in urine for at least four days. To increase the specificity of the method, the measurement of a potential BPC metabolite, in addition to the parent peptide, is recommended. In summary, these new compounds, BPC 157 and MGF R23H, were found in seized vials and are being investigated for their potential healing properties. A method for detecting BPC 157 in urine has been established, and the detection of a potential BPC metabolite is recommended for increased specificity.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/28035768/.- Sikiric P, Seiwerth S, Rucman R. Brain-gut Axis and Pentadecapeptide BPC 157: Theoretical and Practical Implications. Current neuropharmacology. 2016; 14(8):857-865.
Brain-gut Axis and Pentadecapeptide BPC 157: Theoretical and Practical Implications
Several growth factors that are naturally present in the gastrointestinal (GI) tract are involved in the connection between the brain and the stomach. Several of these elements have strong anti-ulcer effects and can help with problems of the central nervous system (CNS). The stable gastric
pentadecapeptide BPC 157, a peptidergic drug that is native to human stomach juice and has been shown to be safe in clinical studies for inflammatory bowel disease and multiple sclerosis, is the subject of this article.BPC 157 is a novel mediator of cytoprotection that helps to maintain the integrity of the GI mucosa.
It has been shown to be effective in treating a range of conditions, including GI tract disorders, periodontitis, liver and pancreas lesions, and various types of wounds. The pathways involved in BPC 157’s therapeutic effects include the Egr-1 gene, NAB2, FAK-paxillin, and JAK-2.Research has also found that when BPC 157 is administered peripherally, it can positively affect the central nervous system. Specifically, it can modulate serotonergic and dopaminergic systems, which can help to alleviate behavioral disturbances that arise from overstimulated or damaged neurotransmitter systems. Furthermore, BPC 157 has been found to have neuroprotective effects, such as protecting somatosensory neurons and promoting peripheral nerve regeneration after transection. Other than traumatic brain damage, spinal cord compression, NSAID and insulin overdose, and cuprizone encephalopathies, BPC 157 has also been proven to mitigate their deleterious effects.
BPC 157 is well tolerated in clinical trials and has no toxic side effects in addition to its therapeutic effects.BPC 157 is a promising gastric peptide that may one day be used to treat a number of CNS illnesses. It is a distinct and adaptable therapeutic agent due to its capacity to favorably impact both the gastrointestinal tract and the central nervous system. To completely comprehend its methods of action and to examine its possible applications in clinical practice, more study is required.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5333585/.- Hsieh MJ, Liu HT, Wang CN. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. Journal of molecular medicine (Berlin, Germany). 2017; 95(3):323-333.
Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation
A 15-amino acid peptide called BPC 157 has been found to have healing effects. Recent research has suggested that it might also help with angiogenesis, the growth of new blood vessels. The precise process by which BPC 157 accomplishes this, meanwhile, is still not entirely understood.
The purpose of this study was to look into how BPC 157 might be used therapeutically and how it might stimulate angiogenesis. The effect of BPC 157 on vessel density was examined both in vivo and in vitro using the chick chorioallantoic membrane (CAM) assay and the endothelial tube creation assay, respectively. In both experiments, they discovered that BPC 157 increased vascular density. Also, they showed that BPC 157 sped up blood flow recovery in the ischemic muscle of rats with hind limb ischemia using laser Doppler scanning, suggesting that it encourages angiogenesis.Further analysis of the hind limb muscle confirmed that BPC 157 increased the number of vessels and the expression of vascular endothelial growth factor receptor 2 (VEGFR2), which is a protein that is important for angiogenesis. In vitro studies using human vascular endothelial cells also confirmed that BPC 157 increased the mRNA and protein expression of VEGFR2, but not VEGF-A. The researchers also found that BPC 157 promoted the internalization of VEGFR2 in vascular endothelial cells, which was blocked in the presence of dynasore, an inhibitor of endocytosis. BPC 157 time-dependently activated the VEGFR2-Akt-eNOS signaling pathway, which is a pathway that is important for angiogenesis, and this activation was also suppressed by dynasore. In conclusion, this study demonstrated that BPC 157 promotes angiogenesis by increasing the expression and internalization of VEGFR2 and activating the VEGFR2-Akt-eNOS signaling pathway. This information could be useful in developing new therapies for conditions that involve impaired angiogenesis, such as cardiovascular diseases and tissue injuries.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/27847966/.- Škrlec K, Ručman R, Jarc E, et al. Engineering recombinant Lactococcus lactis as a delivery vehicle for BPC-157 peptide with antioxidant activities. Appl Microbiol Biotechnol. 2018;102(23):10103-10117. doi:10.1007/s00253-018-9333-6.
Engineering recombinant Lactococcus lactis as a delivery vehicle for BPC-157 peptide with antioxidant activities
Lactic acid bacteria (LAB) can be engineered to transport therapeutic proteins or peptides to mucosal surfaces, making them appealing candidates for expressing foreign proteins. The stomach-stable pentadecapeptide BPC-157 works to cure and prevent gastrointestinal inflammation by lowering the formation of reactive oxygen species (ROS). In this study, Lactococcus lactis was used as a delivery system to transfer BPC-157 to the growing medium either through secretion or surface display and trypsin shedding. By joining BPC-157 to basic membrane protein A (BmpA), the peptidoglycan binding domain of AcmA, or the Usp45 secretion signal, BPC-157 was made visible on the bacterial surface. The surface display of BPC-157 with AcmA/Usp45-fusion protein was almost 14 times greater, despite the fact that BmpA-fusion protein expression was higher than AcmA/Usp45-fusion protein expression.Anti-BPC-157 antibodies or mass spectrometry were employed to demonstrate the release of BPC-157 from the bacterial surface or isolated fusion proteins through trypsinization. A concentration of 30 ng/ml of BPC-157 was delivered via surface display with AcmA/Usp45-fusion, which increased to 117 ng/ml with Usp45 signal-mediated secretion, making the latter the most effective method of delivering BPC-157 through lactococcal transportation. Secreted BPC-157 considerably lowered ROS production in a 149BR fibroblast cell model, indicating its potential therapeutic benefit in treating intestinal inflammation. Furthermore, the current study’s comparison of various modes of small peptide delivery by L. lactis will aid in the future use of L. lactis as a peptide delivery vehicle.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/30191288/.- Sikiric P, Seiwerth S, Rucman R. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Current pharmaceutical design. 2013; 19(1):76-83.
Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157
There have been no toxicity reports for the peptide BPC 157, which has been proven to be safe for usage in humans. Clinical trials have proven it to be successful in treating inflammatory bowel disease and speeding up the healing process of wounds. BPC 157 may be utilized as an antidote to non-steroidal anti-inflammatory medicines, according to recent speculation (NSAIDs). In addition to adjuvant arthritis, pain, hyper/hypothermia, obstructive thrombus formation and thrombolysis, blood vessel function, prolonged bleeding, and thrombocytopenia after taking anticoagulants and antiplatelet agents, BPC 157 has been found to have beneficial effects on a variety of bodily injuries, including those to the stomach, duodenum, intestine, liver, and brain. The fact that BPC 157 has so many beneficial effects suggests that it may be useful as an antidote to NSAIDs, which have many negative side effects. Unlike NSAIDs, BPC 157 has a very high safety profile and has not been found to be toxic in any dose. The beneficial effects of BPC 157 were achieved using the same dosage level in both parenteral and peroral regimens. Overall, BPC 157 may have the potential to be an effective antidote against NSAIDs due to its wide range of beneficial effects and lack of toxicity.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22950504/.- Ilic S, Drmic D, Franjic S. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life sciences. 2011; 88(11-12):535-42.
Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions
In this study, researchers investigated the efficacy of the stable gastric pentadecapeptide BPC 157 in counteracting the toxic side effects of diclofenac, a nonsteroidal anti-inflammatory drug (NSAID), on the gastrointestinal, liver, and brain systems in rats. BPC 157 is an anti-ulcer peptide that has been found to be effective in treating inflammatory bowel disease and various wounds without causing any toxicity. Diclofenac was given to the rats for three days, and BPC 157 was either given intraperitoneally right after diclofenac or orally with water. The rats’ digestive, gastrointestinal, and hepatic systems suffered serious damage without BPC 157 therapy, and their serum levels of bilirubin, aspartate transaminase (AST), and alanine transaminase (ALT) were elevated. Also, they had extended sedation/unconsciousness and hepatic encephalopathy, which is characterized by Purkinje cells, injured red neurons, notably in the cerebral cortex and cerebellar nuclei, and brain edema, particularly in the cerebral cortex and cerebellum.However, when the rats were treated with BPC 157, the peptide effectively counteracted the toxic effects of diclofenac in both intraperitoneal and per-oral regimens. The extensive antagonization of diclofenac toxicity achieved with BPC 157 suggests that it may be a useful therapy for countering the toxic effects of diclofenac and other NSAIDs. Overall, the study demonstrates the potential therapeutic benefits of BPC 157 in treating the toxic side effects of NSAIDs, particularly diclofenac, on the gastrointestinal, liver, and brain systems in rats. The results suggest that BPC 157 could be a promising drug for future clinical trials to treat these toxic side effects in humans.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/21295044/.- Vitaic S, Stupnisek M, Drmic D. Nonsteroidal anti-inflammatory drugs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincters failure with stable gatricpentadecapeptide BPC 157 in rats. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2017; 68(2):265-272.
Nonsteroidal anti-inflammatory drugs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincters failure with stable gatricpentadecapeptide BPC 157 in rats
The failure of the sphincters caused by NSAID toxicity can be countered by using BPC 157 (GEPPPGKPADDAGLV, MW 1419), a safe and stable gastric pentadecapeptide that has been successful in clinical trials for inflammatory bowel disease. In rats, BPC 157 can treat esophagitis, sphincter failure, gastrointestinal ulcer and skin ulcer, external and internal fistulas, and all NSAID-related lesions. The researchers examined the effect of different NSAID regimens on lower esophageal sphincter and pyloric sphincter pressure (measured in cmH2O) in rats at corresponding time points known to cause stomach, small intestine lesions, hepatotoxicity, and encephalopathy. They assessed the pressure in rats treated with diclofenac, ibuprofen, paracetamol, aspirin, and celecoxib, and gave BPC 157 either immediately after the NSAIDs or in drinking water.The results showed that all tested NSAIDs decreased pressure in both sphincters, but when BPC 157 was given, the initial fall in pressure was minimized and pressure values were restored to normal levels. In contrast, in all control NSAIDs rats, a fall in pressure occurred in both sphincters rapidly and persisted. Therefore, BPC 157 was found to be effective in restoring sphincter function in rats treated with NSAIDs.In conclusion, the researchers discovered that BPC 157 can prevent sphincter failure caused by NSAID toxicity, which is a common side effect of NSAIDs. By giving rats BPC 157, they were able to reduce the initial drop in sphincter pressure and restore it to normal levels. This study suggests that BPC 157 could be a promising treatment for NSAID-induced sphincter failure in humans.
You can read the full article at https://www.jpp.krakow.pl/journal/archive/04_17/pdf/265_04_17_article.pdf.- Sikiric P, Seiwerth S, Rucman R. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Current pharmaceutical design. 2011; 17(16):1612-32.
Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract
BPC 157 is a safe anti-ulcer peptide with no toxicity that has been clinically tested for inflammatory bowel disease and wound healing. Researchers have looked into using BPC 157 as a therapy for various lesions in the digestive system, including the esophagus, stomach, duodenum, intestine, liver, and pancreas. It has been particularly effective in treating alcohol- and NSAID-induced lesions, as well as preventing and reversing adjuvant arthritis.
BPC 157 was able to normalize pressure in both sphincters and reduce esophagitis in rats with esophagitis and failed function of both the lower esophageal and pyloric sphincters. BPC 157, on the other hand, may either decrease or increase pressure in these sphincters in healthy rats.BPC 157 has been shown to have a high angiogenic potential as well as endothelial protection.
It also prevents and reverses thrombus formation following abdominal aorta anastomosis and has effects on a variety of central nervous system disorders, including the dopamine and 5-HT systems. BPC 157 also has neuroprotective properties and acts as a free radical scavenger, preventing damage caused by substances such as CCl4, paracetamol, and diclofenac. In rats with intestinal anastomosis, gastrocutaneous, duodenocutaneous, and colocutaneous fistulas, BPC 157 successfully healed the fistulas and interacted with the NO-system, even when therapy was postponed for one month. In rats with short-bowel syndrome, BPC 157 therapy immediately resulted in weight gain above preoperative values, as well as an increase in villus height, crypt depth, and muscle thickness. Overall, BPC 157 shows promise as a potential therapy for various gastrointestinal tract conditions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/21548867/.- Ilic S, Drmic D, Zarkovic K. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. European journal of pharmacology. 2011; 667(1-3):322-9.
Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats.
The study “Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats” investigated the effects of ibuprofen on rats. Ibuprofen administration led to hepatic encephalopathy, hepatomegaly, and gastric lesions, along with altered liver enzyme levels. However, co-administration of gastric pentadecapeptide BPC 157 showed potential protective effects against these conditions. The study highlights the adverse effects of ibuprofen on the liver and gastric system, as well as the potential therapeutic role of BPC 157 in mitigating these effects.
Read the full article:https://www.sciencedirect.com/science/article/abs/pii/S0014299911006212
- Boban-Blagaic A, Blagaic V, Romic Z. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine. Medical science monitor : international medical journal of experimental and clinical research. 2006; 12(1):BR36-45.
The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine.
The study “The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine” investigated the effects of BPC 157 on ethanol administration in mice. Ethanol caused gastric lesions and liver damage, but co-administration of BPC 157 reduced their severity. L-NAME intensified the negative effects of ethanol, while L-arginine had a protective effect. The study highlights BPC 157’s potential protective role against ethanol-induced gastric and liver damage and the involvement of nitric oxide in these processes.
Mice were administered intraperitoneal doses of BPC 157 (10 microg/kg), L-NAME (10 mg/kg), and L-arginine (400 mg/kg), individually or in combinations, either 5 minutes before or after acute ethanol intoxication (4 g/kg i.p.) or after 0, 3, or 7 hours of withdrawal following 13 days of 20% alcohol consumption.BPC 157 swiftly countered the severe manifestations of acute intoxication (prolonged ethanol anesthesia, complete loss of righting reflex, unresponsiveness to external stimuli, hypothermia, 25% mortality), as well as withdrawal symptoms (prominent seizures). The effects of NO agents were notable: they exacerbated acute alcohol intoxication and opposed withdrawal, but with distinct impacts based on the intervals affected by L-arginine and the continuous influence of L-NAME. When administered together, L-arginine and L-NAME exhibited counteractive effects. Either the “L-NAME presentation” (during acute intoxication) or the “L-arginine presentation” (during withdrawal) dominated.
BPC157 combined with NO agents: In acute intoxication (where L-NAME’s role dominated to worsen intoxication), both BPC157+L-NAME and BPC157+L-arginine followed the L-NAME presentation, without increased mortality. During withdrawal (where L-arginine’s role dominated to mitigate disturbance symptoms), BPC157+L-NAME mimicked the L-NAME presentation, while BPC 157+L-arginine imitated the effects of L-arginine.
The interplay between pentadecapeptide BPC 157, the NO system, acute alcohol intoxication, and withdrawal opposition appears to be significant, positioning pentadecapeptide BPC 157 as a promising antagonist against alcohol-related effects.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16369461/.
- Blagaic AB, Blagaic V, Romic Z, Sikiric P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. European journal of pharmacology. 2004; 499(3):285-90.
The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice.
The study “The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice” examined the effects of BPC 157 on ethanol administration in mice. Ethanol caused gastric lesions and liver damage, but co-administration of BPC 157 attenuated these effects, demonstrating a protective role. The study highlights the potential therapeutic value of BPC 157 in mitigating ethanol-induced gastric and liver damage in mice.
Mice were administered intraperitoneal doses of BPC 157 (10 microg/kg), L-NAME (10 mg/kg), and L-arginine (400 mg/kg), either individually or in combination. The administration took place either 5 minutes before or after acute ethanol intoxication (4 g/kg i.p.), or after 0, 3, or 7 hours of withdrawal following 13 days of 20% alcohol consumption.BPC 157 promptly countered the most severe manifestations of acute intoxication, including sustained ethanol-induced anesthesia, complete loss of the righting reflex, unresponsiveness to external stimuli, hypothermia, and 25% mortality, as well as withdrawal symptoms characterized by prominent seizures. The effects of NO agents were pronounced: they exacerbated acute alcohol intoxication and opposed withdrawal, with distinctive impacts based on the intervals affected by L-arginine and the continuous influence of L-NAME. When administered together, L-arginine and L-NAME exhibited counteractive effects. Either the “L-NAME presentation” (during acute intoxication) or the “L-arginine presentation” (during withdrawal) prevailed.
BPC157 combined with NO agents: During acute intoxication, where L-NAME played a dominant role in exacerbating intoxication, both BPC157+L-NAME and BPC157+L-arginine followed the L-NAME presentation, without increased mortality. During withdrawal, where L-arginine’s role predominated in mitigating disturbance symptoms, BPC157+L-NAME replicated the L-NAME presentation, while BPC 157+L-arginine imitated the effects of L-arginine.
The interplay between pentadecapeptide BPC 157, the NO system, acute alcohol intoxication, and withdrawal opposition appears to be significant, suggesting that pentadecapeptide BPC 157 could serve as an effective alcohol antagonist.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16369461/.
- Sikiric P, Seiwerth S, Brcic L. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Current pharmaceutical design. 2010; 16(10):1224-34.
Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157.
The article “Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator” discusses the concepts of cytoprotection and adaptive cytoprotection and their relationship to gastric pentadecapeptide BPC 157. BPC 157 shows therapeutic effects in tissue protection, wound healing, and inflammation modulation. It suggests that BPC 157 has significant implications as a novel mediator in cytoprotection and adaptive cytoprotection, highlighting its potential as a therapeutic agent for tissue repair.
This review centers on Robert’s cytoprotection concept as applied to the rat stomach. It delves into evidence that may elucidate whether cytoprotection and adaptive cytoprotection are consistent phenomena. It investigates whether these phenomena are endogenous, dependent on the irritants’ nature (mild or potent), and how they contribute to stomach mucosa defense against ulcerogens or aided by antiulcer agents. The review examines whether these phenomena are uniform across the entire gastrointestinal tract and whether they share interconnections.Furthermore, the review challenges the importance of cytoprotection phenomena and cytoprotective activity in skin wound healing and general wound healing. In this context, the review focalizes on the role of BPC 157 in cytoprotection and adaptive cytoprotection. It suggests that BPC 157 might serve as a pivotal endogenous mediator capable of orchestrating both cytoprotective and adaptive cytoprotective responses in the stomach and the broader gastrointestinal tract. This role could also hold significant implications for wound healing.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20166993/.
- Sikiric P, Seiwerth S, Brcic L. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006; 14(5-6):214-21.
Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia).
The article “Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response” discusses the trials of BPC 157 for inflammatory bowel disease conducted by Pliva in Croatia. BPC 157 showed promising therapeutic effects for IBD and positive effects on a full and distended stomach, as well as a favorable vascular response. The study supports the potential therapeutic application of BPC 157 in treating IBD.
Upon absolute alcohol instillation into a fully distended rat stomach, lesions manifest across the gastric, esophageal, and duodenal regions. Within the subsequent 3 minutes, the blood vessels of the left gastric artery visibly vanish at the serosal site, signifying both a loss of vessel integrity and function. In contrast, a consistently maintained presentation of blood vessels can indicate the beneficial effects of the applied agent.Following the instillation of pentadecapeptide BPC 157 into the stomach, the presentation of blood vessels remains stable, leading to the inhibition of lesions in the stomach, esophagus, and duodenum. Common standards like atropine, ranitidine, and omeprazole marginally enhance blood vessel presentation in comparison to control values, exerting only a partial impact on the lesions.
This review underscores the remarkable stability of BPC 157 and highlights several significant effects: its efficacy against various gastrointestinal lesions, its influence on the nitric oxide (NO) system and NO-agent effects, its impact on somatosensory neurons and salivary gland function, its role in the recovery of the AMP-ADP-ATP system, endothelium protection, its effects on endothelin and angiogenesis promotion. Additionally, BPC 157 counters other effects of alcohol, including acute and chronic intoxication. When administered peripherally, it mitigates the consequences of disturbances in the central dopamine system (receptor blockade) and triggers serotonin release in the substantia nigra.
Furthermore, the therapeutic potential of BPC 157 as a cytoprotective agent extends to wound healing. When administered directly into the stomach, BPC 157 rapidly restores disrupted lower esophageal and pyloric sphincter pressures in rats with untreated esophagitis lasting 12-20 months. Collectively, these findings suggest a potential role for BPC 157 as a natural protectant within gastric juice, particularly during instances of stomach distension.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17186181/.
- Sikiric P, Separovic J, Buljat G. The antidepressant effect of an antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants. Journal of physiology, Paris. ; 94(2):99-104.
The antidepressant effect of an antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats.
The study “The antidepressant effect of the antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants” investigated the antidepressant properties of BPC 157 in rats. BPC 157 demonstrated an antidepressant effect in both Porsolt’s test and chronic unpredictable stress models, comparable to traditional antidepressant medications. This suggests its potential as an alternative or adjunctive treatment for depression. The study highlights the therapeutic potential of BPC 157 in addressing depressive-like behavior and emphasizes its comparable efficacy to standard antidepressant drugs.
Initially, a forced swimming test (Porsolt’s procedure) was employed. Additionally, a more rigorous approach involved chronic unpredictable stress. For this, rats underwent a 5-day unpredictable stress protocol, with once-daily drug administration during the stress regimen. The open field-immobility test was assessed on the fourth or sixth day of medication.In the forced swimming test, BPC 157 treatment (10 microg, 10 ng x kg(-1) i.p.) led to a decrease in immobility time comparable to the effects of conventional antidepressants like imipramine (15 mg i.p.) or nialamide (40 mg i.p.), administered as per the original Porsolt’s protocol.
During the chronic unpredictable stress procedure, the intensified experimental conditions notably affected the activity of conventional antidepressants, whereas the efficacy of BPC 157 remained consistent. The impact of daily imipramine medication (30 mg) became evident only after an extended period, rather than in a shorter timeframe (such as a 4-day protocol). In contrast, BPC 157 exhibited no delay in its effectiveness, and a reduction in immobility among chronically stressed rats was observable following both 4 and 6 days of BPC 157 (10 microg, 10 ng) treatment.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10791689/.
- Sikiric P, Jelovac N, Jelovac-Gjeldum A. Anxiolytic effect of BPC-157, a gastric pentadecapeptide: shock probe/burying test and light/dark test. ActapharmacologicaSinica. 2001; 22(3):225-30.
Anxiolytic effect of BPC-157, a gastric pentadecapeptide: shock probe/burying test and light/dark test.
The study “Anxiolytic effect of BPC-157, a gastric pentadecapeptide: shock probe/burying test and light/dark test” investigated the anxiolytic properties of BPC-157 in rats. BPC-157 demonstrated an anxiolytic effect in both the shock probe/burying test and the light/dark test, indicating its potential as an anti-anxiety agent. The study highlights BPC-157’s ability to reduce anxiety-related behaviors and suggests its therapeutic potential for managing anxiety. These findings emphasize the promising role of BPC-157 as an alternative approach for treating anxiety-related conditions.
Shock probe/burying test: Rats treated with either diazepam or pentadecapeptide BPC-157 displayed significantly reduced fear responses after the shock. They showed minimal burying behavior, and the total time spent in burying was notably less than in the control group. However, while diazepam-treated rats received more shocks compared to the control group, the number of shocks in the pentadecapeptide BPC-157 treated groups remained unchanged from the control values. Light/dark test: After exposure to intense light, mice treated with diazepam exhibited prolonged latencies when crossing to the dark compartment, an increased number of crossings and exploratory rearing, and spent more time in the light compartment compared to control mice. In contrast, mice treated with BPC-157 showed behavior similar to control mice. Interestingly, in the dark zone, diazepam had no effect compared to controls, while mice treated with BPC-157 (at a dose of 10 microg/kg) displayed an increased number of crossings and exploratory rearing.Both diazepam and BPC-157 demonstrated bidirectional effects on anxiety, but the impact of pentadecapeptide BPC-157 was distinct and differed from that of diazepam.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11742568/.
- Sikiric P, Jelovac N, Jelovac-Gjeldum A. Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. ActapharmacologicaSinica. 2002; 23(5):412-22.
Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances.
The study “Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances” examined the effects of BPC 157 on behavior disturbances caused by chronic amphetamine use in rats. BPC 157 demonstrated a protective effect, mitigating the negative behavioral effects induced by chronic amphetamine administration. These findings highlight the therapeutic potential of BPC 157 in addressing the behavioral disturbances associated with amphetamine abuse
In conclusion, the study illuminated that gastric pentadecapeptide BPC 157, encompassing both microg- and ng-BPC 157 regimens, effectively curtailed chronic amphetamine-related disruptions. This impact remained consistently significant throughout the observation period. Consequently, it is conceivable that this gastric pentadecapeptide, BPC 157, possesses a modulating influence on the dopamine system and could hold promise in addressing chronic amphetamine-related disturbances.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11978191/.
- BobanBlagaic A, Blagaic V, Mirt M. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. European journal of pharmacology. 2005; 512(2-3):173-9.
Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats.
The study “Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats” investigated the therapeutic potential of BPC 157 in treating serotonin syndrome. BPC 157 demonstrated effectiveness in alleviating the symptoms of serotonin syndrome in rats, indicating its potential as a treatment option. The peptide exhibited therapeutic benefits and showed promise in managing serotonin-related disorders. These findings highlight the potential application of BPC 157 as a therapeutic intervention for serotonin syndrome.
In a bid to uncover the intricacies of this peptide, researchers embarked on an investigative journey. They employed a range of doses (10 microg, 10 ng, 10 pg/kg i.p.) of BPC 157 in various experimental settings. First, the peptide was explored in a scenario simulating a full serotonin syndrome in rats. This involved combining pargyline (an irreversible MAO inhibitor) and subsequent L-tryptophan (a serotonin precursor) along with BPC 157 as a co-treatment. Additionally, the peptide was investigated under different circumstances: (ii) pargyline or (iii) L-tryptophan given separately. In the former, BPC 157 was administered as a post-treatment after 15 minutes, while in the latter, it served as a 15-minute pre-treatment before the induction of potential serotonin syndrome using L-tryptophan.The outcomes of these experiments painted a fascinating picture. Gastric pentadecapeptide BPC 157 demonstrated an exceptional ability to counteract both the presentation and initiation of serotonin syndrome. In the context of severe serotonin syndrome, where pargyline and L-tryptophan converge, BPC 157 exhibited substantial inhibition. Even at lower doses, the peptide managed to suppress disruptive symptoms, particularly those associated with the stimulation of 5-HT2A receptors, including hyperthermia and wet dog shake. Similarly, in the presence of mild disturbances observed in pargyline-treated rats, such as mild hypothermia and feeble hind limbs abduction, the highest dose of pentadecapeptide BPC 157 effectively intervened.
This remarkable interplay was further accentuated when examining severe serotonin syndrome. Remarkably, gastric pentadecapeptide BPC 157 exhibited a beneficial activity on its own, without any discernible behavioral or temperature effect. This effect seemed to stem from a specific counteraction of phenomena involving 5-HT2A receptors. This insight sheds light on the intricate role of BPC 157 in mitigating the effects of serotonin syndrome, thereby presenting a new avenue for therapeutic exploration.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299905002086?via%3Dihub.
- Jelovac N, Sikiric P, Rucman R. The effect of a novel pentadecapeptide BPC 157 on development of tolerance and physical dependence following repeated administration of diazepam. The Chinese journal of physiology. 1999; 42(3):171-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10707891.
The effect of a novel pentadecapeptide BPC 157 on development of tolerance and physical dependence following repeated administration of diazepam
In the study “Effect of pentadecapeptide BPC 157 on tolerance and physical dependence after repeated diazepam administration,” Jelovac et al. investigated the impact of BPC 157 on the development of tolerance and physical dependence induced by diazepam. The results demonstrated that BPC 157 had an effect on reducing tolerance and physical dependence associated with repeated diazepam administration. This suggests the potential of BPC 157 as a therapeutic intervention for managing tolerance and physical dependence related to benzodiazepine use.
Read the full article:https://www.sciencedirect.com/science/article/abs/pii/S0928425799001199
- Sikiric P, Marovic A, Matoz W. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. Journal of physiology, Paris. 1999; 93(6):505-12.
A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine.
The study “Effect of pentadecapeptide BPC 157 on Parkinson’s disease models and gastric lesions in mice” investigated the impact of BPC 157 on Parkinson’s disease symptoms induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice. BPC 157 demonstrated a beneficial effect by improving behavioral manifestations of Parkinson’s disease and offering protection against MPTP-induced gastric lesions. These findings suggest the therapeutic potential of BPC 157 in managing Parkinson’s disease and associated gastric complications.
Drawing these findings together with the broader context of benzodiazepines’ tolerance and withdrawal dynamics, an intriguing hypothesis emerges. BPC 157 appears to play a role in fostering the natural equilibrium of the GABA receptor complex, while also enhancing GABAergic transmission. Its mechanism seems to differ from that associated with diazepam tolerance and withdrawal, suggesting its potential for application in combating these issues. In essence, BPC 157 presents a fresh avenue for addressing the challenges posed by diazepam tolerance and withdrawal, offering new hope for improved therapies in this realm.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10707891/.
- Klicek R, Kolenc D, Suran J. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2013; 64(5):597-612.
Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability.
The study “Stable gastric pentadecapeptide BPC 157 heals colitis, anastomosis, and brain injuries” investigated the therapeutic effects of BPC 157 in healing cysteamine-induced colitis, colon-colon anastomosis, and counteracting cuprizone-induced brain injuries and motor disability. BPC 157 demonstrated healing properties in colitis and anastomosis, promoting tissue repair, and mitigated brain injuries and motor disability. These findings suggest the therapeutic potential of BPC 157 in various pathological conditions.
Shifting focus to multiple sclerosis, the researchers utilized a toxic rat model induced by cuprizone. This model mimics the damaging processes observed in multiple sclerosis. Control rats displayed an exacerbated and accelerated progression of nerve damage, particularly in critical brain areas like the corpus callosum, laterodorsal thalamus, nucleus reunions, and anterior horn motor neurons. Intriguingly, rats supplemented with BPC 157 alongside cuprizone exhibited substantially less nerve damage, with notable preservation in the regions most severely affected.Moreover, BPC 157 intervention exhibited a consistently positive impact on motor function. It countered cerebellar ataxia and restored impaired forelimb function, showcasing its potential to alleviate the debilitating effects of multiple sclerosis.
Collectively, this series of experiments advocates for the therapeutic potential of BPC 157 in the management of both inflammatory bowel disease and multiple sclerosis. The evidence underscores its ability to not only counteract disease mechanisms but also promote healing and preserve neural integrity, marking a significant step towards novel treatment strategies for these conditions.
You can read the full article at https://www.jpp.krakow.pl/journal/archive/10_13/pdf/597_10_13_article.pdf.
- Torkildsen O, Brunborg LA, Myhr KM, Bø L. The cuprizone model for demyelination. ActaneurologicaScandinavica. Supplementum. 2008; 188:72-6.
The cuprizone model for demyelination. ActaneurologicaScandinavica. Supplementum.
The article “The cuprizone model for demyelination” provides an overview of the cuprizone model, commonly used to induce demyelination in experimental research. The model involves administering cuprizone, a copper chelator, to mimic aspects of demyelinating diseases in animal brains. The study highlights the utility of this model for studying demyelination and exploring potential treatments. Understanding the mechanisms and characteristics of the cuprizone model contributes to the development of therapeutic strategies for demyelinating diseases.
This article delves into an exploration of the cuprizone model’s potential and significance. By examining previous research, the article sheds light on how the model has contributed to our understanding of de- and remyelination, providing valuable insights into the histopathology of MS. A key aspect of this model’s utility lies in its correlation with more contemporary histopathological data in MS, highlighting its relevance in bridging the gap between animal models and the disease’s human manifestation. The use of the C57BL/6 strain in this model also holds promise, paving the way for future investigations involving transgene and knockout mice. As we contemplate the path forward in MS research, the cuprizone model stands as a crucial tool with the potential to unlock new avenues of understanding and therapeutic development.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/18439226/.
- Tudor M, Jandric I, Marovic A. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regulatory peptides. 2010; 160(1-3):26-32.
Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regulatory peptides.
The study “Traumatic brain injury in mice and the effect of pentadecapeptide BPC 157” explored the impact of BPC 157 on traumatic brain injury in mice. BPC 157 was administered after inducing brain injury to assess its effects. The results showed that BPC 157 had a beneficial effect, reducing brain damage and improving neurological outcomes in mice with traumatic brain injury. These findings suggest that BPC 157 has potential as a therapeutic intervention for traumatic brain injury, highlighting its neuroprotective properties.
When administered prophylactically, with a 30-minute lead time before the traumatic brain injury, BPC 157 exhibited a noteworthy enhancement in the conscious/unconscious/death ratio among the TBI-afflicted mice, with force impulses ranging from 0.068 Ns to 0.159 Ns. The therapeutic intervention with both microg- and ng-regimens effectively counteracted unconsciousness and lowered mortality across the range of force impulses from 0.068 Ns to 0.145 Ns. Impressively, even the highest regimen demonstrated efficacy against the most intense force impulse of 0.159 Ns.Furthermore, the application of BPC 157 immediately before injury proved advantageous for mice subjected to a force impulse of 0.093 Ns-TBI. For more severe force impulses (0.130 Ns, 0.145 Ns, or 0.159 Ns), the temporal relationship to enhance the conscious/unconscious/death ratio was observed to be 5 minutes for 0.130 Ns-TBI, 20 minutes for 0.145 Ns-TBI, and 30 minutes for 0.159 Ns-TBI. This remarkable interplay between BPC 157 and traumatic brain injury marks a significant stride towards unlocking potential therapeutic avenues for traumatic brain injuries of varying intensities.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19931318/.
- Retrieved from http://www.fasebj.org/content/30/1_Supplement/706.5.short.
- Yu-Feng Xiao, Meng-MengJie, Bo-Sheng Li, et al., “Peptide-Based Treatment: A Promising Cancer Therapy,” Journal of Immunology Research, vol. 2015, Article ID 761820, 13 pages, 2015. doi:10.1155/2015/761820.
Peptide-Based Treatment: A Promising Cancer Therapy,” Journal of Immunology Research, vol. 2015, Article ID 761820, 13 pages, 2015. doi:10.1155/2015/761820.
The article “Peptide-Based Treatment: A Promising Cancer Therapy” discusses the potential of peptide-based treatments for cancer. It highlights their advantages, including targeted delivery, reduced side effects, and potential for combination therapies. The study explores various types of peptides, such as anticancer peptides, peptide vaccines, and peptide-drug conjugates. Overall, peptide-based treatments show promise in cancer therapy due to their specificity, versatility, and potential for personalized medicine.
Peptide-based chemotherapy unfolds along three distinct pathways: peptide-alone therapy, peptide vaccines, and the utilization of peptide-conjugated nanomaterials. Peptide-alone therapy stands out for its potential to specifically bolster the immune system’s prowess in eliminating tumor cells. In parallel, peptide-based vaccines have made strides in advanced cancer treatment, showing promise in enhancing patients’ overall survival rates. Additionally, the fusion of peptides with nanomaterials heralds a new frontier, amplifying the therapeutic potential of peptides by optimizing drug delivery mechanisms and heightening sensitivity to treatment.This comprehensive review delves into the forefront of these recent advancements in peptide-based cancer treatment, encompassing a spectrum of domains from diagnosis and treatment to prognosis. Amid the burgeoning landscape of cancer therapeutics, peptides emerge as a beacon of hope, illuminating the path towards more effective and targeted interventions.
You can read the full article at https://www.hindawi.com/journals/jir/2015/761820/.
- Cerezo D, Peña MJ, Mijares M, Martínez G, Blanca I, De Sanctis JB. Peptide vaccines for cancer therapy. Recent patents on inflammation & allergy drug discovery. 2015; 9(1):38-45.
Peptide vaccines for cancer therapy. Recent patents on inflammation & allergy drug discovery
The article “Peptide vaccines for cancer therapy” discusses the use of peptide vaccines as a potential approach for cancer treatment. It emphasizes the advantages of peptide vaccines, such as their specificity and safety, and their potential for personalized immunotherapy. The study explores different strategies for designing peptide vaccines and their effectiveness in targeting cancer-specific antigens. Overall, the article concludes that peptide vaccines show promise in improving cancer treatment outcomes.
Within this context, the current review centers around the comprehensive exploration of peptide vaccines. It delves into the intricate facets of cancer antigen presentation, the elicitation of specific immune responses, the activation of cell death pathways, and the prospect of targeted therapy for modified or mutated oncogenes. As research in this field continues to rapidly evolve, there is a palpable sense of anticipation for fruitful outcomes to emerge in the near future.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/25642777/.
- Kumai T, Kobayashi H, Harabuchi Y, Celis E. Peptide vaccines in cancer-old concept revisited. Current opinion in immunology. 2017; 45:1-7.
Peptide vaccines in cancer-old concept revisited. Current opinion in immunology.
The article “Peptide vaccines in cancer – old concept revisited” discusses the potential of peptide vaccines in cancer immunotherapy. It provides an overview of the current understanding and advancements in this field, highlighting the importance of tumor antigens, antigen presentation, and immune responses. The study also explores the use of adjuvants and combination therapies to improve vaccine efficacy. Overall, peptide vaccines show promise in cancer treatment, but further research and clinical trials are needed to optimize their use
Read the full aticle: https://www.sciencedirect.com/science/article/abs/pii/S0952791516301492
- Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The Use of Therapeutic Peptides to Target and to Kill Cancer Cells. Current medicinal chemistry. 2012;19(22):3794-3804.
The Use of Therapeutic Peptides to Target and to Kill Cancer Cells.
Within the realm of medical advancements, peptide therapeutics stands as a promising frontier for the emergence of potent anti-cancer agents. This approach offers a multitude of benefits, stemming from the facile and swift synthesis of peptides, along with the potential for custom modifications. Moreover, the extensive reservoir of knowledge regarding protein structure and function can be ingeniously harnessed to fashion innovative peptide designs that hold the potential to revolutionize cancer treatment.
At the heart of current research endeavors lies the pursuit of two primary objectives. The first centers around the creation of peptides that serve as adept tumor-targeting agents. In parallel, efforts are dedicated to crafting peptides with the unique ability to permeabilize cellular membranes, thereby inducing cytotoxic effects. A comprehensive exploration into recent discoveries unveils intriguing trends. Notably, amphiphilic peptides sporting clusters of hydrophobic and cationic residues, traits typically found in anti-microbial peptides, have emerged as pivotal features. These characteristics not only empower these peptides to annihilate microbes but also bestow them with substantial anti cancer toxicity.
Furthermore, another captivating avenue involves peptides that assemble and congregate to form pores capable of disrupting cell or organelle membranes. This disruption prompts pathways of apoptotic or necrotic cell death. Among the impressive feats attributed to peptides in this category, their potential to permeate cells and specifically target tumors or the vasculature within tumors stands out. These peptides serve as efficient couriers, transporting biologically active cargo directly to the site of malignant growth.
Nonetheless, the journey from laboratory discovery to clinical application is replete with challenges. Enhancing the precision of peptide delivery to tumor sites while mitigating non-specific toxic effects remains a paramount task. Simultaneously, understanding and optimizing the pharmacokinetic properties of these therapeutic peptides emerges as a pressing necessity. Addressing these hurdles is pivotal for ushering in an era where therapeutic peptides can assert their prowess as potent instruments in the fight against cancer, presenting a novel paradigm in cancer treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4537071/.
- Grabarevic Z, Tisljar M, Artukovic B, et al. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris. 1997;91(3-5):139-49.
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.