GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Book a Free Consultation
Potential Benefits of 5-amino-1MQ
5-amino-1MQ offers a range of notable benefits, including producing dramatic weight loss, lowering cholesterol levels, and improving blood sugar levels, making it potentially valuable for metabolic health. Moreover, it demonstrates anti-inflammatory properties and shows promise in combating various cancer types, suggesting potential applications in both cancer therapy and inflammation-related conditions. Additionally, it enhances aged muscle regeneration, highlighting its potential in promoting muscle health and function.
- Produces dramatic weight loss [1-10]
- Lowers cholesterol levels [3, 11]
- Improves blood sugar levels [3, 12-17]
- Fights inflammation [18-22]
- Combats various cancer types [23-37]
- Enhances aged muscle regeneration [38-41]
What is 5-amino-1MQ?
Obesity may significantly impact one’s quality of life, as it can negatively affect independent movement and exercise. While this condition can cause physical and emotional problems, it can also increase one’s risk for deadly diseases such as heart disease, diabetes, cancer, stroke, and non-alcoholic liver failure.
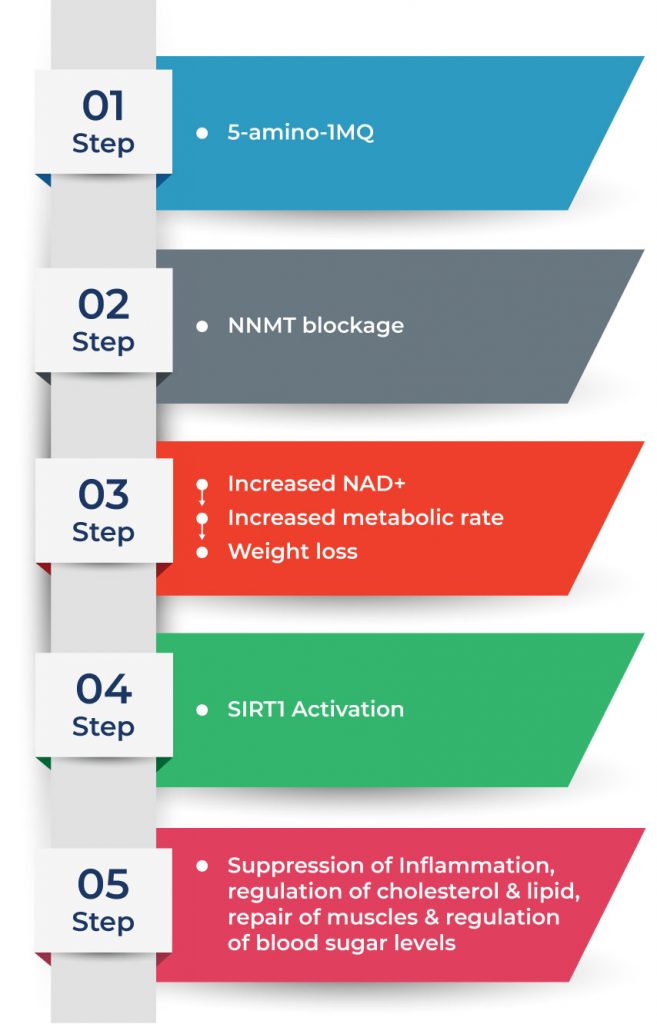
When fat cells grow larger as we gain weight, it causes excess production of NNMT (Nicotinamide N-methyltransferase), a cytosolic enzyme that slows down fat cell metabolism and is predominantly active in fat tissue. Excess production of NNMT can lead to alterations in the body’s metabolism and the regulation of energy balance, potentially contributing to weight gain. In addition, more hormones and pro-inflammatory signals associated with weight gain are produced with increased fat tissue. Therefore, body fat accumulates over time and becomes very challenging to remove without surgical intervention.
Recently, a new breakthrough may help patients with this severe form of obesity achieve a healthier weight. The NNMT inhibitor 5-amino-1-methylquinoline or 5-amino-1MQ has been shown to reduce the production of the enzyme nicotinamide N-methyltransferase (NNMT), a regulator of energy homeostasis in adipose tissue (a type of fat used for energy storage). This mechanism could shrink white adipose tissue and dramatically reduce weight without the need to limit calorie intake. Studies show that there are far more potential benefits of 5-amino-1MQ than just weight loss.
How 5-amino-1MQ Works
5-amino-1MQ, a small, selective, membrane-permeable molecule, blocks the activity of an enzyme in the metabolic cycles called nicotinamide N-methyltransferase (NNMT). This process increases nicotinamide adenine dinucleotide (NAD+), which is involved in a wide array of cellular reactions. The increase in NAD+ levels also increases the body’s metabolic rate, resulting in weight loss. In addition, a gene called sirtuin-1 (SIRT1), also known as ‘the longevity gene’, is also activated. SIRT1 helps lower the risk of debilitating diseases such as diabetes, cardiovascular disease, sleep apnea, and cancer.
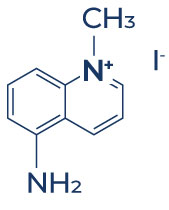
Chemical Structure of 5-amino-1MQ
Research on 5-amino-1MQ
A. Produces Dramatic Weight Loss
5-amino-1MQ has been demonstrated to target and stimulate brown adipose tissue (BAT) activity, a critical player in fat cell metabolism and thermogenesis, responsible for burning stored fat to generate heat. Through a process known as browning, 5-amino-1MQ triggers the conversion of white fat cells into beige fat cells, enhancing their ability to burn fat and thus effectively promoting weight loss. This browning effect not only aids in reducing weight gain but also contributes to the overall shrinkage of fat cells within the body’s fat tissue, thus reducing fat stores.
Furthermore, 5-amino-1MQ has been shown to potentially enhance the basal metabolic rate, which can contribute to increased calorie burning and, consequently, support weight loss efforts. Therefore, 5-amino-1MQ emerges as a promising strategy for reversing obesity and related metabolic disorders, offering a potential means to lose weight and improve fat cell metabolism.
Evidence suggests that by shrinking fat cells, 5-amino-1MQ can significantly reduce weight, burn fat, and improve body composition:
- In U.S. Veterans military service members, the administration of 5-amino-1MQ resulted in significant weight loss without any adverse side effects. [1]
- In diet-induced obese mice that were maintained on a high-fat diet, the administration of an NNMT inhibitor significantly reduced body weight and white adipose tissue mass and decreased the size of adipocyte cells (cells specialized for fat storage) without any observable adverse effects, suggesting enhanced cellular metabolism of adipocytes. [2-3]
- The administration of an NNMT inhibitor in mice on a high-fat diet (HFD) protected against fat accumulation and weight gain by augmenting cellular energy expenditure. [4]
- Studies also showed that NNMT is increased 2-fold in the subcutaneous and abdominal adipose tissue of men and women with type II diabetes compared to healthy subjects, suggesting that the administration of NNMT inhibitors may burn fat and lose weight. [5-7]
- A cell study found that 5-amino-1MQ was able to prevent fat accumulation and shrink fat cells, resulting in less fat tissue. [8]
- Studies found that high levels of NNMT were often found in fat cells and were associated with impaired calorie utilization resulting in increased fat storage – a mechanism related to weight gain. [9-10]
Unlock Your Weight Loss Potential with Peptides! Discover the science-backed power of peptides for weight loss.
B. Lowers Cholesterol Levels
5-amino-1MQ can help lower cholesterol levels by increasing the production of NAD+ in our cells and activating the SIRT1 gene. When NAD+ levels rise, it promotes the activity of SIRT1, a gene that plays a key role in regulating our body’s metabolism. SIRT1 helps in breaking down cholesterol and reducing its buildup in the bloodstream, which ultimately leads to lower cholesterol levels. This can help lower the risk of chronic diseases such as coronary heart disease, kidney disease, and high blood pressure.
Studies show that 5-amino-1MQ’s ability to increase NAD+ and activate the SIRT1 gene can help improve cholesterol levels:
- In diet-induced obese (DIO) mice maintained on a high-fat diet, the administration of an NNMT inhibitor lowered plasma total cholesterol levels. [3]
- A study found that SIRT1 activation by 5-amino-1MQ resulted in the suppression of cholesterol synthesis. [11]
C. Improves Blood Sugar Levels
5-amino-1MQ can lower blood sugar levels by improving the body’s ability to use sugar more effectively. It does this by improving the way cells in the body respond to insulin, which is a hormone that regulates blood sugar. When cells become more responsive to insulin, they can take in and use sugar from the blood more efficiently, leading to lower blood sugar levels. This effect can be particularly helpful for people with conditions like type II diabetes, where blood sugar regulation is a concern, as it can contribute to better overall blood sugar control.
Evidence found that 5-amino-1MQ has beneficial effects on blood sugar levels:
- In diet-induced obese (DIO) mice maintained on a high-fat diet, the administration of a potent NNMT inhibitor dramatically reduced blood sugar levels without altering food intake and without adverse side effects. [3]
- Several studies found that NNMT plays a causative role in the development of type 2 diabetes and insulin resistance, suggesting that NNMT inhibitors may help lower the risk of the disease. [12-16]
- In mice, 5-amino-1MQ altered how fat cells work leading to the production of palmitic acid esters of hydroxy-stearic acids (PAHSAs), a class of lipids that can reduce insulin resistance. [17]
D. Fights Inflammation
5-amino-1MQ can help combat inflammation by acting as an anti-inflammatory agent. It does this by reducing the production and release of molecules in the body that promote inflammation. This, in turn, helps to calm down the body’s inflammatory response and prevent it from becoming overly active. As a result, 5-amino-1MQ contributes to the reduction of inflammation, which is associated with various health issues, including chronic conditions.
Evidence suggests that the enzyme NNMT is highly associated with inflammatory conditions and related injuries, suggesting that NNMT inhibitors like 5-amino-1MQ may have anti-inflammatory effects:
- Significantly increased levels of NNMT have been observed in the lungs and skeletal muscle of patients with chronic obstructive pulmonary disease (COPD) with muscle wasting. [18-19]
- Experimental studies in mice also found an increase in NNMT levels after drug-induced liver injury. [20-21]
- A study found that NNMT production is the body’s protective compensatory response to injury, resulting in inflammatory states. [22]
E. Combats Various Cancer Types
5 amino 1MQ cancer-fighting properties are attributed to the peptide’s ability to disrupt the energy supply to cancer cells, making it difficult for them to grow and spread. Cancer cells often rely heavily on a process called glycolysis to fuel their rapid growth. 5-amino-1MQ interferes with this process, reducing the availability of glucose, a key energy source for cancer cells. This disruption can slow down cancer cell growth and make them more vulnerable to other treatments, potentially enhancing the effectiveness of cancer therapies and contributing to the fight against cancer.
A number of high-quality studies also suggest that excess NNMT is strongly linked with the development of various types of cancer, suggesting a potential therapeutic role for NNMT inhibitors:
- NNMT levels are significantly increased in primary glioblastoma tumors (aggressive brain tumors) compared to normal human brain samples. [23]
- Enhanced NNMT production is also increased in human papillary thyroid cancer cell lines. [24]
- In renal clear cell carcinoma, the production of NNMT is also significantly increased. [25]
- In human bladder cancer, NNMT was found to be a major regulator of cell migration. [26]
- The NNMT protein is also increased in gastric cancers. [27-28]
- NNMT is also identified as a novel serum tumor marker for human colorectal cancers and oral squamous cell carcinoma. [29-30]
- NNMT knockdown (NNMT gene loss) decreased the proliferation and migration of bladder cancer cells. [31]
- NNMT knockdown also inhibited the proliferation and/or metastasis of kidney cancer cells, pancreatic cancer cells, and oral squamous carcinoma. [32-34]
- In non-small-cell lung cancer cells, inhibition of NNMT production resulted in suppression of tumor growth. [35]
- Higher levels of NNMT in tumor tissues are associated with lower overall survival rates in cancer patients, suggesting that NNMT inhibitor administration may help improve survival. [36]
- In a human oral cancer cell line, the administration of an NNMT inhibitor dramatically reduced cancer cell growth and reproduction. [37]
F. Enhances Aged Muscle Regeneration
5-amino 1MQ helps reverse the decline in aged muscle regeneration and promotes muscle mass by boosting the activity of satellite cells, which are essential for muscle repair and growth. In aged muscles, these satellite cells become less active, leading to muscle loss and weakness. 5-amino-1MQ rejuvenates these cells, enhancing their ability to repair and replace damaged muscle fibers. This results in improved muscle regeneration and an increase in muscle mass over time. As a result, 5-amino-1MQ holds promise as a potential solution for combating the muscle loss commonly associated with aging.
Studies suggest that 5-amino-1MQ and other NNMT inhibitors have anti-aging effects on the muscles:
- In old mice (24 months) with acute muscle injury due to barium chloride injection, the NNMT inhibitor-treated group exhibited greater contractile function (indicative of improved muscle function) compared to the saline-treated group. [38]
- In a mouse model of muscular dystrophy (progressive weakness and loss of muscle mass), NAD+ restoration delayed muscle deterioration and increased mouse life span. [39]
- In mice with muscular dystrophy, replenishing NAD+ stores with dietary nicotinamide riboside supplementation significantly improved muscle and heart function. [40]
- A study found that increased NNMT was commonly associated with muscle wasting disorders, suggesting that NNMT inhibitors like 5-amino-1MQ may provide benefits. [41]
5 Amino 1MQ Peptide Therapy
5-amino-1MQ peptide therapy is a promising and innovative approach in the field of health and wellness. This therapy utilizes a specific peptide, 5-amino-1MQ, to target and modulate various metabolic processes in the body, particularly those related to weight management, muscle health, and overall well-being.
What sets 5-amino-1MQ peptide therapy apart is its ability to selectively impact key enzymes within metabolic cycles without interfering with other enzymes, minimizing the risk of adverse effects. This targeted approach has garnered attention for its potential to reverse obesity, improve athletic performance, and improve overall health. As research in this area continues to expand, 5-amino-1MQ peptide therapy holds promise as a valuable tool in the pursuit of better health and fitness outcomes.
5 Amino 1MQ Side Effects
5-amino-1MQ side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on 5-amino-1MQ. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of 5-amino-1MQ. Despite this, it was listed as a side effect associated with 5-amino-1MQ even though these associated side effects are very uncommon.
Side effects associated with 5-amino-1MQ may include the following:
- Difficulty sleeping
- Difficulty participating in exercise
5 Amino 1MQ Dosage
The dosage of 5 Amino 1MQ peptide can vary depending on several factors, including the specific medical condition it’s being used to treat, individual patient factors, and the form in which it is administered (e.g. 5 amino 1mq capsules). It is crucial to follow the prescribed dosage and administration instructions provided by a qualified healthcare professional, such as a doctor or pharmacist. Self-administering or altering the dosage without medical guidance can be dangerous and is not recommended. If you have been prescribed 5-amino-1MQ, please consult your healthcare provider for precise dosing instructions tailored to your unique needs.
5 Amino 1MQ Before and After
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
5 Amino 1MQ Results
The timing for seeing results from 5-amino-1MQ can vary depending on factors such as the individual’s metabolism, dosage, and specific health goals.
FAQ
How much weight will you lose with 5-amino-1MQ?
The amount of weight you will use when using 5-amino-1MQ will depend on various factors such as your exercise regime and eating habits. To achieve the best weight loss outcomes, you must still eat fewer calories than you burn.
Does 5-amino-1MQ affect muscle mass?
5-amino-1MQ may help burn fat and preserve muscle mass for some individuals, but individual responses can vary.
Is 5-amino-1MQ suitable for athletes or bodybuilders?
5-amino-1MQ has the potential to increase muscle mass, resulting in increased athletic power and ability. However, athletes and bodybuilders should consult with a healthcare professional first.
Are there any contraindications for using 5-amino-1MQ?
Yes, there are some contraindications for using 5-amino-1MQ such as pregnancy, breastfeeding, kidney disease, and liver disease.
Can 5-amino-1MQ be taken alongside other supplements or medications?
It’s advisable to consult with a healthcare provider before taking 5-amino-1MQ alongside other supplements or medications to ensure safety and avoid potential interactions.
Does 5-amino-1MQ have any effects on metabolism?
Yes, 5-amino-1MQ may have effects on metabolism, potentially related to its impact on adipose tissue and energy expenditure.
Can 5-amino-1MQ be used as part of a fitness regimen?
Yes, 5-amino-1MQ can potentially be included as part of a fitness regimen to support weight management and muscle health. However, it is important to keep in mind that you should consume fewer calories than you burn to achieve effective and sustainable weight loss.
Are there any specific dietary recommendations while using 5-amino-1MQ?
While using 5-amino-1MQ, it’s recommended to maintain a balanced diet rich in nutrients and stay adequately hydrated to support overall health and optimize its potential benefits.
Reference
Available from https://apps.dtic.mil/dtic/tr/fulltext/u2/1059191.pdf.
Available from https://diabetes.diabetesjournals.org/content/67/Supplement_1/115-LB.
Neelakantan H, Vance V, Wetzel MD, et al. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. BiochemPharmacol. 2018;147:141–152. doi:10.1016/j.bcp.2017.11.007
Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice
The study by Neelakantan et al. in “Biochemical Pharmacology” explores small molecule inhibitors of nicotinamide N-methyltransferase (NNMT) as potential treatments for obesity. NNMT is identified as a key enzyme in cellular metabolism and energy homeostasis. The research focuses on the inhibitors’ properties, like permeability and selectivity, and their effects on obesity measures in mice fed a high-fat diet. Results showed that these inhibitors reduced body weight, adipose mass, and plasma cholesterol levels without affecting food intake or causing adverse effects, supporting the potential of NNMT inhibitors as obesity treatments.
For more details https://www.sciencedirect.com/science/article/pii/S0006295217306718
Kraus D, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014; 508:258–262.
Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity
The study by Kraus et al., published in Nature in 2014, examines the role of Nicotinamide N-methyltransferase (NNMT) in obesity and type 2 diabetes. The research found that NNMT expression is increased in the white adipose tissue (WAT) and liver of obese and diabetic mice. Knocking down NNMT in WAT and liver was shown to protect against diet-induced obesity by enhancing cellular energy expenditure.
The study suggests that NNMT inhibition leads to increased levels of S-adenosylmethionine (SAM) and NAD+ in adipose tissue, which in turn upregulates the activities and expressions of enzymes like ornithine decarboxylase (ODC) and spermidine-spermine N(1)-acetyltransferase (SSAT). These changes are attributed to the impact of NNMT on histone H3 lysine 4 methylation in adipose tissue. Furthermore, the inhibition of NNMT in adipocytes was found to increase oxygen consumption in a manner dependent on ODC, SSAT, and polyamine oxidase (PAO).
For more details https://www.nature.com/articles/nature13198
Kannt A, et al. Association of nicotinamide-N-methyltransferase mRNA expression in humanadipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulinresistance. Diabetologia. 2015; doi: 10.1007/s00125-014-3490-7.
Association of nicotinamide-N-methyltransferase mRNA expression in humanadipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulinresistance
The study by Kannt et al. in “Diabetologia” (2015) found a significant association between the expression of Nicotinamide-N-methyltransferase (NNMT) mRNA in human adipose tissue and insulin resistance in type 2 diabetes. It was observed that higher NNMT expression in white adipose tissue (WAT) correlated with increased plasma concentrations of 1-methylnicotinamide (MNA), which is a product of NNMT activity. This correlation was notably stronger in individuals with type 2 diabetes compared to non-diabetic individuals. The study suggests that plasma MNA and tissue NNMT expression could potentially serve as biomarkers for insulin resistance.
For more details https://link.springer.com/article/10.1007/s00125-014-3490-7
Liu M, et al. Serum N(1)-Methylnicotinamide Is Associated With Obesity and Diabetes in Chinese.J ClinEndocrinolMetab. 2015; 100:3112–3117.
Pissios P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends EndocrinolMetab. 2017;28(5):340–353.
Nicotinamide N-Methyltransferase
The article by Pissios P., titled “Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme,” delves into the multifaceted role of Nicotinamide N-Methyltransferase (NNMT), which extends beyond its primary function of metabolizing vitamin B3. NNMT is shown to have significant involvement in diverse cellular processes such as energy metabolism, glucose regulation, and lipid metabolism. Dysregulation of NNMT is implicated in metabolic disorders like obesity, diabetes, and fatty liver disease, highlighting its potential as a therapeutic target for drug development in these conditions. Overall, the article underscores the importance of understanding NNMT’s broader functions and its relevance in metabolic diseases.
For more details https://academic.oup.com/jcem/article-abstract/100/8/3112/2830342
Neelakantan H, Wang HY, Vance V, Hommel JD, McHardy SF, Watowich SJ. Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase. J Med Chem. 2017;60(12):5015–5028.
Structure–Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase
The paper titled “Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase” by Neelakantan et al. explores the relationship between the chemical structure of small molecules and their ability to inhibit Nicotinamide N-Methyltransferase (NNMT). The study investigates various compounds and their inhibitory effects on NNMT, providing insights into the design and development of potential NNMT inhibitors, which could have therapeutic implications in the context of metabolic disorders and other diseases.
For more details https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.7b00389
Kim, Y. B., Peroni, O. D., Aschenbach, W. G., Minokoshi, Y., Kotani, K., Zisman, A., Kahn, C. R., Goodyear, L. J., & Kahn, B. B. (2005). Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism. Molecular and cellular biology, 25(21), 9713–9723. https://doi.org/10.1128/MCB.25.21.9713-9723.2005.
Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism
The paper titled “Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism” by Kim et al. investigates the impact of muscle-specific deletion of the Glut4 glucose transporter on glycogen metabolism. The study explores how the absence of Glut4 in muscle tissue affects various regulatory aspects of glycogen metabolism. This research provides insights into the role of Glut4 in glucose transport and its downstream effects on glycogen synthesis and breakdown in muscle cells, contributing to our understanding of the molecular mechanisms involved in glucose homeostasis and metabolic regulation. The paper was published in Molecular and Cellular Biology in 2005.
For more details https://www.tandfonline.com/doi/abs/10.1128/MCB.25.21.9713-9723.2005
Carvalho, E., Kotani, K., Peroni, O. D., & Kahn, B. B. (2005). Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. American journal of physiology. Endocrinology and metabolism, 289(4), E551–E561. https://doi.org/10.1152/ajpendo.00116.2005.
Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle
The paper titled “Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle” by Carvalho et al. explores the effects of adipose tissue-specific overexpression of the GLUT4 glucose transporter in mice that lack GLUT4 specifically in muscle tissue. The study investigates how this genetic manipulation can reverse insulin resistance and diabetes in these mice. The research highlights the importance of GLUT4 in glucose homeostasis and demonstrates the potential therapeutic implications of adipose-specific GLUT4 overexpression in the context of insulin resistance and diabetes. The paper was published in the American Journal of Physiology.
For more details https://journals.physiology.org/doi/abs/10.1152/ajpendo.00116.2005
X. Ye, M. Li, T. Hou, T. Gao, W.-g. Zhu, Y. Yang. Sirtuins in glucose and lipid metabolism. Oncotarget, 8 (1) (2016), pp. 1845-1859.
Sirtuins in glucose and lipid metabolism
The article titled “Sirtuins in glucose and lipid metabolism” by Ye et al. discusses the role of sirtuins, a family of proteins with deacetylase activity, in regulating glucose and lipid metabolism. Published in 2016 in Oncotarget, the paper delves into how sirtuins are involved in the modulation of metabolic processes, including glucose utilization and lipid storage. It sheds light on the potential therapeutic implications of targeting sirtuins for metabolic disorders such as diabetes and obesity.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5352102/
H. Yaguchi, K. Togawa, M. Moritani, and M. Itakura, “Identification of candidate genes in the type 2 diabetes modifier locus using expression QTL,” Genomics, vol. 85, no. 5, pp. 591–599, 2005.
Identification of candidate genes in the type 2 diabetes modifier locus using expression QTL
The paper titled “Identification of candidate genes in the type 2 diabetes modifier locus using expression QTL” by Yaguchi et al., published in Genomics in 2005, discusses the identification of potential candidate genes within a locus that modifies type 2 diabetes. The study utilizes expression quantitative trait loci (eQTL) analysis to identify genes whose expression levels are associated with the modifier locus. This research aims to provide insights into the genetic factors and molecular mechanisms underlying type 2 diabetes, with a focus on genes that may play a role in its modification or development.
For more details https://www.sciencedirect.com/science/article/pii/S0888754305000200
A. Kannt, A. Pfenninger, L. Teichert et al., “Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance,” Diabetologia, vol. 58, no. 4, pp. 799–808, 2015.
Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance
The article titled “Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance” by Kannt et al., published in Diabetologia in 2015, explores the relationship between the expression of nicotinamide-N-methyltransferase (NNMT) mRNA in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. The study investigates the potential link between NNMT activity and insulin resistance, providing insights into the molecular mechanisms associated with metabolic disorders such as diabetes.
For more details https://link.springer.com/article/10.1007/s00125-014-3490-7
D. Kraus, Q. Yang, D. Kong et al., “Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity,” Nature, vol. 508, no. 7495, pp. 258–262, 2014.
Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity
The article titled “Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity” by Kraus et al., published in Nature in 2014, presents research on the role of Nicotinamide N-methyltransferase (NNMT) in diet-induced obesity. The study investigates the effects of reducing NNMT expression, suggesting that its knockdown can protect against the development of obesity in the context of a high-fat diet. This research contributes to our understanding of the molecular factors involved in obesity and highlights NNMT as a potential therapeutic target for obesity-related conditions.
For more details https://www.nature.com/articles/nature13198
S. Hong, J. M. Moreno-Navarrete, X. Wei et al., “Nicotinamide N -methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization,” Nature medicine, vol. 21, no. 8, pp. 887–894, 2015.
Nicotinamide N -methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization
The article titled “Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization” by Hong et al., published in Nature Medicine in 2015, investigates the role of Nicotinamide N-methyltransferase (NNMT) in regulating nutrient metabolism in the liver. The study explores how NNMT influences hepatic metabolism by stabilizing the Sirt1 protein. Sirt1 is known to play a crucial role in metabolic processes, and this research provides insights into the molecular mechanisms by which NNMT impacts nutrient metabolism, shedding light on potential therapeutic targets for metabolic disorders.
For more details https://www.nature.com/articles/nm.3882
J. H. Li, Y. H. Wang, X. J. Zhu, Q. Zhou, Z. H. Xie, and T. F. Yao, “Metabolomics study on the association between nicotinamide N-methyltransferase gene polymorphisms and type 2 diabetes,” International Journal of Diabetes in Developing Countries, vol. 38, pp. 409–416, 2018.
Metabolomics study on the association between nicotinamide N-methyltransferase gene polymorphisms and type 2 diabetes
The paper titled “Metabolomics study on the association between nicotinamide N-methyltransferase gene polymorphisms and type 2 diabetes” by Li et al., published in the International Journal of Diabetes in Developing Countries in 2018, presents a study that investigates the potential association between nicotinamide N-methyltransferase (NNMT) gene polymorphisms and type 2 diabetes. The research employs metabolomics to explore the relationship between genetic variations in the NNMT gene and the development of type 2 diabetes, providing insights into the genetic factors that may contribute to the risk of this metabolic disorder.
For more details https://link.springer.com/article/10.1007/s13410-017-0601-2
Moraes-Vieira, P. M., Saghatelian, A., & Kahn, B. B. (2016). GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects. Diabetes, 65(7), 1808–1815. https://doi.org/10.2337/db16-0221.
GLUT4 expression in adipocytes regulates de novo lipogenesis and levels of a novel class of lipids with antidiabetic and anti-inflammatory effects
The article titled “GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects” by Moraes-Vieira et al., published in Diabetes in 2016, discusses the role of GLUT4 (glucose transporter 4) expression in adipocytes in regulating de novo lipogenesis and the production of a novel class of lipids. These lipids are found to have both antidiabetic and anti-inflammatory effects. The study explores how GLUT4 expression influences lipid metabolism in adipocytes and its potential implications for diabetes and inflammation, providing valuable insights into the complex relationship between glucose transport, lipid synthesis, and metabolic regulation.
For more details https://diabetesjournals.org/diabetes/article-abstract/65/7/1808/16104
Kim HC, et al. Expression and functional significance of nicotinamide N-methyl transferase inskeletal muscles of patients with chronic obstructive pulmonary disease. Am J RespirCrit CareMed. 2010; 181:797–805.
Expression and functional significance of nicotinamide N-methyl transferase inskeletal muscles of patients with chronic obstructive pulmonary disease
The study titled “Expression and functional significance of nicotinamide N-methyl transferase in skeletal muscles of patients with chronic obstructive pulmonary disease” by Kim et al., published in the American Journal of Respiratory and Critical Care Medicine in 2010, investigates the expression and functional role of nicotinamide N-methyl transferase (NNMT) in the skeletal muscles of individuals with chronic obstructive pulmonary disease (COPD). The research explores the potential implications of NNMT in the context of muscle function and COPD, shedding light on its role in the disease and its relevance to muscle-related complications in COPD patients.
For more details https://www.atsjournals.org/doi/abs/10.1164/rccm.200906-0936oc
Savarimuthu Francis SM, et al. Genes and gene ontologies common to airflow obstruction andemphysema in the lungs of patients with COPD. PloS One. 2011; 6:e17442.
Genes and gene ontologies common to airflow obstruction andemphysema in the lungs of patients with COPD
The study titled “Genes and gene ontologies common to airflow obstruction and emphysema in the lungs of patients with COPD” by Savarimuthu Francis et al., published in PLOS ONE in 2011, explores the shared genes and gene ontologies associated with airflow obstruction and emphysema in the lungs of individuals with Chronic Obstructive Pulmonary Disease (COPD). The research aims to identify common molecular pathways and genetic factors that contribute to both airflow limitation and emphysema in COPD patients, providing insights into the underlying mechanisms of the disease and potential therapeutic targets.
For more details https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0017442
Sternak M, et al. Nicotinamide N-methyltransferase (NNMT) and 1-methylnicotinamide (MNA) in experimental hepatitis induced by concanavalin A in the mouse. Pharmacol Rep PR. 2010;62:483–493.
Nicotinamide N-methyltransferase (NNMT) and 1-methylnicotinamide (MNA) in experimental hepatitis induced by concanavalin A in the mouse
The study titled “Nicotinamide N-methyltransferase (NNMT) and 1-methylnicotinamide (MNA) in experimental hepatitis induced by concanavalin A in the mouse” by Sternak et al., published in Pharmacological Reports in 2010, investigates the role of Nicotinamide N-methyltransferase (NNMT) and its product 1-methylnicotinamide (MNA) in an experimental model of hepatitis induced by concanavalin A in mice. The research explores the potential involvement of NNMT and MNA in the context of hepatitis, shedding light on their roles in the pathophysiology of liver inflammation and disease.
For more details https://link.springer.com/article/10.1016/S1734-1140(10)70304-2
Fedorowicz A, et al. Activation of the nicotinamide N-methyltransferase (NNMT)-1-methylnicotinamide (MNA) pathway in pulmonary hypertension. Respir Res. 2016; 17:108.
Activation of the nicotinamide N-methyltransferase (NNMT)-1-methylnicotinamide (MNA) pathway in pulmonary hypertension
The article titled “Activation of the nicotinamide N-methyltransferase (NNMT)-1-methylnicotinamide (MNA) pathway in pulmonary hypertension” by Fedorowicz et al., published in Respiratory Research in 2016, explores the activation of the nicotinamide N-methyltransferase (NNMT) pathway and the production of 1-methylnicotinamide (MNA) in the context of pulmonary hypertension. The study investigates the potential role of this pathway in the pathophysiology of pulmonary hypertension, providing insights into its molecular mechanisms and potential implications for the disease.
For more details https://link.springer.com/article/10.1186/s12931-016-0423-7
Jakubowski A, et al. 1-Methylnicotinamide protects against liver injury induced by concanavalin Avia a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α. IntImmunopharmacol. 2016; 31:98–104.
1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: a possible involvement of IL-4 and TNF-α
The study titled “1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: A possible involvement of IL-4 and TNF-α” by Jakubowski et al., published in the International Immunopharmacology journal in 2016, investigates the protective effects of 1-Methylnicotinamide (MNA) against liver injury induced by concanavalin A. The research suggests that MNA’s protection is mediated through a prostacyclin-dependent mechanism and potentially involves the modulation of interleukin-4 (IL-4) and tumor necrosis factor-alpha (TNF-α). This study provides insights into the mechanisms underlying MNA’s protective effects on liver injury and its potential implications for inflammatory processes involving IL-4 and TNF-α.
For more details https://www.sciencedirect.com/science/article/pii/S1567576915302009
Markert JM, et al. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33.
Differential gene expression profiling in human brain tumors
The study titled “Differential gene expression profiling in human brain tumors” by Markert et al., published in Physiological Genomics in 2001, focuses on the analysis of differential gene expression patterns in human brain tumors. The research aims to identify and characterize the unique gene expression profiles associated with various types of brain tumors. By comparing these profiles, the study seeks to gain a better understanding of the molecular mechanisms underlying brain tumor development and progression, potentially leading to insights into novel diagnostic and therapeutic approaches for these conditions.
For more details https://journals.physiology.org/doi/abs/10.1152/physiolgenomics.2001.5.1.21
Xu J, et al. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J ClinEndocrinolMetab. 2003;88:4990–4996.
Enhanced Expression of Nicotinamide N-Methyltransferase in Human Papillary Thyroid Carcinoma Cells
The study titled “Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells” by Xu et al., published in the Journal of Clinical Endocrinology and Metabolism in 2003, investigates the increased expression of nicotinamide N-methyltransferase (NNMT) in human papillary thyroid carcinoma cells. The research explores the potential role of NNMT in thyroid cancer, providing insights into its involvement in the disease and its potential implications for diagnosis and treatment.
For more details https://academic.oup.com/jcem/article-abstract/88/10/4990/2845853
Yao M, et al. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol. 2005;205:377–387.
Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma
The study titled “Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma” by Yao et al., published in the Journal of Pathology in 2005, conducts gene expression analysis in renal carcinoma. The research identifies adipose differentiation-related protein as a potential biomarker for clear-cell renal carcinoma, suggesting its utility in the diagnosis and prognosis of this type of kidney cancer. This study contributes to the understanding of molecular markers associated with clear-cell renal carcinoma and their potential clinical applications.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1002/path.1693
Wu Y, et al. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–6689.
Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration
The study titled “Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration” by Wu et al., published in the journal Oncogene in 2008, investigates the gene expression profiles associated with both cell migration and tumor invasion in human bladder cancer. The research identifies two novel regulators of cell migration, metallothionein 1E and nicotinamide N-methyltransferase, shedding light on their potential roles in bladder cancer progression. This study contributes to the understanding of the molecular mechanisms underlying cancer cell migration and invasion, which are crucial processes in cancer metastasis and progression.
For more details https://www.nature.com/articles/onc2008264
Jang JS, et al. The differential proteome profile of stomach cancer: identification of the biomarker candidates. Oncol Res. 2004;14:491–499.
The differential proteome profile of stomach cancer: identification of the biomarker candidates
The study titled “The differential proteome profile of stomach cancer: identification of the biomarker candidates” by Jang et al., published in Oncology Research in 2004, focuses on identifying differential protein expression patterns in stomach cancer. The research aims to discover potential biomarker candidates that could be used for the diagnosis or monitoring of stomach cancer. By analyzing the proteome profile of stomach cancer, this study contributes to the search for markers that may assist in the early detection and management of this disease.
For more details https://www.ingentaconnect.com/contentone/cog/or/2004/00000014/00000010/art00004?crawler=true&mimetype=application/pdf
Lim BH, et al. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. ExpMol Med. 2006;38:455–465.
Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification
The study titled “Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification” by Lim et al., published in Experimental and Molecular Medicine in 2006, investigates the overexpression of nicotinamide N-methyltransferase (NNMT) in gastric cancer tissues. The research also explores the potential post-translational modifications of NNMT in the context of gastric cancer. This study contributes to our understanding of the role of NNMT in gastric cancer and its potential implications as a molecular marker or target for therapeutic intervention in the disease.
For more details https://www.nature.com/articles/emm200654
Roessler M, et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:6550–6557.
Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer
The study titled “Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer” by Roessler et al., published in Clinical Cancer Research in 2005, identifies nicotinamide N-methyltransferase (NNMT) as a potential serum tumor marker for colorectal cancer. The research suggests that NNMT could be used as a diagnostic marker to detect colorectal cancer in the blood serum, offering a non-invasive means of screening for this type of cancer. This study contributes to the search for reliable biomarkers for the early detection and monitoring of colorectal cancer.
For more details https://aacrjournals.org/clincancerres/article-abstract/11/18/6550/185985
Sartini D, et al. Nicotinamide N-methyltransferaseupregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol Med Camb Mass. 2007;13:415–421.
Nicotinamide N-methyltransferaseupregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma
The study titled “Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma” by Sartini et al., published in Molecular Medicine in 2007, explores the upregulation of Nicotinamide N-methyltransferase (NNMT) and its correlation with lymph node metastasis in oral squamous cell carcinoma. The research suggests that increased NNMT expression is associated with a reduced likelihood of lymph node metastasis in this type of cancer. This finding may have implications for understanding the molecular mechanisms underlying the spread of oral squamous cell carcinoma and could potentially be relevant for prognosis and treatment strategies.
For more details https://link.springer.com/article/10.2119/2007-00035.Sartini
Wu Y, et al. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–6689.
Yu T, et al. Effects of nicotinamide N-methyltransferase on PANC-1 cells proliferation, metastatic potential and survival under metabolic stress. Cell PhysiolBiochemInt J Exp Cell PhysiolBiochemPharmacol. 2015;35:710–721.
Effects of nicotinamide N-methyltransferase on PANC-1 cells proliferation, metastatic potential and survival under metabolic stress
The study titled “Effects of nicotinamide N-methyltransferase on PANC-1 cells proliferation, metastatic potential and survival under metabolic stress” by Yu et al., published in the journal Cellular Physiology and Biochemistry in 2015, investigates the impact of Nicotinamide N-methyltransferase (NNMT) on various aspects of PANC-1 cells, including proliferation, metastatic potential, and survival under metabolic stress conditions. The research explores how NNMT influences the behavior and survival of these cells, shedding light on its potential role in pancreatic cancer biology and response to metabolic stress. This study contributes to the understanding of the molecular mechanisms involved in cancer progression and adaptation to challenging conditions.
For more details https://karger.com/cpb/article/35/2/710/75011
Tang SW, et al. Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis. 2011;32:138–145.
Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells
The study titled “Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells” by Tang et al., published in Carcinogenesis in 2011, investigates how Nicotinamide N-methyltransferase (NNMT) influences cellular invasion in clear cell renal cell carcinoma. The research focuses on NNMT’s role in activating the expression of matrix metalloproteinase-2 (MMP-2), which is associated with cellular invasion. This study provides insights into the molecular mechanisms underlying the invasive properties of clear cell renal cell carcinoma cells, potentially contributing to our understanding of the disease’s progression and suggesting potential therapeutic targets.
For more details https://academic.oup.com/carcin/article-abstract/32/2/138/2391464
Pozzi V, et al. RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells. PloS One. 2013;8:e71272.
RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells
The study titled “RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells” by Pozzi et al., published in PLOS ONE in 2013, investigates the impact of RNA-mediated gene silencing of Nicotinamide N-methyltransferase (NNMT) on the tumorigenicity of human oral carcinoma cells. The research explores how suppressing NNMT expression through RNA interference affects the cancer cells’ tumorigenic potential. This study provides insights into the potential therapeutic strategies targeting NNMT for the treatment of oral carcinoma and highlights its role in cancer development.
For more details https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0071272
Bach DH, Kim D, Bae SY, et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. MolTher Nucleic Acids. 2018;11:455–467. doi:10.1016/j.omtn.2018.03.011.
Targeting nicotinamide N-methyltransferase and miR-449a in EGFR-TKI-resistant non-small-cell lung cancer cells
The study titled “Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells” by Bach et al., published in Molecular Therapy – Nucleic Acids in 2018, explores the potential therapeutic strategy of targeting Nicotinamide N-Methyltransferase (NNMT) and microRNA-449a (miR-449a) in non-small-cell lung cancer cells that have become resistant to EGFR-TKI (Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors). The research investigates how inhibiting NNMT and utilizing miR-449a can potentially overcome resistance to EGFR-TKI treatment in lung cancer cells. This study contributes to the understanding of molecular mechanisms involved in drug resistance and suggests potential strategies for improving the efficacy of targeted therapies in non-small-cell lung cancer.
For more details https://www.cell.com/molecular-therapy-family/nucleic-acids/fulltext/S2162-2531(18)30042-8
Bae S.Y., Park H.J., Hong J.Y., Lee H.J., Lee S.K. Down-regulation of SerpinB2 is associated with gefitinib resistance in non-small cell lung cancer and enhances invadopodia-like structure protrusions. Sci. Rep. 2016;6:32258.
Down-regulation of SerpinB2 is associated with gefitinib resistance in non-small cell lung cancer and enhances invadopodia-like structure protrusions
The study titled “Down-regulation of SerpinB2 is associated with gefitinib resistance in non-small cell lung cancer and enhances invadopodia-like structure protrusions” by Bae et al., published in Scientific Reports in 2016, investigates the role of SerpinB2 in gefitinib resistance in non-small cell lung cancer. The research explores how the down-regulation of SerpinB2 is associated with resistance to gefitinib, a targeted therapy for lung cancer. Additionally, the study examines how this down-regulation may contribute to the formation of invadopodia-like structure protrusions in cancer cells. Understanding the mechanisms of resistance and invasive behavior in lung cancer cells can have implications for the development of more effective treatments.
For more details https://www.nature.com/articles/srep32258
Gao Y, Van haren MJ, Moret EE, et al. Bisubstrate Inhibitors of Nicotinamide -Methyltransferase (NNMT) with Enhanced Activity. J Med Chem. 2019;62(14):6597-6614.
Bisubstrate Inhibitors of Nicotinamide -Methyltransferase (NNMT) with Enhanced Activity
The study titled “Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity” by Gao et al., published in the Journal of Medicinal Chemistry in 2019, focuses on the development of bisubstrate inhibitors targeting Nicotinamide N-Methyltransferase (NNMT). These inhibitors are designed to have enhanced activity compared to traditional inhibitors. The research explores the potential of these compounds to modulate NNMT activity and may have implications for the development of therapeutic agents targeting NNMT for various medical applications.
For more details https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.9b00413
Neelakantan H, Brightwell CR, Graber TG, et al. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. BiochemPharmacol. 2019;163:481-492.
Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle
The study titled “Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle” by Neelakantan et al., published in Biochemical Pharmacology in 2019, investigates the effects of a small molecule inhibitor of Nicotinamide N-methyltransferase (NNMT) on senescent muscle stem cells and its potential to enhance the regenerative capacity of aged skeletal muscle. The research explores the potential of this inhibitor to activate dormant or senescent muscle stem cells, which may have implications for improving muscle regeneration in the context of aging and age-related muscle decline. This study contributes to our understanding of potential strategies for addressing muscle aging and related issues.
For more details https://www.sciencedirect.com/science/article/pii/S0006295219300462
Zhang H, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances lifespan in mice. Science. 2016; 352:1436–1443.
NAD+ repletion improves mitochondrial and stem cell function and enhances lifespan in mice
The study titled “NAD+ repletion improves mitochondrial and stem cell function and enhances lifespan in mice” by Zhang et al., published in Science in 2016, investigates the effects of replenishing nicotinamide adenine dinucleotide (NAD+) on mitochondrial function, stem cell activity, and overall lifespan in mice. The research explores how boosting NAD+ levels can potentially enhance various aspects of cellular and organismal health, ultimately leading to an extension of the lifespan in mice. This study sheds light on the potential therapeutic applications of NAD+ supplementation for improving aging-related health issues and longevity.
For more details https://www.science.org/doi/abs/10.1126/science.aaf2693
Ryu, D., Zhang, H., Ropelle, E. R., Sorrentino, V., Mázala, D. A., Mouchiroud, L., Marshall, P. L., Campbell, M. D., Ali, A. S., Knowels, G. M., Bellemin, S., Iyer, S. R., Wang, X., Gariani, K., Sauve, A. A., Cantó, C., Conley, K. E., Walter, L., Lovering, R. M., Chin, E. R., … Auwerx, J. (2016). NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Science translational medicine, 8(361), 361ra139. https://doi.org/10.1126/scitranslmed.aaf5504.
NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation
The study titled “NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation” by Ryu et al., published in Science Translational Medicine in 2016, investigates the effects of nicotinamide adenine dinucleotide (NAD+) repletion on muscle function in the context of muscular dystrophy. The research explores how boosting NAD+ levels can potentially improve muscle function and counteract excessive poly(ADP-ribosyl)ation (PARylation) in muscular dystrophy. This study provides insights into potential therapeutic strategies for addressing muscle-related issues in the context of muscular dystrophy and highlights the role of NAD+ in muscle health.
For more details https://www.science.org/doi/abs/10.1126/scitranslmed.aaf5504
Pissios P. (2017). Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends in endocrinology and metabolism: TEM, 28(5), 340–353. https://doi.org/10.1016/j.tem.2017.02.004.
Nicotinamide N-methyltransferase: more than a vitamin B3 clearance enzyme
The review article titled “Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme” by Pissios, published in Trends in Endocrinology and Metabolism (TEM) in 2017, provides an in-depth exploration of the multifaceted roles of Nicotinamide N-Methyltransferase (NNMT). Beyond its traditional role in vitamin B3 clearance, the article discusses how NNMT is involved in various cellular processes, including energy metabolism, glucose regulation, and lipid metabolism. Dysregulation of NNMT is linked to metabolic disorders such as obesity and diabetes, emphasizing its potential as a therapeutic target for these conditions. The article highlights the broader functions of NNMT beyond its enzymatic role in vitamin B3 metabolism.
For more details https://www.cell.com/trends/endocrinology-metabolism/fulltext/S1043-2760(17)30020-6
Other Peptides
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.