GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Gonadorelin
- Key Takeaways
- What is Gonadorelin?
- How Gonadorelin Works
- Chemical Structure of Gonadorelin
- Research on Gonadorelin
- Associated Side Effects of GonadorelinPeptide
- What is Gonadorelin Tablet?
- Gonadotropin Releasing Hormone
- Gonadorelin Dosage
- Gonadorelin vs HCG
- Gonadorelin Acetate
- Gonadorelin and Histerelin
- Gonadorelin and Triptorelin
- Gonadorelin and Leuprolide
- Gonadotropin and Follicle Stimulating Hormone
- Gonadotropin and Luteinizing Hormone
- Gonadotropin and Pituitary Gland
- FAQ
- Reference
Book a Free Consultation
Table of Contents
- Overall Health Benefits of Gonadorelin
- Key Takeaways
- What is Gonadorelin?
- How Gonadorelin Works
- Chemical Structure of Gonadorelin
- Research on Gonadorelin
- Associated Side Effects of GonadorelinPeptide
- What is Gonadorelin Tablet?
- Gonadotropin Releasing Hormone
- Gonadorelin Dosage
- Gonadorelin vs HCG
- Gonadorelin Acetate
- Gonadorelin and Histerelin
- Gonadorelin and Triptorelin
- Gonadorelin and Leuprolide
- Gonadotropin and Follicle Stimulating Hormone
- Gonadotropin and Luteinizing Hormone
- Gonadotropin and Pituitary Gland
- FAQ
- Reference
Overall Health Benefits of Gonadorelin
Gonadorelin benefits include stimulating the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are essential for reproductive health and fertility. It is used in diagnostic testing for pituitary gland function and in treating reproductive disorders such as amenorrhea and delayed puberty.
- Treats testicular atrophy [1-2]
- Improves fertility by increasing testosterone production [3-23]
- Increases sexual desire [24-25]
- Prevents cancer [26-35]
- Increases muscle mass [36-39]
- Promotes healthier bones [40-43]
- Improves mood [44-45]
- Treats amenorrhea [46-47]
Key Takeaways
- Hormone Release Stimulation: Gonadorelin stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are critical for reproductive health.
- Diagnostic Use: It is utilized in diagnostic testing to assess pituitary gland function and diagnose conditions related to hormonal imbalances.
- Treatment of Reproductive Disorders: Gonadorelin is used in the treatment of reproductive disorders such as amenorrhea (absence of menstruation) and delayed puberty.
- Fertility Support: By promoting the release of essential hormones, Gonadorelin supports fertility and aids in the management of infertility issues.
- Clinical Applications: It has important clinical applications in endocrinology and gynecology, helping to manage and treat various hormonal and reproductive conditions.
What is Gonadorelin?
Gonadorelin, also known as Gonadotropin-releasing hormone (GnRH), is produced in the brain region called hypothalamus. This hormone stimulates the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland. Gonadorelin is mainly used for treating infertility, delayed puberty, and amenorrhea (absence of menstruation).
How Gonadorelin Works
IMG
Gonadorelin stimulates the pituitary gland to increase the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In women, gonadorelin is used to stimulate the release of an egg from the ovary. This in turn results in regular ovulation and higher chances of pregnancy. In men, gona to stimuate spermatogenesis or sperm production. It also boosts the production of testosterone by the teste. Gonadorelin is used in conjunction with testosterone to minimize testicular atrophy (shrinking of the testicles).
Chemical Structure of Gonadorelin
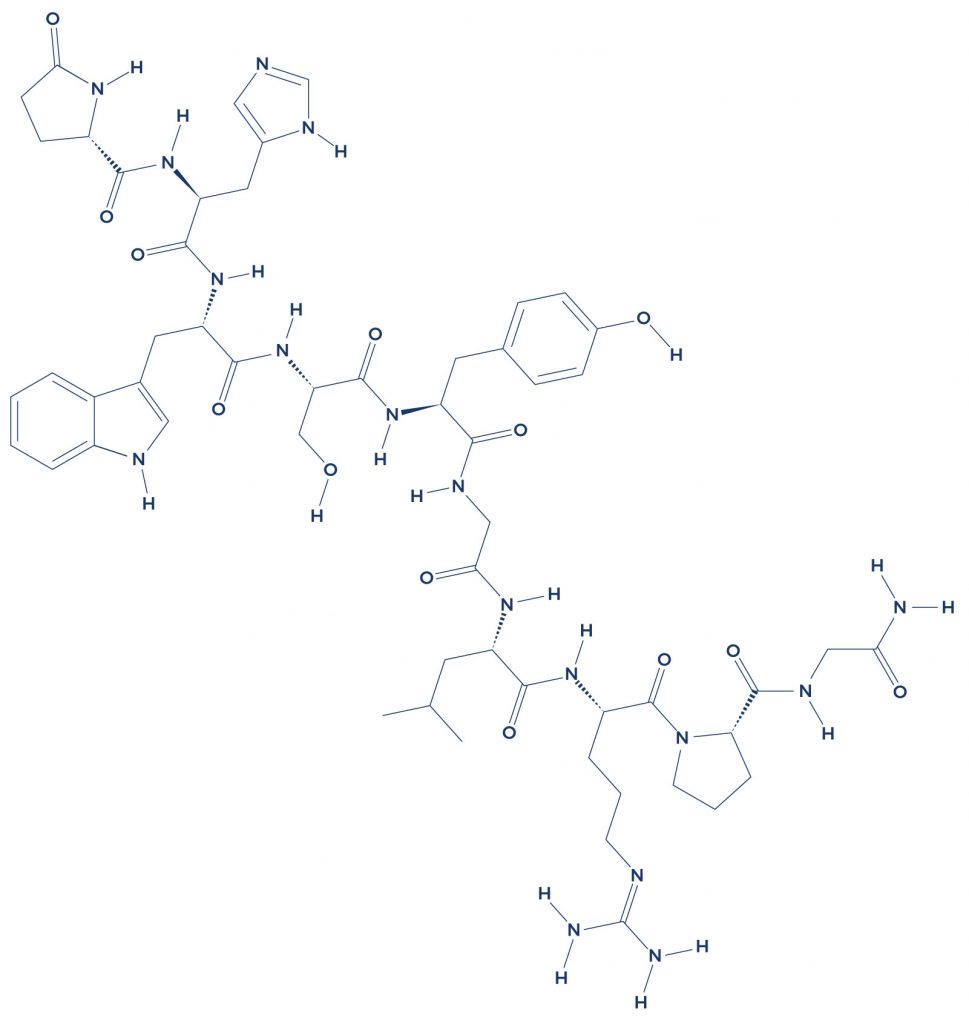
Research on Gonadorelin
A. Treats Testicular Atrophy
IMG
There are studies supporting the beneficial effects of gonadorelin on testicular atrophy, a condition characterized by shrinking of the testicles:
- In infants with undescended testes, gonadorelin therapy through nasal spray significantly increased the testicular volume after 5 years. [1]
- In adolescents who had left varicocelectomy (removal of enlarged veins in the scrotum), gonadorelin treatment increased testicular volume. [2]
B. Improves Fertility by Increasing Testosterone Production
A number of convincing studies suggest that gonadorelin can help improve fertility in both men and women:
- In men with steroid-induced azoospermia (absence of sperm), gonadorelin therapy resulted in increased sperm production. [3]
- In boys and girls with delayed puberty, long-term administration with gonadorelin improved reproductive health. [4]
- In men with testosterone deficiency, gonadorelin was found to be helpful in restoring sperm production. [5]
- In male goats, a single dose of gonadorelin exhibited beneficial effects on testicular blood flow which in turn improved sperm production. [6]
- In men with testosterone deficiency, long-term gonadorelin therapy was successful in stimulating sexual maturation. [7]
- In goats, gonadorelin administration promoted ovulation and resulted in a higher number of embryos. [8]
- In dairy cattles, gonadorelin administration after insemination resulted in an increased pregnancy rate. [9]
- In cows with low sexual cycles, gonadorelin injections were found to be effective in increasing the number of the subjects’ pregnancies. [10]
- In women, pulsatile administration of gonadorelin was successful in improving the pregnancy rate of the subjects with lesser risks than the conventional gonadotropin treatment. [11]
- In breeder cows, conception rates became significantly high after gonadorelin injection. [12]
- A study showed that gonadorelin treatment was effective in restoring sperm production in infertile men. [13]
- In infertile men, gonadorelin treatment increased testosterone and sperm production. [14-17]
- In patients with Kallman’s syndrome (delayed or absent puberty) who failed traditional treatment, gonadorelin treatment restored sperm production. [18]
- In adolescent boys, low-dose administration of gonadorelin induced testicular growth and sperm production. [19]
- A study showed that gonadorelin therapy could be used to trigger testosterone and sperm production in men with sex hormone deficiency. [20-22]
- In cows, gonadorelin treatment resulted in a higher ovulatory period rate. [23]
C. Increases Sexual Desire

Gonadorelin has also been found to increase sexual desire:
- In camel bulls, gonadorelin administration resulted in improved sperm concentration and libido. [24]
- In female monkeys, gonadorelin injection resulted in improved sexual behavior. [25]
D. Prevents Cancer

Evidence suggests that gonadorelin has potent anti-cancer properties:
- In mice, gonadorelin injection resulted in the suppression of intestinal and colonic tumor growth. [26]
- In pre-menopausal women with early and advanced breast cancer, gonadorelin therapy prevented the h of breast cancer cells. [27]
- A study suggested that gonadorelin can help suppress the growth of prostate cancer. [28]
- A study showed that gonadorelin may play an important role in modulating several malignant human tumors. [29]
- Research found that gonadorelin is safe and cost-effective and that using it for 15 years could reduce the risk of breast cancer by 70%. [30]
- Gonadorelin has been found to reduce the growth of estrogen-sensitive cancer and boost the efficacy of receptor-blocking medications. [31]
- In postmenopausal women with high levels of estrogen, long-term GnRH treatment was associated with a reduced risk of breast cancer. [32]
- In men with aggressive prostate cancer, gonadorelin administration has been found to produce similar efficacy to surgical removal of the testicles in preventing the spread of cancer. [33]
- Research suggests that gonadorelin may help treat castration-resistant prostate cancer. [34]
- When used effectively and combined with early detection, the addition of gonadorelin to chemotherapeutic drugs may help cure 99% of all prostate cancer. [35]
E. Increases Muscle Mass

Studies suggest that gonadorelin is essential for muscle health:
- In healthy young men, administration of gonadorelin significantly improved muscle mass and strength. [36]
- In healthy older men, monthly treatment with gonadorelin increased muscle mass and leg strength. [37]
- A study showed that gonadorelin treatment among male subjects resulted in increased muscle mass strength. [38]
- In healthy older men, the increase in testosterone caused by gonadorelin administration induced muscle improvements. [39]
F. Promotes Healthier Bones
Gonadorelin is also important for maintaining a healthy skeletal frame according to studies:
- In young men with sex hormone deficiency, gonadorelin treatment resulted in increased bone mineral density. [40]
- In patients with bone disorders caused by ovarian cysts, gonadorelin treatment increased bone mineral density. [41]
- In women with endometriosis, a condition where the womb tissue starts to grow in other places, gonadorelin treatment combined with physical training resulted in bone formation. [42]
- A study found that surgically-castrated men with prostate cancer who received gonadorelin treatment had a lower rate of bone loss compared to the untreated group. [43]
G. Improves Mood
Studies suggest that gonadorelin can help improve mood through its anti-depressant effects:
- In women with severe premenstrual syndrome, daily intranasal administration of low-dose gonadorelin greatly reduced depression and irritability. [44]
- In men with central hypogonadism, gonadorelin administration produced positive effects on mood by treating sexual dysfunction. [45]
H. Treats Amenorrhea
Gonadorelin can also help restore a normal menstrual cycle according to clinical studies:
- In women with amenorrhea, administration of gonadorelin produced menstruation without any side effects. [46]
- In women with primary and secondary amenorrhea, gonadorelin treatment was extremely effective in inducing ovulation and menstruation. [47]
Associated Side Effects of GonadorelinPeptide
Gonadorelin side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on gonadorelin. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of gonadorelin. Despite this, it was listed as a side effect associated with gonadorelin even though these associated side effects are very uncommon.
Side effects associated with gonadorelin may include the following:
- Abdominal discomfort
- Dizziness
- Flushing
- Headache
- Lightheadedness
- Nausea
- Skin rash
What is Gonadorelin Tablet?
Gonadorelin tablet is a synthetic form of gonadotropin-releasing hormone (GnRH), which is naturally produced in the hypothalamus. This hormone plays a crucial role in regulating the release of two key reproductive hormones from the pituitary gland: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are essential for normal reproductive function, including the regulation of the menstrual cycle in women and the production of sperm in men.
The primary use of Gonadorelin tablets is in the treatment of reproductive disorders. For example, it is prescribed for individuals experiencing amenorrhea (the absence of menstruation) or delayed puberty, helping to stimulate the body’s natural production of LH and FSH. By doing so, Gonadorelin tablets can help restore normal menstrual cycles in women and support the onset of puberty in adolescents with delayed development. Additionally, these tablets are used in diagnostic testing to evaluate the functioning of the pituitary gland and identify potential hormonal imbalances.
In clinical practice, Gonadorelin tablets offer a convenient and effective means of managing various reproductive health issues. They are particularly valuable in cases where natural GnRH production is insufficient or disrupted, providing a targeted approach to hormone regulation. The tablets are typically well-tolerated, but like any medication, they should be used under the supervision of a healthcare professional to ensure proper dosage and to monitor for any potential side effects. Overall, Gonadorelin tablets play a significant role in the field of reproductive endocrinology, helping to address and manage conditions related to hormonal deficiencies and imbalances.
Gonadotropin Releasing Hormone
Gonadotropin-releasing hormone (GnRH) is a peptide hormone produced in the hypothalamus that plays a crucial role in regulating the reproductive system. It stimulates the anterior pituitary gland to release two key gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are essential for the proper functioning of the ovaries in women, including ovulation and the menstrual cycle, and the testes in men, including spermatogenesis. GnRH is released in a pulsatile manner, which is vital for its proper functioning and the subsequent regulation of reproductive hormones and processes.
Gonadorelin Dosage
Gonadorelin dosage varies depending on the condition being treated and the specific needs of the patient. For diagnostic purposes, a single injection of Gonadorelin is often administered to stimulate the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The typical diagnostic dose ranges from 0.1 mg to 0.2 mg, injected subcutaneously or intravenously. This helps healthcare providers evaluate the function of the pituitary gland and diagnose conditions related to hormonal imbalances.
In therapeutic applications, such as the treatment of amenorrhea or delayed puberty, Gonadorelin is administered more frequently and in different dosages. The standard therapeutic dose may vary but typically involves continuous or pulsatile administration to mimic the natural release patterns of gonadotropin-releasing hormone (GnRH). Continuous administration usually involves a dosage of 5 to 20 micrograms per hour delivered via a pump, while pulsatile administration mimics the body’s natural rhythms more closely and may involve doses of 5 to 20 micrograms every 90 minutes.
Dosage adjustments are often necessary based on the patient’s response and any side effects experienced. Healthcare providers monitor hormone levels and clinical symptoms to fine-tune the dosage, ensuring optimal therapeutic outcomes while minimizing adverse effects. It is crucial that Gonadorelin administration and dosage adjustments are conducted under strict medical supervision to ensure safety and efficacy. Consult with your doctor immediately if you experience any adverse effects.
Gonadorelin vs HCG
Gonadorelin and human chorionic gonadotropin (HCG) are both used in medical treatments related to reproductive health, but they have distinct mechanisms and applications. Gonadorelin is a synthetic version of gonadotropin-releasing hormone (GnRH) that stimulates the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are crucial for regulating the reproductive system, making Gonadorelin useful in diagnosing and treating hormonal imbalances, such as amenorrhea and delayed puberty. It is often employed in controlled clinical settings to assess pituitary function and stimulate ovulation in women undergoing fertility treatments.
HCG, on the other hand, mimics the action of LH and is used primarily to trigger ovulation and support the luteal phase of the menstrual cycle in women undergoing fertility treatments, such as in vitro fertilization (IVF). HCG is also used in men to stimulate testosterone production and spermatogenesis, addressing conditions like hypogonadism and infertility. Unlike Gonadorelin, which acts on the pituitary gland to induce the release of LH and FSH, HCG directly stimulates the ovaries and testes, providing a more immediate hormonal response.
While both Gonadorelin and HCG play vital roles in reproductive health, their different mechanisms of action and clinical applications highlight the importance of choosing the appropriate treatment based on the specific medical condition. Gonadorelin is more diagnostic and regulatory in its use, focusing on stimulating the body’s natural hormone production pathways. In contrast, HCG provides direct hormonal stimulation, making it suitable for cases requiring immediate intervention in hormone levels. Understanding these differences is crucial for healthcare providers in tailoring effective treatment plans for patients with reproductive health issues.
Gonadorelin Acetate
Gonadorelin acetate, a synthetic version of Gonadorelin, plays a crucial role in clinical medicine, primarily in the field of endocrinology and reproductive health. This peptide is designed to mimic the natural gonadotropin-releasing hormone (GnRH) produced in the hypothalamus. Its primary function is to stimulate the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones, in turn, stimulate the production of testosterone in males and estrogen in females, essential for normal reproductive function.
In clinical practice, Gonadorelin acetate is utilized for diagnostic purposes to assess pituitary gland function. By administering Gonadorelin acetate and measuring the subsequent rise in LH and FSH levels, healthcare providers can evaluate the integrity of the hypothalamic-pituitary-gonadal axis. This is crucial in diagnosing conditions such as hypothalamic amenorrhea, delayed puberty, and other hormonal disorders affecting fertility.
Moreover, Gonadorelin acetate finds therapeutic applications in managing certain reproductive disorders. It is prescribed to stimulate ovulation in women undergoing assisted reproductive technologies, such as in vitro fertilization (IVF). Additionally, it is used to treat hypogonadotropic hypogonadism, a condition where the gonads produce little or no hormones due to insufficient stimulation by the pituitary gland. This versatile peptide continues to be a cornerstone in reproductive endocrinology, offering diagnostic insights and therapeutic benefits to patients worldwide.
Gonadorelin and Histerelin
Gonadorelin and Histerelin are both synthetic forms of gonadotropin-releasing hormone (GnRH) that play significant roles in reproductive medicine. GnRH is a crucial hormone produced in the hypothalamus, responsible for regulating the release of LH and FSH from the pituitary gland. These hormones, in turn, stimulate the production of sex hormones such as testosterone and estrogen, essential for fertility and reproductive health.
Gonadorelin is commonly used in clinical settings to diagnose and treat conditions related to reproductive health. It stimulates the pituitary gland to release LH and FSH, making it valuable in assessing pituitary function and diagnosing disorders such as infertility, amenorrhea, and delayed puberty. In therapeutic applications, Gonadorelin can help restore normal hormone levels in individuals with hypothalamic or pituitary dysfunction, thereby aiding in fertility treatments.
Similarly, Histerelin functions similarly to Gonadorelin but may have different clinical uses and formulations. Both medications are administered either as injections or nasal sprays, depending on the specific treatment goals and patient needs. Their controlled use under medical supervision is crucial due to their potent effects on hormonal balance and reproductive function. Research continues to explore their efficacy in managing various reproductive disorders and optimizing fertility treatments for both men and women.
Gonadorelin and Triptorelin
Gonadorelin and Triptorelin are both synthetic forms of gonadotropin-releasing hormone (GnRH) used in medical practice for their ability to influence hormonal pathways. Gonadorelin functions by stimulating the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This stimulation is crucial in regulating the reproductive system, making Gonadorelin valuable in diagnosing and treating conditions related to fertility and pituitary function disorders. It is administered via injection and is particularly useful in assessing the integrity of the hypothalamic-pituitary-gonadal axis in both men and women.
Triptorelin, on the other hand, is another synthetic GnRH agonist that works similarly to Gonadorelin but with a longer duration of action. It is frequently used in the treatment of hormone-dependent conditions such as prostate cancer, breast cancer, and endometriosis. By continuously stimulating the pituitary gland, Triptorelin initially causes an increase in LH and FSH release, followed by a decrease due to downregulation of GnRH receptors. This property allows Triptorelin to effectively suppress sex hormone production over extended periods, making it a cornerstone in managing hormone-sensitive cancers and other disorders where hormone reduction is beneficial.
Both Gonadorelin and Triptorelin exemplify the clinical versatility of GnRH agonists in manipulating hormonal pathways for therapeutic purposes. While Gonadorelin is primarily diagnostic and supportive in reproductive health, Triptorelin extends its utility to therapeutic interventions in oncology and gynecology, offering precise control over hormone levels critical for disease management. Their distinct pharmacokinetic profiles cater to specific medical needs, underscoring their importance in modern medical practice.
Gonadorelin and Leuprolide
Gonadorelin and Leuprolide are both synthetic analogs of gonadotropin-releasing hormone (GnRH) used in medicine to regulate reproductive hormones. They function by initially stimulating the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland. Gonadorelin is often employed in diagnostic tests to assess pituitary gland function and evaluate conditions related to hormonal imbalances, whereas Leuprolide is primarily used in therapeutic settings for its potent and sustained suppression of LH and FSH release. This suppression is beneficial in treating hormone-dependent conditions such as prostate cancer, endometriosis, and certain types of infertility.
While both medications share the same basic mechanism of action, their clinical applications differ significantly. Gonadorelin is commonly used in controlled ovarian stimulation protocols during assisted reproductive technologies (ART), helping to induce ovulation in women undergoing fertility treatments. On the other hand, Leuprolide’s ability to suppress LH and FSH production makes it a cornerstone in the management of hormone-sensitive cancers and disorders. Its long-acting formulations are particularly advantageous in providing sustained hormonal suppression without the need for frequent dosing.
Despite their therapeutic benefits, both Gonadorelin and Leuprolide can cause side effects related to hormonal fluctuations. These can include hot flashes, mood swings, and changes in libido. Careful monitoring and adjustment of dosage are essential to minimize these effects and optimize treatment outcomes. Overall, while Gonadorelin and Leuprolide serve distinct roles in medical practice, their shared foundation in regulating reproductive hormones underscores their importance in managing a variety of hormonal disorders and conditions.
Gonadotropin and Follicle Stimulating Hormone
Follicle-stimulating hormone (FSH) is a vital gonadotropin produced and secreted by the anterior pituitary gland. It plays a critical role in regulating the reproductive processes in both males and females. In women, FSH stimulates the growth and maturation of ovarian follicles, which are essential for ovulation and the menstrual cycle. It works in tandem with luteinizing hormone (LH) to ensure the proper development and release of eggs from the ovaries. In men, FSH is essential for spermatogenesis, as it acts on the Sertoli cells in the testes to promote the production and maturation of sperm. FSH levels are regulated by the gonadotropin-releasing hormone (GnRH) from the hypothalamus and are crucial for maintaining fertility and reproductive health.
Gonadotropin and Luteinizing Hormone
Gonadotropin is a general term for hormones that stimulate the activity of the gonads (ovaries and testes), with luteinizing hormone (LH) being one of the primary gonadotropins produced by the anterior pituitary gland. LH plays a crucial role in the reproductive system: in women, it triggers ovulation and stimulates the corpus luteum to produce progesterone, essential for maintaining the uterine lining for potential pregnancy. In men, LH stimulates the Leydig cells in the testes to produce testosterone, which is necessary for the development of male secondary sexual characteristics and spermatogenesis. The release of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus, and its proper function is vital for fertility and reproductive health.
Gonadotropin and Pituitary Gland
Gonadotropins, which include luteinizing hormone (LH) and follicle-stimulating hormone (FSH), are essential hormones produced and secreted by the anterior pituitary gland. The pituitary gland, often termed the “master gland,” plays a pivotal role in regulating the endocrine system, including reproductive functions. Gonadotropins are released in response to gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, LH and FSH regulate the menstrual cycle, ovulation, and the maintenance of the corpus luteum, while in males, they are crucial for spermatogenesis and the production of testosterone. The interplay between the pituitary gland, gonadotropins, and the gonads ensures the proper functioning of reproductive processes and overall hormonal balance.
FAQ
What does gonadorelin do for a man?
Gonadorelin stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the hypothalamic pituitary axis pituitary gland, which in turn stimulates the testes to produce testosterone.
How much will gonadorelin increase testosterone?
Gonadorelin itself does not directly increase testosterone levels. It stimulates the pituitary gland to release LH, which then stimulates testosterone production in the testes. The increase in testosterone can vary depending on individual hormone levels and the specific condition being treated. Gonadorelin is also used to stimulate breast milk production in lactating women.
What are the side effects of gonadorelin peptide?
Common side effects of gonadorelin can include hot flashes, headache, nausea, and local reactions at the naturally released injection site. Rarely, it may cause allergic reactions or changes in mood. Consult with your doctor immediately if you experience any unpleasant side effects.
What does gonadotropin do in males?
Gonadotropins, such as LH and FSH, regulate testicular function in males. LH stimulates testosterone production, while FSH supports sperm production (spermatogenesis). It’s crucial to consult a doctor immediately if there are any concerns about these hormone levels or reproductive health issues. It’s crucial to consult a doctor immediately if there are any concerns about these hormone levels or reproductive health issues. It’s crucial to consult a doctor immediately if there are any concerns about these hormone levels or reproductive health issues.
Does gonadorelin cause erectile dysfunction?
Gonadorelin is not typically associated with causing difficulty breathing or erectile dysfunction. Its primary role is to regulate hormone levels rather than directly affecting difficulty breathing and erectile function. However, in rare cases, individuals may experience difficulty breathing and erectile dysfunction as a side effect.
Does gonadorelin cause weight gain?
Weight gain is not a commonly reported side effect of gonadorelin. GnRH pulses play a crucial role in regulating hormonal balance and reproductive functions. GnRH pulses are essential for maintaining proper endocrine function and reproductive health.
How often should I inject gonadorelin?
The frequency of gonadorelin injections for male patients depends on the specific condition being treated and the formulation used. It is typically administered as needed according to a healthcare provider’s instructions. Male patients should follow their healthcare provider’s guidance closely to ensure optimal treatment outcomes.
What is gonadorelin used for?
The frequency of gonadorelin injections for male patients depends on the specific condition being treated and the formulation used. It is typically administered as needed according to a healthcare provider’s instructions. Male patients should follow their healthcare provider’s guidance closely to ensure optimal treatment outcomes.
Can gonadorelin cause erectile dysfunction?
Gonadorelin is not known to cause erectile dysfunction. Its primary function is to stimulate hormone production rather than affecting erectile function directly. However, it’s important to consult with your healthcare provider about potential interactions with other medicines that could affect its effectiveness.
Is gonadorelin available in the USA?
Yes, gonadorelin is available in the USA under various brand names for medical use. Low testosterone Gonadorelin Low testosterone is a synthetic form of gonadotropin-releasing hormone (GnRH) that stimulates the production of gonadotropins (LH and FSH) from the anterior pituitary gland.
Does gonadorelin increase testosterone in men?
Yes, gonadorelin indirectly increases testosterone levels by stimulating the release of LH, which then stimulates LH response and testosterone production in the testes. This process is crucial for maintaining normal testosterone levels in the body.
What is gonadorelin injection used for?
Gonadorelin injections are used for diagnosing disorders of the pituitary gland and for treating conditions where there is inadequate production of sex hormones. They are also beneficial in managing other drug disorders that affect hormone levels and reproductive health.
Why would a man take gonadorelin?
A man might take gonadorelin to treat conditions such as delayed puberty, infertility due to low testosterone levels, or to stimulate testicular function in certain medical conditions. It is crucial to follow the doctor’s instructions when using gonadorelin to ensure safe and effective treatment. Adherence to the doctor’s instructions is essential for achieving desired health outcomes. Always consult with a healthcare professional for proper guidance on the use of gonadorelin and follow the doctor’s instructions diligently throughout the treatment process.
Does gonadorelin increase seminal fluid?
Gonadorelin primarily affects hormone levels and is not specifically known to increase seminal fluid. However, its impact on functional capacity in relation to hormone regulation is significant, influencing various physiological processes.
Does gonadorelin work as well as HCG?
Benefits include its ability to diagnose and treat hormone-related disorders, support fertility treatment protocols, and regulate reproductive health in both men and women. In addition to these benefits, it can also assist in managing the effects of other medications and hormone therapy.
What are the benefits of gonadorelin?
Benefits include its ability to diagnose and treat hormone-related disorders, support fertility treatment protocols, and regulate reproductive health in both men and women. In addition to these benefits, it can also assist in managing the effects of other medications and hormone therapy.
Will gonadorelin increase testicle size?
Gonadorelin primarily affects hormone levels and is not typically associated with directly increasing testicle size. However, research suggests that Gonadorelin, through its action on the arcuate nucleus, can modulate gonadotropin-releasing hormone (GnRH) secretion, which in turn influences testosterone production. This mechanism involving the arcuate nucleus highlights its role in regulating reproductive hormone levels and, consequently, testicular function.
What does gonadorelin acetate do?
Gonadorelin acetate, also known as gonadorelin, is a synthetic form of gonadorelin used in medical treatments to regulate hormone levels and diagnose pituitary gland disorders. Gonadorelin passes acetate is crucial in stimulating the release of gonadotropins, which are hormones involved in reproductive functions. Gonadorelin is administered via injection and acts by stimulating the pituitary gland, thereby inducing the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This process is essential for maintaining fertility and normal reproductive function in both men and women.
Does gonadorelin raise testosterone?
Gonadorelin indirectly raises testosterone levels by stimulating the release of LH, which then stimulates potential healthy testosterone production in the testes. This mechanism underscores its potential health impact on overall testosterone levels and highlights its relevance in therapeutic contexts aimed at addressing hormonal imbalances and related conditions
How long does the histrelin implant last?
The histrelin implant can last for up to 12 months, providing continuous suppression of LH and FSH response release. This sustained LH and FSH response suppression is particularly beneficial for managing conditions such as precocious puberty.
How much does the histrelin implant cost?
The cost of the histrelin implant, brand name Factrel, can vary, but it generally ranges from several hundred to several thousand dollars, depending on the specific formulation and healthcare provider. The brand name Factrel is known for its efficacy in treatment. The brand name Factrel is recommended by healthcare providers for its quality and effectiveness.
What is the classification of histrelin?
Histrelin is classified as a gonadotropin-releasing hormone (GnRH) agonist that targets kisspeptin neurons in the hypothalamus, regulating reproductive function. Activation of kisspeptin neurons by histrelin suppresses the release of gonadotropins, thereby controlling the reproductive cycle.
Is histrelin acetate a hormone?
Yes, histrelin acetate is a synthetic hormone analog that acts similarly to natural GnRH in the body. It is often prescribed by doctors to manage certain hormone-related conditions, including those involving androgens. This medication requires careful monitoring by a doctor to ensure its effectiveness and safety against androgens.
What is cystorelin used for?
Cystorelin, which contains GnRH (gonadotropin-releasing hormone), is used primarily in veterinary medicine to induce ovulation in cattle and synchronize estrus cycles. GnRH stimulates estrogen production in female cattle, thereby facilitating reproductive processes.
Does Cystorelin contain GnRH?
Yes, Cystorelin contains GnRH (gonadotropin-releasing hormone), which can affect hormone levels. GnRH, a synthetic decapeptide, plays a crucial role in regulating reproductive hormones. It’s important to be aware of potential serious side effects associated with medications like Cystorelin due to their impact on hormone levels. Serious side effects can occur as a result of GnRH’s influence on reproductive hormones. It’s crucial to monitor for any serious side effects that may arise from using medications containing GnRH.
How much Cystorelin to give cows?
The dosage of Cystorelin for cattle can vary based on specific protocols but typically involves a single injection at a specific stage of the estrous cycle to prevent testicular shrinkage. Preventing testicular shrinkage is a crucial consideration when administering Cystorelin. Therefore, ensuring the correct dosage and timing is essential to prevent testicular shrinkage.
What is the use of Cystorelin in dogs?
In dogs, Cystorelin is used similarly to induce ovulation and regulate reproductive cycles in breeding programs or for medical treatment of certain conditions such as testicular shrinkage. Additionally, testicular shrinkage can be a concern in various medical treatments, and using Cystorelin can help manage these reproductive issues. In some cases, testicular shrinkage might necessitate specific interventions, and Cystorelin provides a viable option for addressing such reproductive health challenges in dogs.
What is triptorelin used for?
Triptorelin is used to treat conditions such as prostate cancer, breast cancer, and endometriosis, and to manage precocious puberty by suppressing gonadotropin secretion. Triptorelin can also lead to testicular shrinkage in patients. It is important to monitor for testicular shrinkage during treatment. Patients should discuss the potential for testicular shrinkage with their healthcare provider before starting treatment with Triptorelin.
Is triptorelin a chemotherapy drug?
Triptorelin is not a chemotherapy drug. It is a synthetic analog of GnRH used to manage hormone-related conditions, including those associated with a pituitary tumor. In some cases, treatment for a pituitary tumor may involve hormone therapy. Therefore, triptorelin can be a part of the management strategy for conditions linked to a pituitary tumor.
Where is triptorelin injected?
Triptorelin is typically administered as an injection into the muscle (intramuscular) or under the skin (subcutaneous). The GnRH receptor is involved in the mechanism of action of Triptorelin. Understanding the role of the GnRH receptor helps in optimizing the treatment. Studies on the GnRH receptor have shown promising results in enhancing the efficacy of Triptorelin.
Does triptorelin increase testosterone?
Initially, triptorelin may cause a transient increase in testosterone levels due to a phenomenon known as “flare,” followed by a decrease in testosterone levels with continued treatment. This initial flare is associated with an increase in GnRH secretion. However, with continued treatment, GnRH secretion is suppressed, leading to lower testosterone levels. The reduction in GnRH secretion ultimately helps in managing conditions associated with high testosterone levels.
What is the drug leuprolide used for?
Leuprolide is used in the treatment of prostate cancer, endometriosis, uterine fibroids, and precocious puberty to suppress the production of gonadotropins and other hormones. In treating prostate cancer, other hormones may also be involved in the therapeutic process. Additionally, when addressing conditions like endometriosis and uterine fibroids, other hormones might play a role in the overall management and treatment strategy.
Is leuprolide the same as Lupron?
Yes, Leuprolide is the generic name for the drug commonly known as Lupron. The hypothalamus gland plays a crucial role in regulating the release of hormones, which is why Leuprolide affects its function. Understanding the relationship between Leuprolide and the hypothalamus gland is essential for comprehending its mechanism. Furthermore, the hypothalamus gland’s involvement in hormone regulation highlights the importance of Leuprolide in medical treatments.
What does Lupron do to your body?
Lupron (leuprolide) suppresses the production of sex hormones (testosterone in males and estrogen in females) by acting on the pituitary gland to decrease gonadotropin release. Blood samples are often taken to monitor hormone levels before, during, and after treatment with Lupron. By analyzing these blood samples, doctors can assess the effectiveness of the medication. Regular blood samples help ensure that the suppression of sex hormones is maintained appropriately.
How does leuprolide affect hormones?
Leuprolide works by initially causing a surge in hormone production (known as a “flare” effect) followed by a sustained decrease in hormone levels through desensitization of the hypothalamus and pituitary glands. This is useful in treating conditions dependent on sex hormone production. The interaction between the hypothalamus and pituitary glands is crucial in regulating the body’s hormone levels. By targeting the hypothalamus and pituitary glands, leuprolide effectively manages conditions that rely on controlled hormone production.
What is gonadorelin injection?
Gonadorelin injection is a synthetic form of gonadotropin-releasing hormone (GnRH) that plays a crucial role in regulating the reproductive system. When administered, it stimulates the pituitary gland to release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones are essential for proper reproductive function, including the maturation of ovarian follicles in women and the production of sperm in men. The injection is commonly used in clinical settings to diagnose and treat hormonal imbalances and reproductive disorders.
Reference
Spinelli C, Strambi S, Busetto M, Pucci V, Bianco F. Effects on normalized testicular atrophy index (TAIn) in cryptorchid infants treated with GnRHa pre and post-operative vs surgery alone: a prospective randomized trial and long-term follow-up on 62 cases. Pediatr Surg Int. 2014 Oct;30(10):1061-7. doi: 10.1007/s00383-014-3577-8. Epub 2014 Aug 9. PMID: 25106891.
Pediatric Surgery International
The study conducted by Spinelli C, Strambi S, Busetto M, Pucci V, Bianco F, and published in “Pediatric Surgery International” in October 2014, focuses on the effectiveness of preoperative and postoperative treatment with gonadotropin-releasing hormone analog (GnRHa) in infants with cryptorchidism, compared to surgery alone. This prospective, randomized trial involved 62 cases and included long-term follow-up. The main outcome measure was the normalized testicular atrophy index (TAIn), which is a parameter used to evaluate testicular volume and health.
The study’s findings suggest that patients with a TAIn greater than 20% who were treated with GnRHa both before and after surgery showed a significant increase in testicular volume after 5 years of follow-up, indicated by a relative reduction of TAIn values. This suggests that GnRHa therapy, when used in conjunction with surgery, may have beneficial effects on testicular health in cryptorchid infants, potentially improving long-term outcomes related to fertility and testicular function.
For more details visit https://pubmed.ncbi.nlm.nih.gov/25106891/
Fisch H, Hyun G, Hensle TW. Testicular growth and gonadotrophin response associated with varicocele repair in adolescent males. BJU Int. 2003 Jan;91(1):75-8. doi: 10.1046/j.1464-410x.2003.03078.x. PMID: 12614255.
Testicular growth and gonadotrophin response associated with varicocele repair in adolescent males
The study by Fisch, Hyun, and Hensle, published in “BJU International” in January 2003, aimed to assess testicular growth and the gonadotropin response to gonadotropin-releasing hormone (GnRH) stimulation in adolescents undergoing left varicocelectomy. The research involved 13 adolescents who had their testicular volume and endocrine function evaluated before and after surgery.
This study is significant as it focuses on the outcomes of varicocele repair in adolescent males, a topic of interest due to the potential impact of varicoceles on fertility and testicular function. By evaluating testicular volume and gonadotrophin response pre and post-surgery, the research provides insights into the benefits of varicocele repair beyond symptomatic relief, suggesting potential implications for fertility and hormonal balance.
For more details visit https://pubmed.ncbi.nlm.nih.gov/12614255/
Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertil Steril. 2003 Jun;79 Suppl 3:1659-61. doi: 10.1016/s0015-0282(03)00365-0. PMID: 12801577.
Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin
The study by Menon DK, published in “Fertility and Sterility” in June 2003, documents a clinical case where anabolic steroid-induced azoospermia (absence of sperm in semen) was successfully treated with human chorionic gonadotropin (hCG) and human menopausal gonadotropin (hMG). This case is notable because the azoospermia persisted for one year after the cessation of steroid use, presenting a significant challenge for fertility treatment.
The study outlines a tertiary referral center for infertility’s approach to treating a condition often associated with anabolic steroid abuse. The use of hCG and hMG to stimulate spermatogenesis in a context where traditional recovery methods had failed showcases an innovative therapeutic strategy. This approach may offer hope for individuals facing similar fertility issues due to anabolic steroid abuse.
For more details visit https://pubmed.ncbi.nlm.nih.gov/12801577/
Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J Hum Reprod Sci. 2014;7(3):170-174. doi:10.4103/0974-1208.142476.
Gonadotropin-releasing hormone analogs: Understanding advantages and limitations.
The review article by Kumar P and Sharma A, titled “Gonadotropin-releasing hormone analogs: Understanding advantages and limitations,” published in the “Journal of Human Reproductive Sciences” in 2014, provides a comprehensive overview of the use, benefits, and limitations of gonadotropin-releasing hormone (GnRH) analogs in various clinical settings. The authors discuss how these analogs can be used to manipulate reproductive physiology for therapeutic purposes, such as in the treatment of endometriosis, uterine fibroids, precocious puberty, and certain reproductive cancers. They also cover the mechanism of action of GnRH analogs, which can either stimulate (using pulsatile administration) or suppress (using continuous administration) gonadotropin secretion, depending on the clinical need.
The review highlights the dual nature of GnRH analogs in inducing both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion through pituitary stimulation when administered in a pulsatile fashion. Conversely, continuous administration can lead to pituitary desensitization and a decrease in FSH and LH levels, which is useful for conditions requiring suppression of ovarian or testicular steroidogenesis.
For more details visit https://pubmed.ncbi.nlm.nih.gov/25395741/
McBride JA, Coward RM. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian J Androl. 2016;18(3):373-380. doi:10.4103/1008-682X.173938.
Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use
The study by McBride JA and Coward RM, published in the “Asian Journal of Andrology” in 2016, addresses the recovery of spermatogenesis following testosterone replacement therapy (TRT) or anabolic-androgenic steroid (AAS) use. This research is particularly relevant given the increasing use of TRT in younger men who may wish to remain fertile and the widespread use of AAS in the general population. Both TRT and AAS can suppress the hypothalamic-pituitary-gonadal axis, leading to diminished spermatogenesis and potentially affecting fertility.
The article explores strategies for the recovery of spermatogenesis post-TRT or AAS use, highlighting the clinical challenges and the therapeutic approaches to restore fertility. It underscores the importance of understanding the physiological effects of TRT and AAS on the male reproductive system, as well as the potential for recovery of spermatogenesis with appropriate intervention.
For more details visit https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854084/
Samir, Haney et al. “Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats.” The Journal of veterinary medical science vol. 77,5 (2015): 549-56. doi:10.1292/jvms.14-0633.
Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats
The study titled “Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color Doppler ultrasonography in male Shiba goats” by Samir, Haney, et al., published in “The Journal of Veterinary Medical Science” in 2015, explores the impact of GnRH and hCG on testicular blood flow in Shiba goats. This research is significant as it contributes to the understanding of how these hormones can influence testicular physiology, potentially offering insights into fertility treatments and reproductive health management in veterinary medicine.
This study utilized color Doppler ultrasonography, a non-invasive imaging technique, to measure changes in testicular blood flow following the administration of GnRH and hCG. Such an approach provides valuable data on the vascular effects of these hormones, which could be crucial for developing therapeutic strategies to enhance reproductive function in male livestock and possibly in other species.
For more details visit https://pubmed.ncbi.nlm.nih.gov/25715956/
Nelly Pitteloud, Frances J. Hayes, Andrew Dwyer, Paul A. Boepple, Hang Lee, William F. Crowley, Jr., Predictors of Outcome of Long-Term GnRH Therapy in Men with Idiopathic Hypogonadotropic Hypogonadism, The Journal of Clinical Endocrinology & Metabolism, Volume 87, Issue 9, 1 September 2002, Pages 4128–4136, https://doi.org/10.1210/jc.2002-020518.
Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism
The study “Predictors of Outcome of Long-Term GnRH Therapy in Men with Idiopathic Hypogonadotropic Hypogonadism” by Nelly Pitteloud, Frances J. Hayes, Andrew Dwyer, Paul A. Boepple, Hang Lee, William F. Crowley, Jr., published in “The Journal of Clinical Endocrinology & Metabolism” in September 2002, focuses on identifying factors that predict the effectiveness of long-term gonadotropin-releasing hormone (GnRH) therapy in men with idiopathic hypogonadotropic hypogonadism (IHH).
This comprehensive study examines the response to GnRH therapy in IHH patients, exploring how factors such as the individual’s prior pubertal development and baseline testicular size can influence treatment outcomes. Specifically, the research highlights the variation in the doses of subcutaneous GnRH necessary to normalize levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone (T) among patients. The study found significant differences in GnRH dosing requirements among groups categorized by their stage of pubertal development at the onset of treatment, indicating that prior pubertal development is a critical predictor of therapy success.
For more details visit https://academic.oup.com/jcem/article/87/9/4128/2846495
Hashem NM, Sallam SM. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest Anim Endocrinol. 2020 Apr;71:106390. doi: 10.1016/j.domaniend.2019.106390. Epub 2019 Sep 6. PMID: 31731249.
Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus
The study conducted by Hashem NM and Sallam SM, published in “Domestic Animal Endocrinology” in April 2020, evaluates the reproductive performance of goats treated with either free gonadorelin or nanoconjugated gonadorelin at estrus. This research aimed to assess the effectiveness of using gonadorelin, a GnRH analog, in its traditional form versus a nanoconjugated form, which involves conjugation with chitosan-sodium tripolyphosphate (TPP) nanoparticles, to improve reproductive outcomes in goats.
The study’s findings could provide significant insights into the optimization of reproductive protocols in livestock, especially in improving the efficiency and effectiveness of hormonal treatments for estrus induction and synchronization. By comparing the effects of free and nanoconjugated gonadorelin, the research highlights the potential benefits of nanotechnology in veterinary medicine, particularly in enhancing the delivery and action of reproductive hormones.
For more details visit https://pubmed.ncbi.nlm.nih.gov/31731249/
Freick M, Weber O, Passarge O, Neubert T. Einsatz von Gonadorelin[6-D-Phe] am Tag 0 oder 12 post inseminationem zur Steigerung der Konzeptionsrate in einer sächsischen Milchviehgroßanlage [Use of gonadorelin[6-D-Phe] at day 0 or 12 after insemination to increase the conception rate in a large dairy herd in Saxony/Germany]. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2014;42(6):331-42. German. doi: 10.15653/TPG-140315. Epub 2014 Nov 17. PMID: 25401927.
Einsatz von Gonadorelin [6-D-Phe] am Tag 0 oder 12 post inseminationem zur Steigerung der Konzeptionsrate in einer sächsischen Milchviehgroßanlage
The study by Freick, Weber, Passarge, and Neubert, published in “Tierärztliche Praxis Ausgabe G: Großtiere/Nutztiere” in 2014, investigates the use of Gonadorelin[6-D-Phe] on days 0 or 12 post-insemination to increase the conception rate in a large dairy herd in Saxony, Germany. This research explores the efficacy of administering gonadorelin, a GnRH analog, at specific times following artificial insemination to enhance fertility outcomes in dairy cattle.
The study’s focus on days 0 and 12 post-insemination is strategic, aiming to optimize the timing of hormonal intervention to improve reproductive performance. Gonadorelin is used to stimulate the release of LH (luteinizing hormone) from the pituitary gland, which is critical for ovulation and subsequent conception. By examining the effects of gonadorelin administration at these key points in the reproductive cycle, the research provides valuable insights into fertility management practices that could significantly impact dairy herd productivity.
For more details visit https://pubmed.ncbi.nlm.nih.gov/25401927/
Rodrigues WB, Silva AS, Silva JCB, Anache NA, Silva KC, Cardoso CJT, Garcia WR, Sutovsky P, Nogueira E. Timed artificial insemination plus heat II: gonadorelin injection in cows with low estrus expression scores increased pregnancy in progesterone/estradiol-based protocol. Animal. 2019 Oct;13(10):2313-2318. doi: 10.1017/S1751731119000454. Epub 2019 Mar 27. PMID: 30915942.
Timed artificial insemination plus heat II: gonadorelin injection in cows with low estrus expression scores increased pregnancy in progesterone/estradiol-based protocol
The study “Timed Artificial Insemination plus heat II: Gonadorelin injection in cows with low estrus expression scores increased pregnancy in progesterone/estradiol-based protocol” by Rodrigues WB et al., published in “Animal” in 2019, investigated the impact of gonadorelin injection in cows with low estrus expression undergoing a progesterone/estradiol-based timed artificial insemination protocol. This research aimed to enhance pregnancy rates by improving the estrus synchronization process, showcasing a practical approach to addressing low estrus expression in cattle for better reproductive outcomes.
Fenichel P, Guedj AM, Verdino P, Brucker F, Strulo S, Mehouas C, Harter M. Aménorrhée hypothalamique. Intérêts diagnostique et thérapeutique de l’apport pulsatile de gonadoréline [Hypothalamic amenorrhea. Diagnostic and therapeutic values of pulsatile administration of gonadorelin]. Presse Med. 1988 Jan 23;17(2):61-4. French. PMID: 2964021.
Aménorrhée hypothalamique: intérêts diagnostique et thérapeutique de l’apport pulsatile de gonadoréline
The study by Fenichel P, Guedj AM, et al., published in “Presse Médicale” in 1988, evaluates the diagnostic and therapeutic benefits of the pulsatile administration of gonadorelin in cases of hypothalamic amenorrhea. This condition, characterized by the absence of menstrual periods due to a disruption in the hypothalamic release of GnRH, impacts fertility and menstrual regularity. The research highlights how pulsatile gonadorelin can mimic the natural hormone release patterns, potentially restoring normal reproductive function and offering a valuable treatment and diagnostic approach for affected women.
For more details visit https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=7832682
Lee CN, Maurice E, Ax RL, Pennington JA, Hoffman WF, Brown MD. Efficacy of gonadotropin-releasing hormone administered at the time of artificial insemination of heifers and postpartum and repeat breeder dairy cows. Am J Vet Res. 1983 Nov;44(11):2160-3. PMID: 6359982.
Efficacy of gonadotropin-releasing hormone administered at the time of artificial insemination of heifers and postpartum and repeat breeder dairy cows
The study by Lee CN, Maurice E, et al., published in the “American Journal of Veterinary Research” in 1983, investigates the effectiveness of administering gonadotropin-releasing hormone (GnRH) at the time of artificial insemination in heifers, as well as in postpartum and repeat breeder dairy cows. It aimed to improve fertility outcomes in these groups by optimizing hormonal support during insemination, which is critical for successful conception. This research provides insights into reproductive management practices that could enhance the breeding efficiency of dairy and beef cattle operations.
For more details visit https://europepmc.org/article/med/6359982
Ide, V.; Vanderschueren, D.; Antonio, L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. Int. J. Mol. Sci. 2021, 22, 21. https://doi.org/10.3390/ijms22010021.
Treatment of men with central hypogonadism: Alternatives for testosterone replacement therapy
The article by Ide, Vanderschueren, and Antonio, published in the “International Journal of Molecular Sciences” in 2021, discusses alternatives to testosterone replacement therapy for treating men with central hypogonadism. It critically examines the potential benefits and limitations of different therapeutic approaches, aiming to provide a comprehensive overview of the available strategies beyond traditional testosterone supplementation. This review contributes significantly to the field by exploring innovative treatments that could offer improved outcomes for patients with central hypogonadism.
For more details visit https://www.mdpi.com/1422-0067/22/1/21
Dabaja AA, Schlegel PN. Medical treatment of male infertility. Transl Androl Urol. 2014;3(1):9-16. doi:10.3978/j.issn.2223-4683.2014.01.06.
Medical treatment of male infertility
The article by Dabaja and Schlegel, published in “Translational Andrology and Urology” in 2014, reviews the medical treatments available for male infertility, emphasizing non-surgical approaches. It outlines the effectiveness of various pharmacological therapies aimed at improving sperm quality, enhancing testicular function, and addressing underlying hormonal imbalances. This comprehensive review provides insights into the current and emerging treatments for male infertility, offering guidance for clinicians in the management of this condition
For more details visit https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4708300/
Rajkanna J, Tariq S, Oyibo SO. Successful fertility treatment with gonadotrophin therapy for male hypogonadotrophic hypogonadism. Endocrinol Diabetes Metab Case Rep. 2016;2016:150124. doi:10.1530/EDM-15-0124.
Successful fertility treatment with gonadotrophin therapy for male hypogonadotrophic hypogonadism
The case report by Rajkanna, Tariq, and Oyibo in “Endocrinology, Diabetes & Metabolism Case Reports” in 2016 documents successful fertility treatment using gonadotrophin therapy for a male with hypogonadotrophic hypogonadism. This condition, characterized by low levels of gonadotrophins leading to reduced testicular function and infertility, was effectively managed through hormonal therapy, highlighting the potential for gonadotrophin treatment to restore fertility in affected individuals.
For more details visit https://edm.bioscientifica.com/view/journals/edm/2016/1/EDM15-0124.xml
Iwamoto H, Yoshida A, Suzuki H, Tanaka M, Watanabe N, Nakamura T. A man with hypogonadotropic hypogonadism successfully treated with nasal administration of the low-dose gonadotropin-releasing hormone analog buserelin. Fertil Steril. 2009 Sep;92(3):1169.e1-1169.e3. doi: 10.1016/j.fertnstert.2009.05.090. Epub 2009 Jul 9. PMID: 19591988.
A man with hypogonadotropic hypogonadism successfully treated with nasal administration of the low-dose gonadotropin-releasing hormone analog buserelin.
The study by Iwamoto et al., published in “Fertility and Sterility” in 2009, reports the successful treatment of a man with hypogonadotropic hypogonadism using the nasal administration of a low-dose gonadotropin-releasing hormone analog, buserelin. This treatment approach is noteworthy because it provides an alternative to traditional injection methods, potentially offering a more convenient and patient-friendly option for managing this condition.
For more details visit https://www.sciencedirect.com/science/article/pii/S001502820901228X
Madhukar, D. and Rajender, S. (2009), Hormonal Treatment of Male Infertility: Promises and Pitfalls. Journal of Andrology, 30: 95-112. https://doi.org/10.2164/jandrol.108.005694.
Hormonal treatment of male infertility: promises and pitfalls
The article by Madhukar and Rajender, published in the “Journal of Andrology” in 2009, reviews the hormonal treatments available for male infertility, discussing their potential benefits and associated risks. It provides a critical analysis of various hormonal therapies, including their mechanisms of action and effectiveness in improving male reproductive health. The authors highlight the importance of understanding the underlying causes of infertility to select appropriate treatment strategies.
For more details visit https://onlinelibrary.wiley.com/doi/abs/10.2164/jandrol.108.005694
Berezin M, Weissenberg R, Rabinovitch O, Lunenfeld B. Successful GnRH treatment in a patient with Kallmann’s syndrome, who previously failed HMG/HCG treatment. Andrologia. 1988 Jul-Aug;20(4):285-8. doi: 10.1111/j.1439-0272.1988.tb00687.x. PMID: 3143274.
Successful GnRH treatment in a patient with Kallmann’s syndrome, who previously failed HMG/HCG treatment
The study by Berezin, Weissenberg, Rabinovitch, and Lunenfeld, published in “Andrologia” in 1988, reports a successful treatment of a patient with Kallmann’s syndrome using GnRH after a failed attempt with HMG/HCG therapy. This case highlights the potential of GnRH treatment in patients with this syndrome, who do not respond to more traditional therapies. It underscores the importance of personalized treatment plans in reproductive endocrinology.
For more details visit https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1439-0272.1988.tb00687.x
Delemarre-Van de Waal HA, Odink RJ. Pulsatile GnRH treatment in boys and girls with idiopathic hypogonadotrophic hypogonadism. Hum Reprod. 1993 Nov;8 Suppl 2:180-3. doi: 10.1093/humrep/8.suppl_2.180. PMID: 8276956.
Pulsatile GnRH treatment in boys and girls with idiopathic hypogonadotrophic hypogonadism
The study by Delemarre-Van de Waal and Odink, published in “Human Reproduction” in 1993, examines the effectiveness of pulsatile GnRH treatment for boys and girls with idiopathic hypogonadotrophic hypogonadism (IHH). This research provides valuable insights into the therapeutic potential of GnRH in stimulating pubertal development and enhancing gonadal function in patients with IHH, offering a significant contribution to the management of this condition.
For more details visit https://academic.oup.com/humrep/article-abstract/8/suppl_2/180/617309
Matsumoto AM. Hormonal therapy of male hypogonadism. Endocrinol Metab Clin North Am. 1994 Dec;23(4):857-75. PMID: 7705324.
Hormonal therapy of male hypogonadism
The article by Matsumoto AM, published in “Endocrinology and Metabolism Clinics of North America” in December 1994, provides a comprehensive review of the hormonal therapy options available for treating male hypogonadism. It discusses the physiological basis of hypogonadism, the clinical approaches to diagnosis, and the various treatment strategies, including the benefits and potential side effects of hormonal replacement therapy. This work is crucial for understanding the management of male hypogonadism and the role of hormone therapy in improving patient outcomes.
For more details visit https://www.sciencedirect.com/science/article/pii/S0889852918300720
Layman LC. Hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am. 2007 Jun;36(2):283-96. doi: 10.1016/j.ecl.2007.03.010. PMID: 17543719.
Hypogonadotropic hypogonadism. Endocrinol Metab Clin North Am
The article by Layman LC, published in “Endocrinology and Metabolism Clinics of North America” in June 2007, offers an in-depth review of hypogonadotropic hypogonadism, covering its causes, diagnosis, and treatment options. It delves into the underlying genetic and physiological mechanisms, highlighting the complexity of this condition and the importance of a tailored therapeutic approach for affected individuals.
For more details visit https://www.sciencedirect.com/science/article/pii/S0889852907000278
Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002 Sep;87(9):4128-36. doi: 10.1210/jc.2002-020518. PMID: 12213860.
Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism
The study by Pitteloud et al., published in the “Journal of Clinical Endocrinology & Metabolism” in September 2002, investigates the predictors of successful long-term GnRH therapy outcomes in men with idiopathic hypogonadotropic hypogonadism (IHH). It emphasizes the importance of individual patient characteristics in determining the effectiveness of therapy, offering valuable insights for clinicians managing IHH.
For more details visit https://doi.org/10.1210/jc.2002-020518
Martínez M, Mapletoft RJ, Kastelic JP, Carruthers T. The effects of 3 gonadorelin products on luteinizing hormone release, ovulation, and follicular wave emergence in cattle. Can Vet J. 2003 Feb;44(2):125-31. PMID: 12650040; PMCID: PMC340045.
The effects of 3 gonadorelin products on luteinizing hormone release, ovulation, and follicular wave emergence in cattle
The study by Martínez et al., published in the “Canadian Veterinary Journal” in February 2003, evaluated the effects of three gonadorelin products on luteinizing hormone release, ovulation, and follicular wave emergence in cattle. It aimed to determine the efficacy and potential differences between these products in managing reproductive events in cattle, which is crucial for optimizing breeding programs and improving reproductive efficiency in livestock.
For more details visit https://www.ncbi.nlm.nih.gov/pmc/articles/PMC340045/
Monaco D, Fatnassi M, Padalino B, Aubé L, Khorchani T, Hammadi M, Lacalandra GM. Effects of a GnRH administration on testosterone profile, libido and semen parameters of dromedary camel bulls. Res Vet Sci. 2015 Oct;102:212-6. doi: 10.1016/j.rvsc.2015.08.011. Epub 2015 Sep 2. PMID: 26412546.
ACCEPTED M
The study by Monaco et al., published in “Research in Veterinary Science” in October 2015, investigated the effects of GnRH administration on testosterone levels, libido, and semen parameters in dromedary camel bulls. This research aimed to understand how GnRH influences reproductive functions in camels, potentially offering insights for improving breeding strategies in these animals.
For more details visit https://cris.unibo.it/bitstream/11585/729022/4/Padalino%20postprint.pdf
Deborah K. Barnett, Tina M. Bunnell, Robert P. Millar, David H. Abbott, Gonadotropin-Releasing Hormone II Stimulates Female Sexual Behavior in Marmoset Monkeys, Endocrinology, Volume 147, Issue 1, 1 January 2006, Pages 615–623, https://doi.org/10.1210/en.2005-0662.
Gonadotropin-releasing hormone II stimulates female sexual behavior in marmoset monkeys
The study by Barnett et al., published in “Endocrinology” in January 2006, explores the effects of Gonadotropin-Releasing Hormone II (GnRH II) on female sexual behavior in marmoset monkeys. The research demonstrates that GnRH II administration stimulates sexual behavior in these primates, suggesting a potential role for GnRH II in modulating reproductive behaviors. This finding contributes to our understanding of the hormonal regulation of sexual behavior in non-human primates.
For more details visit https://academic.oup.com/endo/article-abstract/147/1/615/2501152
Janakiram NB, Mohammed A, Brewer M, Bryant T, Biddick L, Lightfoot S, Pathuri G, Gali H, Rao CV. Raloxifene and antiestrogenic gonadorelin inhibits intestinal tumorigenesis by modulating immune cells and decreasing stem-like cells. Cancer Prev Res (Phila). 2014 Mar;7(3):300-9. doi: 10.1158/1940-6207.CAPR-13-0345. Epub 2014 Jan 15. PMID: 24431404; PMCID: PMC3951612.
Raloxifene and antiestrogenic gonadorelin inhibits intestinal tumorigenesis by modulating immune cells and decreasing stem-like cells
The study by Janakiram NB et al., published in “Cancer Prevention Research” in March 2014, investigates the effects of raloxifene and an antiestrogenic gonadorelin on intestinal tumorigenesis. The research found that these compounds inhibited tumor growth by modulating immune cell activity and reducing the number of stem-like cells in the intestine. This suggests a potential therapeutic strategy for preventing intestinal cancer.
For more details visit https://aacrjournals.org/cancerpreventionresearch/article-abstract/7/3/300/50334
Robertson JF, Blamey RW. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur J Cancer. 2003 May;39(7):861-9. doi: 10.1016/s0959-8049(02)00810-9. PMID: 12706354.
The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre-and perimenopausal women
The article by Robertson and Blamey reviews the application of gonadotrophin-releasing hormone (GnRH) agonists in treating early and advanced breast cancer in pre- and perimenopausal women. It discusses the therapeutic potential of GnRH agonists in managing hormone-responsive breast cancers by inhibiting ovarian function, thereby reducing estrogen levels that can fuel tumor growth. This treatment approach offers an alternative for women who are not candidates for traditional hormone therapies.
For more details visit https://www.sciencedirect.com/science/article/pii/S0959804902008109
Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J, Candas B. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005 May;26(3):361-79. doi: 10.1210/er.2004-0017. Epub 2005 May 2. PMID: 15867098.
Gonadotropin-releasing hormone agonists in the treatment of prostate cancer
The review by Labrie et al. in “Endocrine Reviews” highlights the role of gonadotropin-releasing hormone (GnRH) agonists in treating prostate cancer. It discusses how these agents, by suppressing testosterone production, serve as a cornerstone for managing advanced prostate cancer. The article delves into the mechanisms, benefits, and outcomes of GnRH agonist therapy, underscoring its importance in the therapeutic landscape of prostate cancer.
For more details visit https://academic.oup.com/edrv/article-abstract/26/3/361/2355237
Gründker C, Emons G. The Role of Gonadotropin-Releasing Hormone in Cancer Cell Proliferation and Metastasis. Front Endocrinol (Lausanne). 2017;8:187. Published 2017 Aug 4. doi:10.3389/fendo.2017.00187.
The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis
The article by Gründker and Emons in “Frontiers in Endocrinology” explores the significant role of gonadotropin-releasing hormone (GnRH) in the proliferation and metastasis of cancer cells. It emphasizes the potential of GnRH and its analogs in cancer treatment, particularly their ability to inhibit cancer cell growth and spread, offering a promising avenue for therapeutic intervention in various cancers.
For more details visit https://www.frontiersin.org/articles/10.3389/fendo.2017.00187/full
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001 Dec;281(6):E1172-81. doi: 10.1152/ajpendo.2001.281.6.E1172. PMID: 11701431.
Testosterone dose-response relationships in healthy young men
The study by Bhasin et al., published in “American Journal of Physiology – Endocrinology and Metabolism” in December 2001, investigates the dose-response relationships of testosterone in healthy young men. It examines how different doses of testosterone affect body composition, muscle strength, and various health parameters, providing valuable insights into the physiological impacts of testosterone supplementation.
For more details visit https://journals.physiology.org/doi/abs/10.1152/ajpendo.2001.281.6.E1172?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org
Secreto, G., Muti, P., Sant, M., Meneghini, E., & Krogh, V. (2017). Medical ovariectomy in menopausal breast cancer patients with high testosterone levels: a further step toward tailored therapy. Endocrine-related cancer, 24(11), C21–C29. https://doi.org/10.1530/ERC-17-0251.
Medical ovariectomy in menopausal breast cancer patients with high testosterone levels: a further step toward tailored therapy
The study by Secreto et al., published in “Endocrine-Related Cancer” in 2017, discusses the use of medical ovariectomy in menopausal breast cancer patients with high testosterone levels. This approach represents a step toward tailored therapy for breast cancer, aiming to better address the hormonal profiles of individual patients. The research explores the potential benefits of targeting testosterone levels as part of breast cancer treatment.
For more details visit https://erc.bioscientifica.com/view/journals/erc/24/11/C21.xml
Vollaard, E. S., van Beek, A. P., Verburg, F. A., Roos, A., & Land, J. A. (2011). Gonadotropin-releasing hormone agonist treatment in postmenopausal women with hyperandrogenism of ovarian origin. The Journal of clinical endocrinology and metabolism, 96(5), 1197–1201. https://doi.org/10.1210/jc.2010-1991
The Journal of Clinical Endocrinology & Metabolism
The study by Vollaard et al., published in “The Journal of Clinical Endocrinology & Metabolism” in 2011, investigates the use of gonadotropin-releasing hormone (GnRH) agonist treatment in postmenopausal women with hyperandrogenism of ovarian origin. This research explores the potential of GnRH agonists to manage hyperandrogenism in this specific patient population. The findings provide insights into the effectiveness of this therapeutic approach for addressing hormonal imbalances in postmenopausal women.
For more details visit https://academic.oup.com/jcem/article-abstract/96/5/1197/2833196
Labrie F. (2014). GnRH agonists and the rapidly increasing use of combined androgen blockade in prostate cancer. Endocrine-related cancer, 21(4), R301–R317. https://doi.org/10.1530/ERC-13-0165
GnRH agonists and the rapidly increasing use of combined androgen blockade in prostate cancer
The article by Labrie in “Endocrine-Related Cancer” in 2014 discusses the use of GnRH (Gonadotropin-Releasing Hormone) agonists and the increasing adoption of combined androgen blockade in the treatment of prostate cancer. It examines the role of GnRH agonists in modulating hormonal pathways and their effectiveness in prostate cancer therapy when combined with androgen blockade. The research provides insights into the evolving strategies for managing prostate cancer and their impact on patient outcomes.
For more details visit https://doi.org/10.1530/ERC-13-0165
Labrie F. (2015). Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology. The Journal of steroid biochemistry and molecular biology, 145, 144–156. https://doi.org/10.1016/j.jsbmb.2014.05.012.
Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology
The article by Labrie in “The Journal of Steroid Biochemistry and Molecular Biology” in 2015 discusses the combined blockade of testicular and locally produced androgens in the context of prostate cancer treatment. This approach represents significant progress in the field of medicine, particularly in the domain of intracrinology, which involves understanding and targeting hormone production within tissues. The research explores the implications and benefits of this combined blockade strategy for managing prostate cancer
For more details visit https://doi.org/10.1016/j.jsbmb.2014.05.012
Labrie F. (2006). Rôle clé de l’endocrinologie dans la victoire contre le cancer de la prostate [Keyrole of endocrinology in the victory against prostate cancer]. Bulletin du cancer, 93(9), 949–958.
Keyrole of endocrinology in the victory against prostate cancer
The article by Labrie, published in the “Bulletin du Cancer” in 2006, highlights the key role of endocrinology in the fight against prostate cancer. The article discusses the importance of understanding the hormonal mechanisms involved in prostate growth and developing therapeutic approaches based on this understanding. It underscores significant advancements in prostate cancer management through an endocrinological approach.
For more details visit https://www.jle.com/en/revues/bdc/e-docs/role_cle_de_lendocrinologie_dans_la_victoire_contre_le_cancer_de_la_prostate_270343/article.phtml?cle_doc=00042007
Spicer, D. V., & Pike, M. C. (1994). Sex steroids and breast cancer prevention. Journal of the National Cancer Institute. Monographs, (16), 139–147.
Sex steroids and breast cancer prevention
The article by Spicer and Pike, published in the “Journal of the National Cancer Institute. Monographs” in 1994, discusses the role of sex steroids in breast cancer prevention. It explores the relationship between sex hormones and the risk of developing breast cancer, providing insights into potential strategies for breast cancer prevention.
For more details visit https://europepmc.org/article/med/7999456
Storer TW, Woodhouse L, Magliano L, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56(11):1991-1999. doi:10.1111/j.1532-5415.2008.01927.x.
Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men
The article by Spicer and Pike, published in the “Journal of the National Cancer Institute. Monographs” in 1994, discusses the role of sex steroids in breast cancer prevention. It explores the relationship between sex hormones and the risk of developing breast cancer, providing insights into potential strategies for breast cancer prevention.
For more details visit https://europepmc.org/article/med/7999456
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005 Feb;90(2):678-88. doi: 10.1210/jc.2004-1184. Epub 2004 Nov 23. PMID: 15562020.
Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle
The study by Bhasin et al., published in the “Journal of Clinical Endocrinology & Metabolism” in February 2005, investigates the response of older men to graded doses of testosterone on skeletal muscle. The research findings suggest that older men exhibit similar responsiveness to the anabolic effects of testosterone on muscle when compared to young men. This study has implications for understanding the potential benefits of testosterone therapy in older individuals.
For more details visit https://academic.oup.com/jcem/article-abstract/90/2/678/2836564
Shalender Bhasin, Testosterone Supplementation for Aging-Associated Sarcopenia, The Journals of Gerontology: Series A, Volume 58, Issue 11, November 2003, Pages M1002–M1008, https://doi.org/10.1093/gerona/58.11.M1002.
The Journals of Gerontology: Series A
The article by Shalender Bhasin, published in “The Journals of Gerontology: Series A” in November 2003, discusses testosterone supplementation as a potential intervention for aging-associated sarcopenia, which is the age-related loss of muscle mass and strength. The research explores the use of testosterone to address the decline in muscle function associated with aging and provides insights into its potential benefits in managing sarcopenia.
For more details visit https://academic.oup.com/biomedgerontology/article-abstract/58/11/M1002/640336
Available at https://www.hindawi.com/journals/ije/2015/324524/.
Uemura T, Mohri J, Osada H, Suzuki N, Katagiri N, Minaguchi H. Effect of gonadotropin-releasing hormone agonist on the bone mineral density of patients with endometriosis. Fertil Steril. 1994 Aug;62(2):246-50. doi: 10.1016/s0015-0282(16)56873-3. PMID: 8034067.
Effect of gonadotropin-releasing hormone agonist on the bone mineral density of patients with endometriosis
The study by Uemura et al., published in “Fertility and Sterility” in August 1994, examines the effect of gonadotropin-releasing hormone (GnRH) agonist on the bone mineral density of patients with endometriosis. This research investigates how GnRH agonist treatment impacts bone health in individuals with endometriosis, as hormonal therapies like GnRH agonists can have effects on bone density.
For more details visit https://www.sciencedirect.com/science/article/pii/S0015028216568733
Bergström I, Freyschuss B, Jacobsson H, Landgren BM. The effect of physical training on bone mineral density in women with endometriosis treated with GnRH analogs: a pilot study. Acta Obstet Gynecol Scand. 2005 Apr;84(4):380-3. doi: 10.1111/j.0001-6349.2005.00558.x. PMID: 15762970.
The effect of physical training on bone mineral density in women with endometriosis treated with GnRH analogs: a pilot study
The study by Bergström et al., published in “Acta Obstetricia et Gynecologica Scandinavica” in April 2005, investigates the effect of physical training on bone mineral density in women with endometriosis who are treated with GnRH (Gonadotropin-Releasing Hormone) analogs. This pilot study explores whether physical training can help mitigate potential reductions in bone density associated with the use of GnRH analogs in women with endometriosis.
For more details visit https://www.tandfonline.com/doi/abs/10.1080/j.0001-6349.2005.00558.x
Bergström I, Gustafsson H, Sjöberg K, Arver S. Changes in bone mineral density differ between gonadotrophin-releasing hormone analogue- and surgically castrated men with prostate cancer–a prospective, controlled, parallel-group study. Scand J Urol Nephrol. 2004;38(2):148-52. doi: 10.1080/00365590310018810. PMID: 15204403.
Changes in bone mineral density differ between gonadotrophin-releasing hormone analogue- and surgically castrated men with prostate cancer–a prospective, controlled, parallel-group study
The study by Bergström et al., published in the “Scandinavian Journal of Urology and Nephrology” in 2004, compares changes in bone mineral density between men with prostate cancer who were treated with gonadotropin-releasing hormone (GnRH) analogs and those who underwent surgical castration. This prospective, controlled, parallel-group study examines the impact of different treatments on bone density in men with prostate cancer, shedding light on the potential differences in bone health outcomes.
For more details visit https://www.tandfonline.com/doi/abs/10.1080/00365590310018810
Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998 Sep;139(3):298-303. doi: 10.1530/eje.0.1390298. PMID: 9758439.
Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases
The study by Büchter et al., published in the “European Journal of Endocrinology” in September 1998, reviews the effectiveness of pulsatile gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin/human menopausal gonadotropin (hCG/hMG) treatments in men with hypogonadotropic hypogonadism. This review analyzes 42 cases and assesses the efficacy of these treatment approaches in addressing hypogonadism in men.
Zhang L, Cai K, Wang Y, Ji W, Cheng Z, Chen G, Liao Z. The Pulsatile Gonadorelin Pump Induces Earlier Spermatogenesis Than Cyclical Gonadotropin Therapy in Congenital Hypogonadotropic Hypogonadism Men. Am J Mens Health. 2019 Jan-Feb;13(1):1557988318818280. doi: 10.1177/1557988318818280. Epub 2018 Dec 20. PMID: 30569789; PMCID: PMC6775549.
The Pulsatile Gonadorelin Pump Induces Earlier Spermatogenesis Than Cyclical Gonadotropin Therapy in Congenital Hypogonadotropic Hypogonadism Men
The study by Zhang et al., published in “The American Journal of Men’s Health” in January-February 2019, compares the effects of the pulsatile gonadorelin pump therapy with cyclical gonadotropin therapy in men with congenital hypogonadotropic hypogonadism. The research findings suggest that the pulsatile gonadorelin pump induces earlier spermatogenesis compared to cyclical gonadotropin therapy in this patient population. This study provides insights into treatment options for individuals with this condition.
For more details visit https://journals.sagepub.com/doi/abs/10.1177/1557988318818280
Fenichel P, Guedj AM, Verdino P, Brucker F, Strulo S, Mehouas C, Harter M. Aménorrhée hypothalamique. Intérêts diagnostique et thérapeutique de l’apport pulsatile de gonadoréline [Hypothalamic amenorrhea. Diagnostic and therapeutic values of pulsatile administration of gonadorelin]. Presse Med. 1988 Jan 23;17(2):61-4. French. PMID: 2964021.
Aménorrhée hypothalamique: intérêts diagnostique et thérapeutique de l’apport pulsatile de gonadoréline
The article by Fenichel et al., published in “Presse Médicale” on January 23, 1988, discusses hypothalamic amenorrhea and explores the diagnostic and therapeutic value of pulsatile administration of gonadorelin. This approach involves administering gonadorelin in a pulsatile manner for the diagnosis and treatment of hypothalamic amenorrhea. The article is in French.
For more details visit https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=7832682
Santoro N. Efficacy and safety of intravenous pulsatile gonadotropin-releasing hormone: Lutrepulse for injection. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 2):1759-64. doi: 10.1016/0002-9378(90)91441-e. PMID: 2122733.
Efficacy and safety of intravenous pulsatile gonadotropin-releasing hormone: Lutrepulse for injection
The article by Santoro, published in the “American Journal of Obstetrics and Gynecology” in November 1990, evaluates the efficacy and safety of intravenous pulsatile gonadotropin-releasing hormone (GnRH) using Lutrepulse for injection. This research assesses the use of intravenous pulsatile GnRH for various purposes, and it provides insights into its effectiveness and safety.
For more details visit https://www.sciencedirect.com/science/article/pii/000293789091441E
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

