GENEMEDICS APP
GENEMEDICS NUTRITION
Elampretide (SS-31)
Author: Dr. George Shanlikian, M.D. | Last Updated: November 26th, 2024
- Home
- >
- Health Library
- >
- Elampretide (SS-31)
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Elampretide
- Key Takeaways
- What is Elampretide?
- How Elampretide Works
- Chemical Structure of Elamipretide
- Research on Elamipretide
- Elamipretide Mechanism of Action
- Elamipretide FDA Approval
- SS-31 Dosage
- SS 31 Elamipretide
- Elamipretide Side Effects
- Elamipretide and Inner Mitochondrial Membrane
- Elamipretide and Mitochondrial Dysfunction
- Elamipretide and Reactive Oxygen Species
- Elamipretide Purchase
- Elamipretide Dosage
- Elamipretide Price
- FAQ
- References
Book a Free Consultation
Table of Contents
- Overall Health Benefits of Elampretide
- Key Takeaways
- What is Elampretide?
- How Elampretide Works
- Chemical Structure of Elamipretide
- Research on Elamipretide
- Elamipretide Mechanism of Action
- Elamipretide FDA Approval
- SS-31 Dosage
- SS 31 Elamipretide
- Elamipretide Side Effects
- Elamipretide and Inner Mitochondrial Membrane
- Elamipretide and Mitochondrial Dysfunction
- Elamipretide and Reactive Oxygen Species
- Elamipretide Purchase
- Elamipretide Dosage
- Elamipretide Price
- FAQ
- References
Overall Health Benefits of Elampretide
Elamipretide benefits include improving cardiovascular health, boosting brain power, preventing cancer, and protecting against kidney and lung injuries.
- Improves cardiovascular health [1-29]
- Boosts brain power [30-42]
- Prevents cancer [43-48]
- Prevents kidney injury [49-63]
- Treats lung injury [64-66]
Key Takeaways
- Mitochondrial Protection: Elampretide binds to cardiolipin, a lipid crucial for maintaining the structural integrity of mitochondria, protecting it from oxidative damage.
- Energy Production: By safeguarding cardiolipin and preventing mitochondrial dysfunction, Elampretide helps maintain the mitochondria’s role as the powerhouse of the cell, ensuring continued energy production for various cellular processes.
- Disease Mitigation: Elampretide’s protective effects on mitochondria can help prevent or reduce the impact of inherited mitochondrial diseases and some age-related diseases, which are often linked to mitochondrial dysfunction.
- Reduction of Oxidative Stress: By minimizing the production of harmful oxidative factors, Elampretide reduces oxidative stress, which is a significant contributor to cellular damage and aging.
- Cellular Health Restoration: Through its action on mitochondria, Elampretide aids in restoring normal cellular functions and processes, potentially improving overall cellular health and longevity.
What is Elampretide?
Elampretide, also known as elamipretide or SS-31, is a synthetic tetrapeptide that reduces the production of harmful reactive oxygen species in the body. It works by targeting the cell’s powerhouse known as mitochondria which in turn restores various important cellular processes. Studies show that SS-31 has anti-aging effects that are beneficial for the treatment of age-related diseases, genetic disorders, impaired blood circulation, kidney injury, and heart disease.
How Elampretide Works
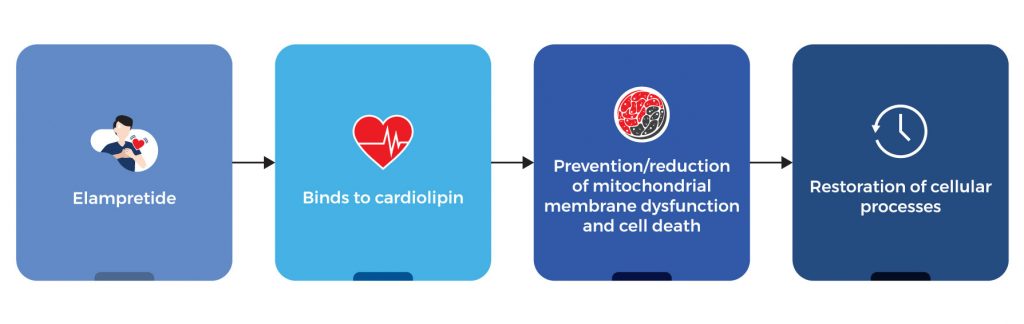
The mitochondria are known as the “powerhouse of the cell” as they produce the energy required for various cellular processes. Dysfunctional mitochondria can lead to a wide array of inherited mitochondrial diseases and some age-related diseases. In addition, dysfunctional mitochondria produce abnormally high levels of oxidative factors that can significantly damage cardiolipin, a lipid that plays an integral role in the maintenance of the structural integrity of the mitochondria. By binding to cardiolipin, elampretide protects it from the harmful effects of oxidative stress. This in turn prevents or reduces mitochondrial membrane dysfunction and cell death. In addition, the protective effects of elampretide on the mitochondria help restore various important cellular processes.
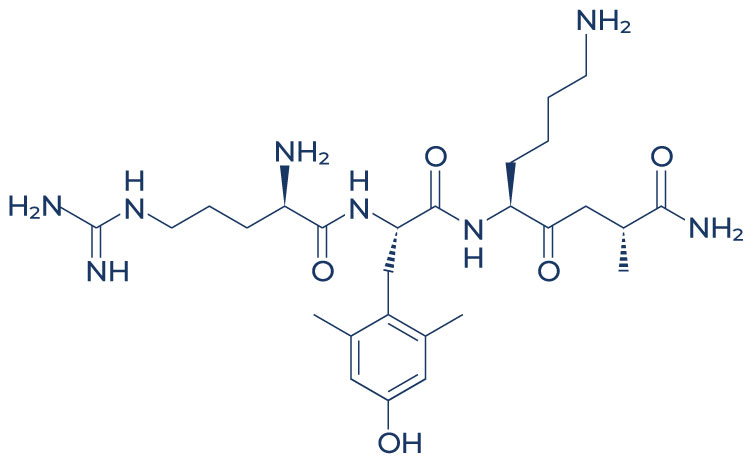
Chemical Structure of Elamipretide
Research on Elamipretide
A. Improves Cardiovascular Health
img
Studies show that elampretide (SS-31) can help protect against heart disease via different mechanisms:
- In the failing human heart, SS-31 improved mitochondrial function. [1]
- In aged mouse hearts, SS-31 significantly improved mitochondrial function. [2]
- In patients with type 2 diabetes, SS-31 treatment protected against cardiovascular disease by reducing reactive oxygen species levels. [3]
- In patients with dilated cardiomyopathy, SS-31 reversed the mitochondrial fragmentation. [4]
- A study showed that SS-31 can help re-energize the mitochondria of heart cells. [5]
- In adult male mice, SS-31 therapy protected against heart damage caused by blood vessel constriction. [6]
- In mice exposed to systemic inflammation, SS-31 effectively protected the heart from infection-induced heart damage by inhibiting oxidative stress and inflammation. [7]
- In mice with heart failure, SS-31 ameliorated cardiomyopathy, a condition that affects the pumping ability of the heart. [8]
- In rats suffering from cardiac arrest, SS-31 treatment increased survival time. [9]
- A study showed that SS-31’s effect on the cell’s mitochondria can be considered a therapeutic strategy for treating heart failure. [10]
- In healthy dogs and healthy donor human hearts, long-term SS-31 therapy normalized the critical abnormalities of the mitochondria. [11]
- In patients with heart enlargement and weakening, 12 weeks of SS-31 treatment improved heart function. [12]
- In patients with narrowing of the heart arteries, SS-31 treatment resulted in improvement of the symptoms. [13]
- In patients with heart disease, treatment with SS-31 increased exercise tolerance. [14]
- In rats, SS-31 administration for 6 weeks effectively treated heart disease caused by poor blood circulation. [15]
- In mouse hearts, SS-31 treatment for 8 weeks significantly reduced the levels of oxidative stress. [16]
- In cells derived from children with dilated cardiomyopathy with ataxia syndrome (DCMA), the abnormalities in the mitochondria were reversed by incubation with SS-31 for 24 hours. [17]
- In old mice that received SS-31 for 8 weeks, significant improvements in skeletal muscle function and endurance, cardiac function, and exercise endurance were observed. [18]
- In mice with cancer receiving SS-31 in both the heart and diaphragm muscle, a significant improvement in cardiorespiratory function and respiratory chain was seen. [19]
- In old mice and rats, SS-31 reversed cardiac dysfunction by reducing excessive proton leak. [20-21]
- In old mouse hearts, SS-31 was effective at restoring different aspects of mitochondrial and heart health via the reduction of oxidative stress. [22-24]
- In dogs with advanced heart failure, elamipretide improved left ventricular and mitochondrial function. [25-26]
- In rats with insufficient blood flow to the heart, elamipretide mitigated impairments in mitochondrial structure function. [27]
- In patients with Barth syndrome (characterized by an enlarged heart, muscle weakness, fatigue, and low blood cell count), elamipretide improved knee extensor strength, patient global impression of symptoms, and some cardiac parameters. [28]
- In aged mice, SS-31 reversed age-related redox stress and improved exercise tolerance. [29]
B. Boosts Brain Power
img
SS-31 has also been found to improve cognitive function through its ability to protect the neurons (nerve cells) in the brain:
- In mice with cognitive deficits, SS-31 treatment rescued learning and memory deficits. [30]
- In developing rat brains, SS-31 protected against isoflurane-induced cognitive deficits through the reversal of mitochondrial dysfunction and regulation of BDNF (brain-derived neurotrophic factor) signaling. [31-32]
- In aged mice, treatment with SS-31 improved working memory, motor skill learning, and gait coordination. [33]
- A study showed that SS-31 has therapeutic potential in preventing damage from oxidative stress and brain cell inflammation. [34]
- In aged mice, treatment with elamipretide significantly enhanced working memory and motor skill learning by increasing the blood flow to the brain. [35]
- In aged mice, SS-31 treatment was associated with significant improvements in learning and spatial working memory and lower levels of inflammatory cytokines. [36]
- Treatment of aged mice with the mitochondrial-targeted peptide elamipretide protected against mitochondrial dysfunction and attenuated surgery-induced cognitive deficits. [37]
- In hypertensive rats with traumatic brain injury, SS-31 treatment reversed cerebrovascular oxidative stress which is linked to dysregulation of blood flow to the brain and cognitive dysfunction. [38]
- In animal models of Parkinson’s disease (PD), SS-31 exerted neuroprotective effects on dopamine neurons (which play a role in memory, movement, motivation, mood, and attention) by targeting both mitochondrial dysfunction and oxidative damage. [39]
- In a model of Alzheimer’s disease, SS-31 protected the brain cells against programmed cell death (apoptosis) and significantly reduced mitochondrial dysfunction. [40]
- In murine cultured microglial cells (nervous system cells), SS-31 protected the cells against lipopolysaccharide (LPS)-induced inflammation and oxidative stress by stabilizing the mitochondrial structure. [41-42]
C. Prevents Cancer
Evidence also found that SS-31’s antioxidant properties and ability to protect the mitochondria from damage may play a role in preventing cancer:
- In mice, SS-31 prevented mitochondrial dysfunction which in turn reduced cancer prevalence. [43]
- Studies also suggest that restoring the function of the cell’s mitochondria, which is the primary action of SS-31, can help prevent cancer. [44-46]
- In cancer patients, SS-31 prevented cancer cachexia (muscle wasting) and improved systemic energy homeostasis. [48]
D. Prevents Kidney Injury
Studies also show that SS-31 is essential for kidney health:
- In mice with acute kidney injury, SS-31 treatment effectively suppressed mitochondrial reactive oxygen species which can contribute to further kidney damage. [49]
- In murine models of kidney injury, SS-31 was able to increase the production of angiotensin AT2 receptor (AT2R) which in turn reduced kidney damage. [50]
- In rats with unilateral ureteral obstruction, SS-31 significantly attenuated the effects of obstruction on all aspects of kidney damage. [51-52]
- In patients with severe atherosclerotic renal artery stenosis (narrowing of the kidney arteries due to blockage), intravenous infusion of elamipretide at 0.05 mg/kg per hour increased blood flow to the kidney and improved kidney function compared with placebo treatment. [53]
- In mice with diabetes, elamipretide protected against the progression of diabetic kidney disease. [54]
- In animal and cell models, SS-31 ameliorated kidney disease by preventing programmed cell death, inflammation, and scarring. [55]
- In pregnant mice, elamipretide was found to ameliorate the progression of kidney disease without any adverse effects. [56]
- In mice models of chronic kidney disease (CKD), SS-31 preserved mitochondrial integrity, ameliorated the increase in the levels of all inflammatory markers, restored glomerular filtration rate (filtering ability of the kidneys), and prevented kidney scarring. [57-58]
- In mice of advanced age, SS-31 improved glomerular filtration rate and mitochondrial structure. [59]
- In murine models of acute tubular injury and glomerular damage, SS-31 reduced damage to the kidneys by increasing the levels of the antibody known as AT2R. [60]
- In swine with atherosclerotic renal artery stenosis, treatment with subcutaneous elamipretide (5d/wk) for 4 weeks improved kidney function and alleviated fibrosis (scarring) and oxidative stress. [61]
- In mice fed with a high-fat diet, SS-31 prevented the loss of kidney cells and lipid accumulation. [62]
- In rats with insufficient blood flow to the kidneys (ischemia), SS-31 prevented mitochondrial swelling and protected against scarring and loss of blood vessels. [63]
E. Treats Lung Injury
SS-31 has also been found to treat various forms of lung injury:
- In a mouse model, SS-31 attenuated mitochondrial dysfunction, reduced inflammatory responses and alleviated the severity of lung damage. [64]
- In mice, SS-31 protected against spinal cord injury-induced lung injury. [65]
- In mice with acute liver injury due to blood infection (sepsis), the mitochondria-targeted antioxidant SS-31 reduced oxidative stress and inflammatory markers and alleviated sepsis-related acute liver injury. [66]
Elamipretide Mechanism of Action
Elamipretide, also known as SS-31 or Bendavia, exerts its therapeutic effects primarily through its unique mechanism of action targeting mitochondria. This mitochondria-targeted peptide is designed to interact with cardiolipin, a phospholipid found exclusively in the inner mitochondrial membrane. Cardiolipin plays a crucial role in maintaining mitochondrial structure and function, particularly in energy production and cellular signaling processes. In damaged or stressed mitochondria, such as those seen in various diseases and conditions, cardiolipin becomes oxidized and disrupts normal mitochondrial function. The primary outcome of many clinical trials involving Elamipretide is to assess its efficacy in restoring mitochondrial function and improving clinical symptoms.
Elamipretide binds selectively to cardiolipin and stabilizes its structure, thereby preserving mitochondrial integrity. By doing so, it helps maintain mitochondrial membrane potential and reduces the production of reactive oxygen species (ROS). ROS are byproducts of cellular respiration and can cause oxidative damage to mitochondria and other cellular components if not properly controlled. Elamipretide’s ability to reduce ROS production contributes to its antioxidant properties and helps protect mitochondria from further damage. In clinical studies, various treatment groups are established to compare the efficacy of Elamipretide against placebos or other treatments. These treatment groups allow researchers to observe the specific effects of elamipretide on mitochondrial function and overall cellular health, ensuring that any observed benefits can be attributed to the drug itself. This structured comparison is crucial for validating the therapeutic potential of Elamipretide across different patient populations and conditions.
Furthermore, elamipretide has been shown to inhibit the opening of the mitochondrial permeability transition pore (mPTP). The mPTP is a channel in the inner mitochondrial membrane whose opening can lead to mitochondrial swelling, disruption of membrane potential, and ultimately cell death. By preventing mPTP opening, elamipretide maintains mitochondrial function and promotes cell survival in conditions where mitochondrial integrity is compromised. Overall, elamipretide’s mechanism of action underscores its potential therapeutic value in various diseases characterized by mitochondrial dysfunction, including cardiovascular disorders, neurodegenerative diseases, and metabolic syndromes.
Elamipretide FDA Approval
The path to FDA approval involves rigorous phases of clinical testing. Elamipretide therapy has shown encouraging results in Phase I and II trials, demonstrating its ability to improve mitochondrial function and alleviate symptoms in patients with mitochondrial diseases. However, in Phase III trials, the outcomes have been mixed, with some studies meeting their primary endpoints and others not achieving statistically significant results. These mixed results have necessitated further investigation to better understand the drug’s efficacy across different patient populations and disease conditions. Elamipretide therapy has been particularly promising in improving energy production and reducing oxidative stress, offering hope for conditions that currently lack effective treatments.
To strengthen the case for FDA approval, researchers are focusing on refining the criteria for patient selection and optimizing dosing regimens. Additionally, long-term studies are being conducted to assess the safety and sustained efficacy of elamipretide therapy. These efforts aim to provide comprehensive data that can support the broader application of elamipretide therapy in treating a range of mitochondrial disorders, ultimately leading to its approval and widespread clinical use.
Stealth BioTherapeutics continues to work closely with the FDA, incorporating feedback and conducting additional studies to address the unmet needs in mitochondrial disease treatment. Stealth BioTherapeutics is also exploring potential biomarkers and patient-reported outcomes to strengthen its case for approval. The process underscores the complexities and challenges inherent in developing therapies for rare and multifaceted diseases. Despite the hurdles, the ongoing commitment to elamipretide research by Stealth BioTherapeutics reflects a broader hope within the scientific and medical communities that this innovative treatment will eventually gain FDA approval, providing new hope for patients suffering from debilitating mitochondrial disorders. Stealth BioTherapeutics’ dedication to advancing elamipretide therapy involves extensive collaboration with researchers and healthcare professionals. The company is focused on refining the criteria for patient selection and optimizing dosing regimens to enhance the efficacy of elamipretide therapy.
Additionally, Stealth BioTherapeutics is conducting long-term studies to assess the safety and sustained efficacy of the treatment, ensuring that it meets the highest standards for clinical application. The efforts of Stealth BioTherapeutics highlight the potential impact of elamipretide therapy on improving energy production and reducing oxidative stress in mitochondrial diseases. By addressing the specific needs of different patient populations, Stealth BioTherapeutics aims to provide comprehensive data that can support the broader application of elamipretide therapy. The ultimate goal of Stealth BioTherapeutics is to secure FDA approval and bring this promising therapy to market, offering new treatment options for patients with mitochondrial disorders. Through its ongoing research and development initiatives, Stealth BioTherapeutics is paving the way for significant advancements in mitochondrial medicine. The commitment of Stealth BioTherapeutics to addressing the challenges in this field demonstrates their dedication to improving patient outcomes and enhancing the quality of life for individuals affected by mitochondrial diseases.
SS-31 Dosage
SS-31, also known as Elamipretide, is a mitochondria-targeted peptide designed to treat mitochondrial dysfunction. Determining the appropriate dosage of SS-31 is crucial for its efficacy and safety. In preclinical studies and initial clinical trials, SS-31 has been administered in various dosages to evaluate its pharmacokinetics, therapeutic effects, and potential side effects. Typical dosages in animal studies range from 0.1 mg/kg to 10 mg/kg, tailored according to the specific model and the desired outcome. These studies have provided a foundational understanding of how SS-31 interacts with biological systems and its potential therapeutic window.
In human clinical trials, the dosage of SS-31 has been meticulously adjusted and monitored. Early phase studies often start with lower doses to ensure safety, gradually increasing to identify the maximum tolerated dose. For example, in trials involving patients with mitochondrial myopathies or heart failure, dosages have ranged from 0.25 mg/kg to 1.0 mg/kg per day, administered via daily subcutaneous injections or intravenous infusion. These trials aim to find the optimal balance between efficacy and minimizing adverse effects. The variations in dosage also help in understanding the pharmacodynamic responses in different patient populations, guiding the development of tailored treatment protocols.
The dosage regimen of SS-31 is influenced by several factors, including the specific condition being treated, the severity of the disease, and individual patient responses. Ongoing and future studies continue to refine these dosages, focusing on long-term safety and effectiveness. The goal is to establish a standardized dosage that maximizes therapeutic benefits while minimizing risks, ultimately leading to potential approval and widespread use of SS-31 in clinical practice. As research progresses, the insights gained from these studies will be crucial in informing guidelines and recommendations for the clinical use of SS-31.
SS 31 Elamipretide
SS-31, also known as elamipretide, is a synthetic tetrapeptide designed to target and protect mitochondria from oxidative damage and dysfunction. Its sequence, D-Arg-Dmt-Lys-Phe-NH2, allows it to selectively bind to cardiolipin, a phospholipid located on the inner mitochondrial membrane. By stabilizing cardiolipin, Elamipretide helps maintain mitochondrial integrity and function, preventing the formation of reactive oxygen species (ROS) and mitigating mitochondrial depolarization. This mechanism is particularly important in conditions where mitochondrial dysfunction plays a critical role, such as cardiovascular diseases, neurodegenerative disorders, and certain metabolic syndromes.
Elamipretide’s potential therapeutic applications have been explored extensively in preclinical and clinical studies. In models of heart failure, elamipretide has demonstrated the ability to improve mitochondrial function, reduce ROS production, and enhance overall cardiac function. Similarly, in models of kidney disease, it has shown promise in reducing oxidative stress and preserving renal function. Clinical trials have further highlighted its benefits; for instance, in patients with primary mitochondrial myopathy, Elamipretide has been observed to improve muscle performance and reduce fatigue. These studies suggest that by protecting mitochondria, Elamipretide can address the underlying causes of various mitochondrial-related diseases and improve patient outcomes.
One of the most significant areas of research for elamipretide has been its impact on primary mitochondrial myopathy, a condition characterized by impaired mitochondrial function leading to muscle weakness and fatigue. In clinical settings, patients with primary mitochondrial myopathy treated with Elamipretide have shown marked improvements in muscle strength and endurance, suggesting that the drug can effectively target the mitochondrial deficiencies at the heart of the disease. These findings are particularly encouraging as they offer hope for a condition with limited treatment options.
Elamipretide Side Effects
Elamipretide, also known by its research name SS-31, is a mitochondria-targeted peptide with promising therapeutic potential for various conditions linked to mitochondrial dysfunction. However, like all medications, it can have side effects. Clinical trials have reported that Elamipretide is generally well-tolerated, but some patients have experienced adverse effects. Common side effects include injection site reactions such as pain, redness, and swelling, which are typical for medications administered via injection. Additionally, some patients have reported gastrointestinal issues, including nausea and vomiting, as well as headaches and dizziness.
In some studies, patients have also experienced changes in low-luminance visual acuity, necessitating careful monitoring of visual function during treatment. Further investigation into the long-term impact of elamipretide on low-luminance visual acuity is ongoing, as understanding these effects is crucial for ensuring the safety of patients. Despite these side effects, the potential benefits of elamipretide for improving mitochondrial function and addressing related conditions make it a promising therapeutic option, with its impact on low-luminance visual acuity being an important area of focus in ongoing clinical research. Monitoring low-luminance visual acuity is essential to ensure that the benefits of elamipretide outweigh any potential risks to visual health.
Beyond these relatively mild and transient side effects, there are concerns about the potential long-term impacts of elamipretide. Since the drug is designed to alter mitochondrial function, it is crucial to monitor any unintended consequences on cellular metabolism and overall energy production. In some clinical trials, patients have experienced fatigue and muscle pain, which might be related to the drug’s impact on mitochondrial activity. While these effects were generally not severe, they underscore the need for careful monitoring and more extensive research to fully understand the safety profile of elamipretide, especially over prolonged periods of use.
Elamipretide and Inner Mitochondrial Membrane
Elamipretide is a mitochondrial-targeted peptide that has shown promise in protecting and enhancing the function of the inner mitochondrial membrane. This membrane is crucial for the mitochondria’s ability to produce ATP, the primary energy currency of the cell. By binding to cardiolipin, a lipid unique to the inner mitochondrial membrane, Elamipretide helps stabilize and protect this vital component from oxidative damage. This action preserves the structural integrity and functionality of the mitochondria, ensuring efficient energy production and overall cellular health.
The inner mitochondrial membrane houses the electron transport chain (ETC), which is responsible for oxidative phosphorylation and ATP synthesis. When oxidative stress damages the membrane, the ETC’s efficiency is compromised, leading to reduced ATP production and increased production of reactive oxygen species (ROS). Elamipretide’s protective effect on cardiolipin helps maintain the proper functioning of the ETC, reducing the generation of ROS and preventing the cascade of cellular damage that can result from mitochondrial dysfunction. This protective mechanism is particularly beneficial in conditions characterized by high oxidative stress and mitochondrial impairment.
Elamipretide and Mitochondrial Dysfunction
Elamipretide is a peptide designed to combat mitochondrial dysfunction by targeting and protecting the mitochondria, the cell’s powerhouse. It binds to cardiolipin, a crucial lipid in the inner mitochondrial membrane, preserving its integrity and preventing oxidative damage. This action helps maintain the mitochondria’s structural and functional stability, which is essential for effective energy production.
Mitochondrial dysfunction is a hallmark of many degenerative diseases and aging, characterized by reduced ATP production and increased reactive oxygen species (ROS) generation. By protecting the mitochondria from oxidative stress, Elamipretide enhances ATP synthesis and reduces ROS levels. This mitigation of oxidative damage helps prevent the cascade of cellular dysfunction that can lead to various diseases.
Elamipretide and Reactive Oxygen Species
Elamipretide is a peptide that targets mitochondria and plays a crucial role in managing reactive oxygen species (ROS). ROS are byproducts of cellular metabolism that can cause significant oxidative damage to cellular structures, including the mitochondria. By binding to cardiolipin in the inner mitochondrial membrane, Elamipretide stabilizes and protects the mitochondria, reducing the excessive production of ROS.
One of the key benefits of elamipretide is its ability to maintain the efficiency of the electron transport chain (ETC) within the mitochondria. The ETC is a primary site for ROS generation, especially when it becomes dysfunctional due to oxidative stress. Elamipretide helps ensure that the ETC operates smoothly, thereby minimizing the leakage of electrons that lead to the formation of harmful ROS.
The reduction in ROS levels brought about by Elamipretide has broad therapeutic implications. Lower ROS levels mean less oxidative damage to cellular components, which helps prevent cell death and supports overall cellular health. This protective effect is particularly beneficial in conditions characterized by high oxidative stress, such as cardiovascular diseases, neurodegenerative disorders, and age-related conditions, making Elamipretide a promising therapeutic agent.
Elamipretide Purchase
Elamipretide, a mitochondrial-targeted peptide, is primarily available for purchase through clinical research channels rather than over-the-counter or standard pharmaceutical retailers. Given its investigational status, Elamipretide is typically supplied to institutions, laboratories, and researchers conducting studies on mitochondrial dysfunction and related conditions. One key area of focus in these studies is its role in preventing cardiolipin peroxidation, a process that can damage mitochondrial membranes and impair function. Accessing this peptide for clinical or experimental purposes generally requires adherence to specific regulatory and ethical guidelines, including obtaining appropriate approvals from relevant health authorities or institutional review boards. Preventing cardiolipin peroxidation is crucial because it helps maintain mitochondrial integrity and supports overall cellular health. Continued research on Elamipretide’s ability to inhibit cardiolipin peroxidation may provide further insights into its therapeutic potential for mitochondrial diseases.
For individuals or organizations interested in purchasing Elamipretide, the process often involves directly contacting the pharmaceutical company responsible for its development, Stealth BioTherapeutics. Potential buyers might need to provide detailed information regarding the intended use, study protocols, and any necessary compliance documentation. This is particularly crucial given the role of Elamipretide in enhancing mitochondrial energy generation, which is vital for research focused on mitochondrial dysfunction. Stealth BioTherapeutics may also require assurances that the peptide will be used solely for legitimate scientific research or therapeutic purposes, aligning with their mission to advance the understanding and treatment of mitochondrial diseases. Ensuring that Elamipretide is used appropriately helps maintain the integrity of studies investigating its effects on energy generation in various cellular processes. By adhering to these guidelines, researchers can contribute to significant advancements in mitochondrial medicine, potentially improving therapeutic options for conditions related to impaired energy generation.
The cost of Elamipretide can be substantial, reflecting its specialized nature and the extensive research and development investments behind its creation. Researchers and institutions must budget accordingly and may seek funding or grants to support their acquisition and use of the peptide. While Elamipretide shows promise in various therapeutic applications, its availability remains tightly controlled to ensure its use is consistent with ongoing scientific investigations and potential clinical development. This is particularly important for adults with primary mitochondrial diseases, where precise and regulated use of Elamipretide can contribute to better understanding and management of these conditions. Furthermore, ongoing studies specifically aim to assess the benefits of elamipretide for adults with primary mitochondrial disorders, potentially paving the way for targeted therapies that can significantly improve patient outcomes.
Elamipretide Dosage
Elamipretide, also known by its investigational names such as SS-31, MTP-131, and Bendavia, has been studied across a variety of clinical settings to determine the most effective and safe dosage for its potential therapeutic benefits. The peptide is primarily administered via intravenous infusion or daily subcutaneous injections, with the dosage and frequency tailored to the specific condition being treated. Clinical trials have investigated a range of dosages, often starting with a lower dose to monitor safety and gradually increasing to assess efficacy. For example, in studies involving patients with mitochondrial myopathy, dosages have typically ranged from 0.25 mg/kg to 0.75 mg/kg administered daily.
In cardiovascular and renal studies, the dosage regimen of Elamipretide has varied based on the acute or chronic nature of the condition being addressed. For instance, in acute settings like ischemia-reperfusion injury, higher doses administered over a shorter period may be required to mitigate immediate oxidative damage. Conversely, chronic conditions such as heart failure or chronic kidney disease might necessitate prolonged administration at lower doses to achieve sustained mitochondrial protection and functional improvement. The pharmacokinetics of Elamipretide support flexibility in dosing, as it is rapidly distributed to tissues and has a relatively short half-life, necessitating regular administration to maintain therapeutic levels. Placebo-controlled trials have been essential in determining these dosing strategies, ensuring that the benefits observed are attributable to the drug and not to placebo effects. Additionally, placebo-controlled trials are critical in validating the efficacy and safety of Elamipretide across various conditions. These trials help to fine-tune the dosing regimens by comparing different dosages and administration schedules against a placebo, providing a clear understanding of the drug’s impact. The data gathered from placebo-controlled trials thus play a pivotal role in guiding clinical decisions and optimizing treatment protocols for both acute and chronic conditions.
While the exact optimal dosage of elamipretide is still under investigation, ongoing and completed clinical trials have provided valuable insights into its safety and efficacy profile. These studies have demonstrated the beneficial effects of Elamipretide in improving mitochondrial function and reducing oxidative stress. Common side effects observed at varying doses include injection site reactions, gastrointestinal disturbances, and transient increases in blood pressure. These trials underscore the importance of balancing efficacy with tolerability, highlighting the beneficial effects of Elamipretide in various patient populations. As research progresses, more refined dosing strategies are likely to emerge, optimizing the therapeutic benefits of elamipretide while minimizing adverse effects. Understanding the beneficial effects of elamipretide on cellular energy production and overall health is crucial for developing effective treatment protocols.
Elamipretide Price
Elamipretide, known by various names including SS-31 and Bendavia, is a mitochondria-targeting peptide currently under investigation for its potential therapeutic benefits in various conditions, such as mitochondrial diseases, heart failure, and kidney diseases. The pricing of elamipretide is not straightforward to pinpoint due to its status as an investigational drug. As it is still in clinical trials and has not yet received full regulatory approval, its price is primarily speculative and dependent on the outcomes of these trials, the approval process, and subsequent market dynamics. Typically, the cost of developing and bringing such a drug to market is high, which can significantly influence its eventual pricing.
For patients and healthcare providers, the cost of elamipretide will ultimately be influenced by multiple factors, including the cost of production, the pricing strategies of the pharmaceutical company, Stealth BioTherapeutics, and the extent to which insurance companies or national health services will cover the medication. Given the complex manufacturing process of peptides and the specialized nature of mitochondrial-targeted therapies, the drug could be expected to have a high price point upon market release. Additionally, the rarity of the conditions it aims to treat could also lead to higher prices, as is often the case with orphan drugs designed for rare diseases.
Patients interested in accessing Elamipretide before its commercial release might explore options such as enrolling in clinical trials, which can provide the medication at no cost while also contributing to valuable research. Compassionate use programs, if available, might offer another route for patients with severe or life-threatening conditions to access the drug. As Elamipretide progresses through the clinical trial phases and moves closer to potential approval, clearer information about its pricing structure and accessibility will likely become available, enabling patients, healthcare providers, and payers to better plan for its integration into therapeutic regimens.
As Elamipretide progresses through the clinical trial phases and moves closer to potential approval, clearer information about its pricing structure and accessibility will likely become available, enabling patients, healthcare providers, and payers to better plan for its integration into therapeutic regimens. Clinical trials not only help in determining the optimal dosage and potential side effects but also play a crucial role in the regulatory approval process. By participating in these trials, patients can contribute to the scientific understanding of elamipretide and expedite its journey to market availability.
FAQ
What is the function of elamipretide?
The protection of cell membranes ensures that the cells can effectively regulate the passage of ions, nutrients, and waste products, thus maintaining proper cellular homeostasis. Furthermore, Elamipretide’s ability to preserve mitochondrial function also indirectly supports the stability of cell membranes, as healthy mitochondria are essential for providing the energy required for various cellular processes, including those that reinforce the structure and function of cell membranes.
What are the other names for Elamipretide?
Other names for Elamipretide include MTP-131, SS-31, and Bendavia. This peptide is known for its ability to modulate cytochrome c peroxidase activity, which plays a crucial role in reducing oxidative stress within cells. By enhancing cytochrome c peroxidase activity, Elamipretide helps to protect cells from oxidative damage and improve overall mitochondrial function. Additionally, the increase in cytochrome c peroxidase activity facilitated by Elamipretide can lead to more efficient removal of reactive oxygen species (ROS), thus preserving cellular health and preventing apoptosis. This property makes Elamipretide a promising candidate for treating conditions associated with mitochondrial dysfunction and oxidative stress.
Who makes Elamipretide?
Elamipretide is developed and made by Stealth BioTherapeutics. This novel therapeutic agent targets the outer mitochondrial membrane, stabilizing its structure and improving mitochondrial bioenergetics. By doing so, Elamipretide enhances overall mitochondrial function and offers potential benefits for a range of mitochondria-related diseases.
What are the other names for SS-31?
Other names for SS-31 include Elamipretide, MTP-131, and Bendavia. SS-31 works by targeting and stabilizing the inner mitochondrial membrane, which helps to protect and restore mitochondrial function. This stabilization is crucial for maintaining the production of adenosine triphosphate (ATP), the primary energy currency of cells. By preserving ATP levels, SS-31 helps to reduce oxidative stress and prevent cellular damage in various disease conditions. The peptide’s ability to support mitochondrial integrity and ensure efficient ATP production makes it a valuable therapeutic agent for diseases characterized by mitochondrial dysfunction, such as mitochondrial myopathies, neurodegenerative disorders, and heart failure. Additionally, the restoration of ATP levels by SS-31 can enhance overall cellular energy metabolism, leading to improved function and vitality of affected tissues. Maintaining adequate adenosine triphosphate levels is essential for the proper functioning of high-energy-demand organs such as the heart, brain, and muscles.
What is the use of Elamipretide?
Elamipretide is used to protect and improve mitochondrial function, potentially benefiting conditions like heart failure, kidney diseases, and diabetic complications by reducing oxidative stress and cellular damage. One of its key properties is that it readily penetrates cell membranes, allowing it to reach the mitochondria efficiently and exert its protective effects. Moreover, the fact that Elamipretide readily penetrates cell membranes ensures that it can be utilized in a wide range of tissues and organs affected by mitochondrial dysfunction. This broad applicability makes it a versatile therapeutic agent for addressing multiple pathologies linked to impaired mitochondrial function.
What is the mechanism of action of Elamipretide?
Elamipretide works by selectively binding to cardiolipin on the inner mitochondrial membrane, stabilizing mitochondrial function, reducing reactive oxygen species production, and preventing mitochondrial depolarization and swelling. This mechanism of action makes Elamipretide particularly beneficial for a diverse patient population suffering from mitochondrial dysfunction. In clinical trials, Elamipretide has demonstrated the potential to improve outcomes in patient populations with various mitochondrial diseases, heart failure, and other conditions characterized by impaired mitochondrial function. Understanding the specific needs and responses of different patient populations will be essential in optimizing the therapeutic applications of elamipretide. Ongoing research aims to further elucidate how Elamipretide can be tailored to benefit specific patient populations, enhancing its efficacy and safety profiles across diverse medical conditions.
What class of drug is elamipretide?
Elamipretide is a mitochondria-targeted antioxidant peptide. It specifically interacts with the electron transport chain to enhance its efficiency and reduce the production of reactive oxygen species. By stabilizing the electron transport chain, Elamipretide helps maintain optimal mitochondrial function and bioenergetics. Additionally, the peptide’s interaction with the electron transport chain supports the prevention of mitochondrial dysfunction, which is crucial in the management of various mitochondria-related diseases.
What is the FDA-granted priority review?
FDA priority review is a designation given to drugs that, if approved, would offer significant improvements in the treatment, diagnosis, or prevention of serious conditions. This process ensures that promising therapies reach patients sooner, provided they demonstrate safety and efficacy with minimal adverse reactions. The FDA closely monitors clinical trials to assess any potential adverse reactions that could impact patient safety. Furthermore, during the post-marketing phase, the FDA continues to evaluate the drug to identify any long-term adverse reactions.
What are the side effects of SS-31 peptide?
FDA priority review is a designation that expedites the review process for drugs offering significant improvements in the treatment, diagnosis, or prevention of serious conditions. This designation is crucial for therapies designed to restore mitochondrial bioenergetics, as it can accelerate the availability of these treatments to patients in need. Drugs that restore mitochondrial bioenergetics can play a vital role in managing conditions associated with mitochondrial dysfunction.
What is the mechanism of action of SS-31?
The mechanism of action of SS-31 involves targeting and binding to cardiolipin in the inner mitochondrial membrane, thereby stabilizing mitochondrial function and reducing oxidative stress. This interaction is a significant aspect of mitochondrial biology, highlighting the peptide’s role in maintaining mitochondrial integrity. Understanding this process is crucial in the field of mitochondrial biology as it underscores how SS-31 can mitigate cellular damage. By stabilizing mitochondrial function, SS-31 helps to preserve cellular energy production and prevent mitochondrial damage, which is a key focus within mitochondrial biology research.
What are the 5 symptoms of mitochondrial disease?
Common symptoms of mitochondrial disease include muscle weakness, fatigue, neurological problems (such as seizures or strokes), gastrointestinal disorders, and cardiac abnormalities. Patients with mitochondrial disease may also experience serious adverse events, particularly when the condition progresses or is left untreated. Managing these serious adverse events is crucial in improving the quality of life for these patients. In addition, treatments need to be monitored closely for any potential serious adverse events to ensure patient safety and efficacy of the therapeutic approach.
How long can a person live with mitochondrial disease?
The life expectancy of a person with mitochondrial disease can vary widely depending on the severity and type of the disease, ranging from infancy to normal adulthood. This variability is similar to that observed in other neurodegenerative diseases, which also show a wide range of life expectancy based on disease progression and individual health factors. Understanding the overlap between mitochondrial disease and other neurodegenerative diseases can provide insights into potential treatment approaches. Research into mitochondrial disease can often inform studies on other neurodegenerative diseases, highlighting the interconnected nature of these conditions.
What is the life expectancy of someone with CPEO?
The life expectancy of someone with Chronic Progressive External Ophthalmoplegia (CPEO) is typically near normal, though it can vary based on the presence of other systemic complications. This condition primarily affects the eye muscles but can also involve other muscles and organs due to mitochondrial DNA mutations. These mutations often impact the mitochondrial matrix, where critical reactions for energy production occur. Consequently, while life expectancy remains generally stable, monitoring and managing systemic issues arising from mitochondrial dysfunction are crucial for maintaining overall health.
What are the symptoms of mitochondrial toxicity?
Symptoms of mitochondrial toxicity include muscle weakness, fatigue, gastrointestinal issues, neuropathy, lactic acidosis, and organ dysfunction. These symptoms can significantly impact individuals with underlying conditions such as Duchenne muscular dystrophy, a severe form of muscular dystrophy characterized by progressive muscle degeneration. Patients with Duchenne muscular dystrophy are particularly vulnerable to mitochondrial toxicity, which can exacerbate their existing muscle weakness and fatigue. Effective management of mitochondrial toxicity is crucial in improving the quality of life for those affected by Duchenne muscular dystrophy. Therefore, understanding the interplay between mitochondrial toxicity and Duchenne muscular dystrophy is essential for developing targeted therapeutic strategies.
What does SS-31 do?
SS-31 is a mitochondria-targeted peptide that reduces oxidative stress, protects mitochondrial structure, and improves cellular function in various disease models. Studies using isolated mitochondria have shown that SS-31 can directly interact with cardiolipin, a crucial component of the inner mitochondrial membrane, thereby stabilizing mitochondrial function. In isolated mitochondria experiments, SS-31 has been observed to prevent the opening of the mitochondrial permeability transition pore, a key event leading to cell death. Furthermore, isolated mitochondria treated with SS-31 exhibit enhanced ATP production, which is vital for energy-demanding tissues such as the heart and brain. The protective effects of SS-31 on isolated mitochondria have been demonstrated in models of ischemia-reperfusion injury, where it mitigates damage and improves recovery. Additionally, SS-31’s ability to maintain the integrity of isolated mitochondria has shown promise in neurodegenerative diseases by preserving neuronal function. Research on isolated mitochondria continues to elucidate the broad therapeutic potential of SS-31 in various pathological conditions, highlighting its role as a versatile mitochondrial protectant.
What does Humanin do?
Humanin is a peptide that protects cells from stress and apoptosis, providing neuroprotective and cytoprotective effects in various disease models. One of the mechanisms by which Humanin exerts its protective effects is by modulating the immune response and reducing the production of proinflammatory cytokines. This reduction in proinflammatory cytokines helps to mitigate inflammation-related damage in tissues and supports cellular resilience against stressors. Additionally, Humanin’s ability to decrease the levels of proinflammatory cytokines has been shown to be beneficial in conditions such as neurodegenerative diseases and cardiovascular disorders, where inflammation plays a crucial role in disease progression. Overall, Humanin’s multifaceted protective actions make it a promising candidate for therapeutic development in a wide range of diseases.
What are the side effects of ss31 peptide?
The side effects of SS-31 peptide, also known as elamipretide, may include mild to moderate injection site reactions, headache, and gastrointestinal symptoms such as nausea and vomiting. Despite these side effects, SS-31 shows promise in treating conditions associated with dysfunctional mitochondria. By targeting and stabilizing dysfunctional mitochondria, SS-31 helps improve cellular function and reduces oxidative stress. This effect is particularly beneficial in diseases where dysfunctional mitochondria play a key role, such as mitochondrial myopathies, heart failure, and neurodegenerative disorders. Ongoing clinical trials aim to further evaluate the efficacy and safety of SS-31 in various patient populations with mitochondrial diseases and other related disorders, emphasizing its potential to improve outcomes by addressing the root cause of these conditions: dysfunctional mitochondria.
What is the use of SS-31?
SS-31 is used to protect and restore mitochondrial function, thereby reducing oxidative stress and preventing cellular damage in various disease conditions. By specifically targeting the inner membrane of mitochondria, SS-31 helps to stabilize and preserve mitochondrial integrity. This stabilization is crucial for maintaining proper mitochondrial function and energy production. In addition, SS-31’s ability to support the inner membrane structure makes it a valuable therapeutic agent for diseases characterized by mitochondrial dysfunction, such as mitochondrial myopathies, neurodegenerative disorders, and heart failure. The peptide’s effectiveness in reducing cellular damage and oxidative stress highlights its potential to improve patient outcomes across a range of mitochondrial-related conditions.
Reference
Obi C, Smith AT, Hughes GJ, Adeboye AA. Targeting mitochondrial dysfunction with elamipretide. Heart Fail Rev. 2022 Sep;27(5):1925-1932. doi: 10.1007/s10741-021-10199-2. Epub 2022 Jan 17. PMID: 35037146.
Targeting mitochondrial dysfunction with elamipretide
Current heart failure therapies improve patient quality of life and decrease mortality but do not halt disease progression, indicating incomplete understanding and a need for new treatments. Research is focusing on mitochondria, particularly the drug elamipretide, which targets and stabilizes the cardiolipin-cytochrome c supercomplex to maintain cellular bioenergetics and prevent cell damage. Elamipretide has shown promising results in animal models and early-phase clinical trials, improving cardiac function without severe adverse events, but further studies are needed to confirm its long-term safety and efficacy.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s10741-021-10199-2.Nhu NT, Xiao SY, Liu Y, Kumar VB, Cui ZY, Lee SD. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front Integr Neurosci. 2022 Jan 17;15:747901. doi: 10.3389/fnint.2021.747901. PMID: 35111001; PMCID: PMC8801496.
Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration
Elamipretide, a mitochondrially-targeted tetrapeptide, shows promise in treating neurodegenerative diseases by enhancing mitochondrial functions, reducing oxidative stress and neuroinflammation, preventing toxic protein accumulation, and inhibiting neural apoptosis. It improves mitochondrial respiration and biogenesis, promotes mitochondrial fusion, and mitigates mitochondrial fission. Additionally, elamipretide reduces neural oxidative stress, neuroinflammation, and toxic protein buildup, thus enhancing neural cell survival and preventing apoptosis. These effects suggest that elamipretide could be a targeted therapy to slow neurodegenerative disease progression.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8801496/.Jiang W, He F, Ding G, Wu J. Elamipretide reduces pyroptosis and improves functional recovery after spinal cord injury. CNS Neurosci Ther. 2023 Oct;29(10):2843-2856. doi: 10.1111/cns.14221. Epub 2023 Apr 20. PMID: 37081763; PMCID: PMC10493668.
Elamipretide reduces pyroptosis and improves functional recovery after spinal cord injury
Elamipretide (EPT), a mitochondria-targeted peptide, shows protective effects in spinal cord injury (SCI) by improving locomotor recovery, reducing neuronal loss, inhibiting NLRP3 inflammasome activation and pyroptosis, decreasing pro-inflammatory cytokines, and alleviating mitochondrial dysfunction and oxidative stress.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10493668/.Pharaoh G, Kamat V, Kannan S, Stuppard RS, Whitson J, Martín-Pérez M, Qian WJ, MacCoss MJ, Villén J, Rabinovitch P, Campbell MD, Sweet IR, Marcinek DJ. The mitochondrially targeted peptide elamipretide (SS-31) improves ADP sensitivity in aged mitochondria by increasing uptake through the adenine nucleotide translocator (ANT). Geroscience. 2023 Dec;45(6):3529-3548. doi: 10.1007/s11357-023-00861-y. Epub 2023 Jul 18. PMID: 37462785; PMCID: PMC10643647.
The mitochondrially targeted peptide elamipretide (SS-31) improves ADP sensitivity in aged mitochondria by increasing uptake through the adenine nucleotide translocator (ANT)
Elamipretide (ELAM) enhances mitochondrial ADP sensitivity and physiological function in aging muscles by improving ADP uptake through the adenine nucleotide translocator (ANT) without altering protein abundance but reducing protein s-glutathionylation, thereby increasing ATP production and rescuing muscle force and heart systolic function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10643647/.Karanjia R, Sadun AA. Elamipretide Topical Ophthalmic Solution for the Treatment of Subjects with Leber Hereditary Optic Neuropathy: A Randomized Trial. Ophthalmology. 2024 Apr;131(4):422-433. doi: 10.1016/j.ophtha.2023.10.033. Epub 2023 Nov 3. PMID: 37923251.
Elamipretide Topical Ophthalmic Solution for the Treatment of Subjects with Leber Hereditary Optic Neuropathy: A Randomized Trial
Elamipretide (ELAM) enhances mitochondrial ADP sensitivity and physiological function in aging muscles by improving ADP uptake through the adenine nucleotide translocator (ANT) without altering protein abundance but reducing protein s-glutathionylation, thereby increasing ATP production and rescuing muscle force and heart systolic function.
You can read the full article at https://www.aaojournal.org/article/S0161-6420(23)00802-3/fulltext.Grosser JA, Fehrman RL, Keefe D, Redmon M, Nickells RW. The effects of a mitochondrial targeted peptide (elamipretide/SS31) on BAX recruitment and activation during apoptosis. BMC Res Notes. 2021 May 22;14(1):198. doi: 10.1186/s13104-021-05613-9. PMID: 34022923; PMCID: PMC8141144.
The effects of a mitochondrial targeted peptide (elamipretide/SS31) on BAX recruitment and activation during apoptosis
Elamipretide (SS31) stabilizes mitochondrial cristae structure and enhances mitochondrial bioenergetics. It also plays a crucial role in cytochrome c regulation, which is integral to mitochondrial function and apoptosis. While it accelerates mitochondrial enlargement and moderately slows BAX protein recruitment during apoptosis in ARPE-19 cells, it does not significantly affect BAX recruitment, cytochrome c release, or mitochondrial fragmentation, indicating its protective effects do not interfere with BAX activity during cell death. Cytochrome c release is a pivotal event in the intrinsic pathway of apoptosis, but SS31’s mechanism ensures that mitochondrial integrity is maintained without altering this process. The regulation of cytochrome c by SS31 underscores its role in protecting cells from oxidative stress and maintaining mitochondrial stability. Despite these protective actions, SS31 does not inhibit the necessary release of cytochrome c required for apoptosis, thereby maintaining a balance between cell survival and programmed cell death. By influencing cytochrome c dynamics and mitochondrial structure, SS31 emerges as a promising therapeutic agent in managing mitochondrial dysfunction and related pathologies.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8141144/.Chatfield KC, Sparagna GC, Chau S, et al. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl Sci. 2019;4(2):147-157. Published 2019 Apr 29. doi:10.1016/j.jacbts.2018.12.005.
Elamipretide Improves Mitochondrial Function in the Failing Human Heart
Elamipretide improves mitochondrial function in failing human hearts by enhancing mitochondrial oxygen flux, complex I and IV activities, and supercomplex-associated complex IV activity in freshly explanted ventricular tissue from both children and adults, addressing mitochondrial impairments in heart failure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6488757/.
Available from https://www.pnas.org/content/117/26/15363.
Mitochondrial protein interaction landscape of SS-31
Mitochondrial dysfunction is linked to various diseases, and restoring mitochondrial health holds therapeutic potential. Elamipretide (SS-31), a synthetic peptide, enhances mitochondrial function by interacting with cardiolipin in the inner mitochondrial membrane. Using chemical cross-linking and mass spectrometry, SS-31 was found to bind proteins involved in ATP production and 2-oxoglutarate metabolism, providing insight into its therapeutic mechanisms.
You can read the full article at https://www.pnas.org/content/117/26/15363.
Escribano-Lopez, I., Diaz-Morales, N., Iannantuoni, F. et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci Rep 8, 15862 (2018). https://doi.org/10.1038/s41598-018-34251-8.
The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes
Mitochondrial dysfunction in type 2 diabetes (T2D) contributes to cardiovascular disease risk, and antioxidants like SS-31 may offer protection. In a study of T2D patients, SS-31 reduced oxidative stress, inflammation, and leukocyte-endothelium interactions while increasing mitochondrial function and SIRT1 levels. These findings suggest SS-31 as a potential therapeutic agent for mitigating cardiovascular risk in T2D.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6203778/.
Machiraju P, Wang X, Sabouny R, Huang J, Zhao T, Iqbal F, King M, Prasher D, Lodha A, Jimenez-Tellez N, Ravandi A, Argiropoulos B, Sinasac D, Khan A, Shutt TE and Greenway SC (2019) SS-31 Peptide Reverses the Mitochondrial Fragmentation Present in Fibroblasts From Patients With DCMA, a Mitochondrial Cardiomyopathy. Front. Cardiovasc. Med. 6:167. doi: 10.3389/fcvm.2019.00167.
SS-31 Peptide Reverses the Mitochondrial Fragmentation Present in Fibroblasts From Patients With DCMA, a Mitochondrial Cardiomyopathy
This study used dermal fibroblasts from patients with dilated cardiomyopathy with ataxia syndrome (DCMA) to investigate mitochondrial abnormalities and assess the therapeutic potential of the peptide SS-31. DCMA, caused by mutations in DNAJC19, leads to severe symptoms including cardiomyopathy and neurological issues, often resulting in early childhood death. DCMA fibroblasts exhibited fragmented mitochondria and increased ROS production, which were reversed by SS-31 treatment, suggesting that SS-31 could be a promising therapy for DCMA.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6873783/.
Available from https://jasn.asnjournals.org/content/24/8/1250.
Lu HI, Lee FY, Wallace CG, et al. SS31 therapy effectively protects the heart against transverse aortic constriction-induced hypertrophic cardiomyopathy damage. Am J Transl Res. 2017;9(12):5220-5237. Published 2017 Dec 15.
SS31 therapy effectively protects the heart against transverse aortic constriction-induced hypertrophic cardiomyopathy damage
This study evaluated whether SS31 therapy could protect against hypertrophic cardiomyopathy (HCM) induced by transverse aortic constriction (TAC) in mice. Mice treated with SS31 after TAC showed improved heart function, reduced markers of inflammation, oxidative stress, fibrosis, and apoptosis compared to untreated TAC mice. SS31 treatment resulted in higher left ventricular ejection fraction and sarcomere length, suggesting it effectively mitigates TAC-induced cardiac damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752876/.
Liu Y, Yang W, Sun X, Xie L, Yang Y, Sang M, Jiao R. SS31 Ameliorates Sepsis-Induced Heart Injury by Inhibiting Oxidative Stress and Inflammation. Inflammation. 2019 Dec;42(6):2170-2180. doi: 10.1007/s10753-019-01081-3. PMID: 31494795.
SS31 Ameliorates Sepsis-Induced Heart Injury by Inhibiting Oxidative Stress and Inflammation
This study evaluated whether SS31 therapy could protect against hypertrophic cardiomyopathy (HCM) induced by transverse aortic constriction (TAC) in mice. Mice treated with SS31 after TAC showed improved heart function, reduced markers of inflammation, oxidative stress, fibrosis, and apoptosis compared to untreated TAC mice. SS31 treatment resulted in higher left ventricular ejection fraction and sarcomere length, suggesting it effectively mitigates TAC-induced cardiac damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752876/.
Dai DF, Chen T, Szeto H, et al. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58(1):73-82. doi:10.1016/j.jacc.2010.12.044.
Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy
This study examined the effects of the mitochondrial-targeted antioxidant peptide SS-31 on hypertensive cardiomyopathy. SS-31, unlike the nontargeted antioxidant NAC, effectively reduced mitochondrial oxidative stress, NOX4 up-regulation, oxidative damage, and apoptosis in models of angiotensin II-induced cardiomyopathy and Gαq overexpression-induced heart failure. Although SS-31 did not lower blood pressure, it significantly ameliorated cardiac hypertrophy, diastolic dysfunction, and fibrosis, suggesting its potential for protecting against hypertensive cardiovascular diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3742010/.
Zhang W, Tam J, Shinozaki K, Yin T, Lampe JW, Becker LB, Kim J. Increased Survival Time With SS-31 After Prolonged Cardiac Arrest in Rats. Heart Lung Circ. 2019 Mar;28(3):505-508. doi: 10.1016/j.hlc.2018.01.008. Epub 2018 Feb 7. PMID: 29503242; PMCID: PMC6081272.
Increased Survival Time With SS-31 After Prolonged Cardiac Arrest in Rats
Cardiac arrest, a leading cause of death with high mortality, currently lacks effective drugs for use during resuscitation. Mitochondrial dysfunction is critical in its pathogenesis. This study tested the peptide SS-31, known for protecting mitochondria from ischemia/reperfusion injury, in a rat model of prolonged cardiac arrest. Rats treated with SS-31 during resuscitation had significantly improved survival rates and lower blood lactate levels, indicating enhanced mitochondrial function, suggesting SS-31’s potential as a novel cardiac arrest therapy.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6081272/.
Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, Keller A, Zhang H, Rabinovitch PS, Marcinek DJ, Bruce JE. Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):15363-15373. doi: 10.1073/pnas.2002250117. Epub 2020 Jun 17. PMID: 32554501; PMCID: PMC7334473.
Mitochondrial protein interaction landscape of SS-31
Mitochondrial dysfunction contributes to various diseases, and restoring mitochondrial health is promising for treatment. The synthetic peptide SS-31 enhances mitochondrial function by interacting with cardiolipin in the inner mitochondrial membrane. Using chemical cross-linking and mass spectrometry, SS-31 was found to bind proteins involved in ATP production and 2-oxoglutarate metabolism, providing insights into its therapeutic mechanisms.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7334473/.
Sabbah, H.N., Gupta, R.C., Singh-Gupta, V. et al. Abnormalities of Mitochondrial Dynamics in the Failing Heart: Normalization Following Long-Term Therapy with Elamipretide. Cardiovasc Drugs Ther 32, 319–328 (2018). https://doi.org/10.1007/s10557-018-6805-y.
Abnormalities of Mitochondrial Dynamics in the Failing Heart: Normalization Following Long-Term Therapy with Elamipretide
This study explored mitochondrial (MITO) dysfunction in heart failure with reduced ejection fraction (HF) and assessed the effects of long-term treatment with the peptide elamipretide (ELAM). In both dogs and humans with HF, there were significant abnormalities in MITO biogenesis, fission, fusion, and cardiolipin synthesis. Long-term ELAM therapy normalized these mitochondrial dysfunctions, supporting its potential as a treatment for HF.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6133191/.
Available from https://www.medrxiv.org/content/10.1101/2020.11.20.20235580v2.full.
Available from https://www.ahajournals.org/doi/full/10.1161/circinterventions.117.005487.
Available from https://europepmc.org/articles/pmc6588449/bin/nihms1005643-supplement-supplemental_material.pdf.
Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice
This study tested whether the peptide SS-31 (elamipretide) could improve mitochondrial function and reduce oxidative stress to enhance skeletal muscle function in aged mice. Treatment with SS-31 reversed age-related declines in mitochondrial ATP production and oxidative phosphorylation efficiency, restored redox balance, increased muscle mass, and improved fatigue resistance and treadmill endurance. These findings suggest that SS-31 could enhance exercise tolerance and quality of life in the elderly, supporting its potential clinical application.
You can read the full article at https://europepmc.org/articles/pmc6588449/bin/nihms1005643-supplement-supplemental_material.pdf.
Szeto HH, Liu S, Soong Y, et al. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD. J Am Soc Nephrol. 2017;28(5):1437-1449. doi:10.1681/ASN.2016070761.
Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD
The innate immune system plays a role in both acute kidney injury (AKI) and chronic kidney disease (CKD), with mitochondrial damage driving chronic inflammation. A 9-month study in rats after renal ischemia showed persistent mitochondrial damage, inflammation, and fibrosis. Treatment with the mitochondrial-protective agent SS-31, starting one month post-ischemia, preserved mitochondrial integrity, reduced inflammation, and halted fibrosis and glomerulosclerosis. SS-31’s benefits persisted for six months after treatment ended, suggesting it as a promising therapy for CKD by mitigating sustained inflammasome activation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5407729/.
Whitson, J. A., Martín-Pérez, M., Zhang, T., Gaffrey, M. J., Merrihew, G. E., Huang, E., White, C. C., Kavanagh, T. J., Qian, W. J., Campbell, M. D., MacCoss, M. J., Marcinek, D. J., Villén, J., & Rabinovitch, P. S. (2021). Elamipretide (SS-31) treatment attenuates age-associated post-translational modifications of heart proteins. GeroScience, 43(5), 2395–2412. https://doi.org/10.1007/s11357-021-00447-6.
Elamipretide (SS-31) treatment attenuates age-associated post-translational modifications of heart proteins
Elamipretide (SS-31) has been shown to improve age-related heart dysfunction, potentially by influencing post-translational modifications of heart proteins. This study used proteomics to analyze the S-glutathionylation and phosphorylation of heart proteins in aged mice. Aging increased protein oxidation, evidenced by more S-glutathionylation, which was almost fully reversed by elamipretide treatment. Changes in phosphorylation associated with aging were also partially restored by elamipretide, particularly in proteins involved in mitochondrial and cardiac function. These findings suggest that elamipretide restores heart function by reversing age-related oxidative and phosphorylation changes in proteins.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8599536/.
Rohani, L., Machiraju, P., Sabouny, R., Meng, G., Liu, S., Zhao, T., Iqbal, F., Wang, X., Ravandi, A., Wu, J. C., Khan, A., Shutt, T., Rancourt, D., & Greenway, S. C. (2020). Reversible Mitochondrial Fragmentation in iPSC-Derived Cardiomyocytes From Children With DCMA, a Mitochondrial Cardiomyopathy. The Canadian journal of cardiology, 36(4), 554–563. https://doi.org/10.1016/j.cjca.2019.09.021.
Reversible Mitochondrial Fragmentation in iPSC-Derived Cardiomyocytes From Children With DCMA, a Mitochondrial Cardiomyopathy
Dilated cardiomyopathy with ataxia syndrome (DCMA) is a rare autosomal recessive disease caused by mutations in DNAJC19, leading to heart failure and early death in children. This study reprogrammed blood cells from DCMA patients into induced pluripotent stem cells (iPSCs) and differentiated them into cardiomyocytes. These patient-derived cardiomyocytes exhibited abnormal mitochondrial structure and function, which were improved with the peptide SS-31. The findings suggest SS-31 as a potential treatment for DCMA, providing a novel cellular model for studying the disease.
You can read the full article at https://onlinecjc.ca/article/S0828-282X(19)31286-3/abstract.
Rabinovitch P, Marcinek DJ. THE ROLE OF MITOCHONDRIAL ENERGETICS IN CARDIAC AND SKELETAL MUSCLE AGING. Innov Aging. 2018 Nov 11;2(Suppl 1):348. doi: 10.1093/geroni/igy023.1278. PMCID: PMC6227127
THE ROLE OF MITOCHONDRIAL ENERGETICS IN CARDIAC AND SKELETAL MUSCLE AGING
Aging in humans and mice leads to declines in skeletal and cardiac muscle function. This study found that short-term treatment with the mitochondrial protective peptide SS-31 (elamipretide) improves muscle ATP generation, fatigue resistance, and overall function in aged mice. SS-31 enhances mitochondrial efficiency, reduces proton leak, and leads to improved muscle energetics, cardiac function, and exercise endurance, demonstrating its potential to reverse age-related muscle deficits.
You can read the abstract of the article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6227127/.
Smuder AJ, Roberts BM, Wiggs MP, Kwon OS, Yoo JK, Christou DD, Fuller DD, Szeto HH, Judge AR. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness. Oncotarget. 2020 Sep 22;11(38):3502-3514. doi: 10.18632/oncotarget.27748. PMID: 33014286; PMCID: PMC7517961.
Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness
Cancer cachexia leads to severe cardiac and diaphragm muscle wasting, increasing morbidity in cancer patients due to cardiorespiratory failure. Mitochondrial dysfunction, contributing to muscle weakness and fatigue, and increased ROS production are implicated in this condition. This study found that treating tumor-bearing mice with the mitochondria-targeting peptide SS-31 reduced ROS production, improved mitochondrial function, and rescued both cardiac and respiratory muscle function, highlighting its potential to prevent cancer cachexia-induced muscle dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7517961/.
Zhang, H., Alder, N. N., Wang, W., Szeto, H., Marcinek, D. J., & Rabinovitch, P. S. (2020). Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. eLife, 9, e60827. https://doi.org/10.7554/eLife.60827.
Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte
Aging-associated cardiac dysfunction is common and lacks effective treatment, with mechanisms remaining poorly understood. In aging mice and rats, increased proton leak in heart mitochondria was observed, mediated by ANT1. The peptide SS-31 was found to prevent excess proton entry, reduce mitochondrial permeability transition pore opening, and rejuvenate mitochondrial function, leading to substantial reversal of diastolic dysfunction, uncovering excessive proton leak as a key mechanism in age-related cardiac dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7738186/.
Available from https://www.ahajournals.org/doi/abs/10.1161/res.125.suppl_1.287.
Chiao, Y. A., Zhang, H., Sweetwyne, M., Whitson, J., Ting, Y. S., Basisty, N., Pino, L. K., Quarles, E., Nguyen, N. H., Campbell, M. D., Zhang, T., Gaffrey, M. J., Merrihew, G., Wang, L., Yue, Y., Duan, D., Granzier, H. L., Szeto, H. H., Qian, W. J., Marcinek, D., … Rabinovitch, P. (2020). Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. eLife, 9, e55513. https://doi.org/10.7554/eLife.55513.
Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice
Diastolic dysfunction is a key feature of cardiac aging. An 8-week treatment with the mitochondrial-targeted peptide SS-31 (elamipretide) in old mice significantly reversed this dysfunction by normalizing proton leak, reducing mitochondrial ROS, decreasing protein oxidation, and shifting protein thiol redox state towards reduction. These improvements, associated with increased phosphorylation of cMyBP-C Ser282, were similar to those seen with late-life viral expression of mitochondrial-targeted catalase (mCAT), suggesting that normalizing mitochondrial oxidative stress is a key mechanism. Thus, targeting mitochondrial dysfunction shows promise for reversing cardiac aging phenotypes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7377906/.
Whitson JA, Bitto A, Zhang H, Sweetwyne MT, Coig R, Bhayana S, Shankland EG, Wang L, Bammler TK, Mills KF, Imai SI, Conley KE, Marcinek DJ, Rabinovitch PS. SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging Cell. 2020 Oct;19(10):e13213. doi: 10.1111/acel.13213. Epub 2020 Aug 11. PMID: 32779818; PMCID: PMC7576234.
SS-31 and NMN: Two paths to improve metabolism and function in aged hearts
The effects of mitochondrial-targeted drugs SS-31 and NMN were tested on old mouse hearts. SS-31 partially reversed age-related diastolic function decline, while NMN fully reversed systolic function deficiency under higher workload. Combined treatment increased NAD(H) levels and normalized energetic capacity, restoring PCr/ATP dynamics. Both drugs improved mitochondrial NAD(P)H production, with NMN also increasing NAD+ in response to workload. The combined treatment synergistically rejuvenated heart function to a youthful state, enhancing both mitochondrial and heart health.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7576234/.
Whitson, J. A., Johnson, R., Wang, L., Bammler, T. K., Imai, S. I., Zhang, H., Fredrickson, J., Latorre-Esteves, E., Bitto, A., MacCoss, M. J., & Rabinovitch, P. S. (2022). Age-related disruption of the proteome and acetylome in mouse hearts is associated with loss of function and attenuated by elamipretide (SS-31) and nicotinamide mononucleotide (NMN) treatment. GeroScience, 44(3), 1621–1639. https://doi.org/10.1007/s11357-022-00564-w.
Age-related disruption of the proteome and acetylome in mouse hearts is associated with loss of function and attenuated by elamipretide (SS-31) and nicotinamide mononucleotide (NMN) treatment
We analyzed the impact of aging on protein abundance and acetylation in mouse hearts, and evaluated the ability of mitochondrial-targeted drugs elamipretide (SS-31) and nicotinamide mononucleotide (NMN) to reverse these changes. Both drugs modestly restored age-related alterations in protein abundance and acetylation, particularly affecting mitochondrial pathways like oxidative phosphorylation and the TCA cycle. These age-related changes were associated with diastolic and systolic function, highlighting key protein modifications essential for maintaining diastolic function in aged hearts.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9213586/.
Sabbah, H. N., Gupta, R. C., Kohli, S., Wang, M., Hachem, S., & Zhang, K. (2016). Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circulation. Heart failure, 9(2), e002206. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002206.
Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure
Elamipretide (MTP-131), a mitochondria-targeting peptide, was found to improve left ventricular (LV) systolic function, normalize plasma biomarkers, and reverse mitochondrial abnormalities in dogs with heart failure (HF) after three months of treatment. In a study with 14 dogs, those treated with elamipretide showed significant improvements in LV ejection fraction and reductions in n-terminal pro-brain natriuretic peptide, tumor necrosis factor-α, and C-reactive protein compared to controls. These findings support the potential of elamipretide for treating heart failure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4743543/.
Sabbah HN, Gupta RC, Singh-Gupta V, Zhang K. Effects of elamipretide on skeletal muscle in dogs with experimentally induced heart failure. ESC Heart Fail. 2019 Apr;6(2):328-335. doi: 10.1002/ehf2.12408. Epub 2019 Jan 28. PMID: 30688415; PMCID: PMC6437430.
Effects of elamipretide on skeletal muscle in dogs with experimentally induced heart failure
Elamipretide (ELAM) treatment in dogs with chronic heart failure (HF) improved skeletal muscle function by restoring near-normal muscle fiber composition and enhancing mitochondrial function. This included increased mitochondrial respiration, membrane potential, ATP synthesis, and cytochrome c oxidase activity. ELAM was effective in reversing HF-related mitochondrial dysfunction, suggesting its potential to restore skeletal muscle function and improve exercise tolerance in HF. Additionally, ATP synthesis was significantly boosted, contributing to the overall enhancement of muscle energy metabolism. This indicates that ELAM’s ability to improve ATP synthesis is a crucial factor in its therapeutic effects. Thus, ELAM’s role in promoting ATP synthesis highlights its promise as a treatment to mitigate the impacts of heart failure on skeletal muscle function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6437430/.
Allen, M. E., Pennington, E. R., Perry, J. B., Dadoo, S., Makrecka-Kuka, M., Dambrova, M., Moukdar, F., Patel, H. D., Han, X., Kidd, G. K., Benson, E. K., Raisch, T. B., Poelzing, S., Brown, D. A., & Shaikh, S. R. (2020). The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Communications biology, 3(1), 389. https://doi.org/10.1038/s42003-020-1101-3.
The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats
Mitochondrial dysfunction is a key factor in cardiac diseases, and elamipretide, a cardiolipin-binding peptide, shows promise in mitigating these effects. In a rat model of cardiac ischemia-reperfusion, elamipretide improved mitochondrial complex activity, preserved cristae ultrastructure, and enhanced the biophysical properties of mitochondrial membranes despite not protecting cardiolipin concentration. This indicates that elamipretide sustains mitochondrial structure and bioenergetic function, supporting its potential as a therapeutic agent for mitochondrial dysfunction-related cardiac pathologies.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7368046/.
Reid Thompson, W., Hornby, B., Manuel, R., Bradley, E., Laux, J., Carr, J., & Vernon, H. J. (2021). A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genetics in medicine : official journal of the American College of Medical Genetics, 23(3), 471–478. https://doi.org/10.1038/s41436-020-01006-8.
A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism
In a randomized, double-blind, placebo-controlled trial followed by an open-label extension, daily administration of the mitochondrial peptide elamipretide in Barth syndrome (BTHS) patients initially showed no significant improvements in primary endpoints. However, after 36 weeks, significant improvements were observed in the 6-minute walk test, BTHS Symptom Assessment scale, knee extensor strength, patient global impression of symptoms, and some cardiac parameters, indicating that elamipretide may effectively alleviate BTHS symptoms.Barth syndrome, a rare genetic disorder that affects mitochondrial function, presents unique challenges in treatment. In clinical trials involving Barth syndrome patients, Elamipretide has shown promise in improving various clinical outcomes despite initial results. The open-label extension of these trials demonstrated that prolonged administration of Elamipretide led to noticeable benefits for Barth syndrome patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7935714/.
Campbell, M. D., Duan, J., Samuelson, A. T., Gaffrey, M. J., Merrihew, G. E., Egertson, J. D., Wang, L., Bammler, T. K., Moore, R. J., White, C. C., Kavanagh, T. J., Voss, J. G., Szeto, H. H., Rabinovitch, P. S., MacCoss, M. J., Qian, W. J., & Marcinek, D. J. (2019). Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free radical biology & medicine, 134, 268–281. https://doi.org/10.1016/j.freeradbiomed.2018.12.031.
Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice
In a study on aged female mice, treatment with the mitochondrial-targeting peptide SS-31 (elamipretide) for eight weeks restored redox balance, improved mitochondrial function, and enhanced skeletal muscle performance. SS-31 reversed age-related declines in mitochondrial ATP production and oxidative phosphorylation efficiency, reduced oxidative stress, and increased muscle mass and endurance, despite no change in mitochondrial content. These findings suggest that SS-31, currently in clinical trials, could potentially improve exercise tolerance and quality of life in the elderly by addressing mitochondrial dysfunction and oxidative stress.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6588449/.
Jia YL, Sun SJ, Chen JH, Jia Q, Huo TT, Chu LF, Bai JT, Yu YJ, Yan XX, Wang JH. SS31, a Small Molecule Antioxidant Peptide, Attenuates β-Amyloid Elevation, Mitochondrial/Synaptic Deterioration and Cognitive Deficit in SAMP8 Mice. Curr Alzheimer Res. 2016;13(3):297-306. doi: 10.2174/1567205013666151218150004. PMID: 26679857.
SS31, a Small Molecule Antioxidant Peptide, Attenuates β-Amyloid Elevation, Mitochondrial/Synaptic Deterioration and Cognitive Deficit in SAMP8 Mice
SS-31, a small molecule antioxidant peptide, was found to reduce β-amyloid levels, protect against mitochondrial and synaptic damage, and improve cognitive function in SAMP8 mice, a model for aging and Alzheimer’s disease.
You can read the abstract of the article at https://www.eurekaselect.com/article/72617.
Wu J, Hao S, Sun XR, et al. Elamipretide (SS-31) Ameliorates Isoflurane-Induced Long-Term Impairments of Mitochondrial Morphogenesis and Cognition in Developing Rats. Front Cell Neurosci. 2017;11:119. Published 2017 Apr 25. doi:10.3389/fncel.2017.00119.
Elamipretide (SS-31) Ameliorates Isoflurane-Induced Long-Term Impairments of Mitochondrial Morphogenesis and Cognition in Developing Rats
This study demonstrates that elamipretide (SS-31), a mitochondrion-targeted antioxidant, protects against isoflurane-induced neurotoxicity in developing rat brains by reducing oxidative stress, preventing mitochondrial deformation, and attenuating cognitive deficits, suggesting its potential therapeutic benefits for pediatric patients undergoing general anesthesia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5403826/.
Wu, J., Zhang, M., Li, H., Sun, X., Hao, S., Ji, M., Yang, J., & Li, K. (2016). BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behavioural brain research, 305, 115–121. https://doi.org/10.1016/j.bbr.2016.02.036.
BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice
In aging mice, isoflurane-induced cognitive impairments are linked to mitochondrial dysfunction in the hippocampus, including reduced complex I activity, increased reactive oxygen species, decreased ATP production, and disrupted mitochondrial membrane potential. The mitochondrion-targeted peptide SS-31 reverses these dysfunctions, enhances BDNF signaling, regulates synaptic plasticity-related proteins, and rescues cognitive deficits, suggesting its potential therapeutic benefits for elderly patients undergoing anesthesia. By stabilizing the mitochondrial membrane potential, SS-31 helps to maintain mitochondrial function and prevent cognitive decline. Additionally, the improvement in mitochondrial membrane potential contributes to overall mitochondrial health and efficiency. Thus, SS-31’s ability to restore mitochondrial membrane potential is a crucial factor in its therapeutic efficacy.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0166432816301127?via%3Dihub.
Tarantini, S, Valcarcel‐Ares, NM, Yabluchanskiy, A, et al. Treatment with the mitochondrial‐targeted antioxidant peptide SS‐31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018; 17:e12731. https://doi.org/10.1111/acel.12731.
Treatment with the mitochondrial‐targeted antioxidant peptide SS‐31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice
This study found that age-related cognitive decline is linked to impaired neurovascular coupling due to increased mitochondrial oxidative stress and cerebromicrovascular endothelial dysfunction. Treatment with the mitochondria-targeted antioxidant peptide SS-31 in aged mice significantly improved neurovascular coupling, enhanced NO-mediated cerebromicrovascular dilation, and boosted spatial working memory, motor skills, and gait coordination, suggesting SS-31’s potential for preventing or treating age-related vascular cognitive impairment (VCI).
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5847870/.
Zhao, W., Xu, Z., Cao, J. et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation 16, 230 (2019). https://doi.org/10.1186/s12974-019-1627-9.
Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice
This study found that the mitochondrion-targeted antioxidant elamipretide (SS-31) can significantly mitigate LPS-induced memory impairment in mice by protecting against mitochondrial dysfunction, oxidative stress, and inflammation. Elamipretide also improved learning and memory performance, facilitated BDNF signaling, and enhanced synaptic structural complexity, suggesting its potential therapeutic benefits for preventing oxidative stress and neuroinflammation-related cognitive disorders, such as perioperative neurocognitive disorders (PND).
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6865061/.
Tarantini, S., Valcarcel-Ares, N. M., Yabluchanskiy, A., Fulop, G. A., Hertelendy, P., Gautam, T., Farkas, E., Perz, A., Rabinovitch, P. S., Sonntag, W. E., Csiszar, A., & Ungvari, Z. (2018). Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging cell, 17(2), e12731. https://doi.org/10.1111/acel.12731.
Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice
Elamipretide (SS-31), a mitochondrion-targeted antioxidant, was found to significantly reduce LPS-induced memory impairment in mice by protecting against mitochondrial dysfunction, oxidative stress, and inflammation. The treatment improved hippocampus-related learning and memory performance and facilitated BDNF signaling, enhancing synaptic structural complexity. These findings suggest that elamipretide may have therapeutic potential for preventing oxidative stress and neuroinflammation-related cognitive disorders, such as perioperative neurocognitive disorders (PND).
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6865061/.
Liu, Y., Fu, H., Wu, Y., Nie, B., Liu, F., Wang, T., Xiao, W., Yang, S., Kan, M., & Fan, L. (2021). Elamipretide (SS-31) Improves Functional Connectivity in Hippocampus and Other Related Regions Following Prolonged Neuroinflammation Induced by Lipopolysaccharide in Aged Rats. Frontiers in aging neuroscience, 13, 600484. https://doi.org/10.3389/fnagi.2021.600484.
Elamipretide (SS-31) Improves Functional Connectivity in Hippocampus and Other Related Regions Following Prolonged Neuroinflammation Induced by Lipopolysaccharide in Aged Rats
This study investigated the impact of hippocampal neuroinflammation on brain functional connectivity (FC) in aged rats, using lipopolysaccharide (LPS) to induce inflammation and elamipretide (SS-31) for treatment. LPS exposure resulted in impaired memory, increased oxidative stress, inflammatory cytokines, and astrocyte activation in the hippocampus, along with decreased FC in specific brain regions. Treatment with SS-31 reduced these inflammatory markers, improved memory performance, and increased FC in various brain areas. The findings suggest that early anti-inflammatory treatment with SS-31 can mitigate the effects of neuroinflammation and improve brain connectivity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7956963/.
Zuo, Y., Yin, L., Cheng, X., Li, J., Wu, H., Liu, X., Gu, E., & Wu, J. (2020). Elamipretide Attenuates Pyroptosis and Perioperative Neurocognitive Disorders in Aged Mice. Frontiers in cellular neuroscience, 14, 251. https://doi.org/10.3389/fncel.2020.00251.
Elamipretide Attenuates Pyroptosis and Perioperative Neurocognitive Disorders in Aged Mice
Pyroptosis, an inflammatory form of programmed cell death, contributes to perioperative neurocognitive disorders (PND). This study examined the effects of elamipretide (SS-31), a mitochondrial-targeted peptide, on pyroptosis in a PND mouse model induced by anesthesia and surgery. The findings revealed that elamipretide protected against mitochondrial dysfunction, reduced pyroptosis, and improved cognitive function by maintaining synaptic integrity and mitigating neuroinflammation. This suggests that elamipretide may offer a promising treatment strategy for PND involving mitochondrial dysfunction and pyroptosis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7439217/.
Czigler, A., Toth, L., Szarka, N., Berta, G., Amrein, K., Czeiter, E., Lendvai-Emmert, D., Bodo, K., Tarantini, S., Koller, A., Ungvari, Z., Buki, A., & Toth, P. (2019). Hypertension Exacerbates Cerebrovascular Oxidative Stress Induced by Mild Traumatic Brain Injury: Protective Effects of the Mitochondria-Targeted Antioxidative Peptide SS-31. Journal of neurotrauma, 36(23), 3309–3315. https://doi.org/10.1089/neu.2019.6439.
Hypertension Exacerbates Cerebrovascular Oxidative Stress Induced by Mild Traumatic Brain Injury: Protective Effects of the Mitochondria-Targeted Antioxidative Peptide SS-31
Traumatic brain injury (TBI) causes transient cerebrovascular oxidative stress, but in conditions like hypertension, it can lead to prolonged oxidative damage. In a study on hypertensive rats with mild TBI, increased long-term production of cytoplasmic and mitochondrial superoxide in cerebral arteries was observed, along with elevated NADPH oxidase subunit Nox4 expression. Treatment with the antioxidant peptide SS-31 reversed these oxidative effects and improved cerebrovascular function, suggesting that SS-31 may mitigate persistent oxidative stress and cognitive dysfunction associated with TBI and hypertension.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6857460/.
Yang, L., Zhao, K., Calingasan, N. Y., Luo, G., Szeto, H. H., & Beal, M. F. (2009). Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxidants & redox signaling, 11(9), 2095–2104. https://doi.org/10.1089/ars.2009.2445.
Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity.
Mitochondrial dysfunction and oxidative damage are implicated in Parkinson’s disease (PD) pathogenesis. This study evaluated two mitochondria-targeted peptides, SS-31 and SS-20, for their neuroprotective effects against MPTP-induced neurotoxicity in mice. SS-31 provided dose-dependent protection against dopamine loss and neuron damage in the brain, while SS-20 also showed significant neuroprotective effects despite lacking intrinsic antioxidant properties. Both peptides prevented MPP+-induced cell death, mitochondrial dysfunction, and swelling, indicating their potential as promising treatments for PD by targeting mitochondrial and oxidative damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819801/.
Reddy, P. H., Manczak, M., Yin, X., & Reddy, A. P. (2018). Synergistic Protective Effects of Mitochondrial Division Inhibitor 1 and Mitochondria-Targeted Small Peptide SS31 in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD, 62(4), 1549–1565. https://doi.org/10.3233/JAD-170988.
Synergistic Protective Effects of Mitochondrial Division Inhibitor 1 and Mitochondria-Targeted Small Peptide SS31 in Alzheimer’s Disease
This study investigated the combined protective effects of the mitochondria-targeted antioxidant SS31 and the mitochondrial division inhibitor Mdivi1 in Alzheimer’s disease (AD). Using biochemical methods, the researchers assessed mitochondrial function, cell survival, and apoptosis in mutant AβPP cells. They found that treatment with SS31, Mdivi1, and especially the combination of both significantly reduced amyloid-β levels, improved mitochondrial function, and increased cell survival and mitochondrial DNA copy number. The combined treatment was more effective than individual treatments, suggesting it as a superior therapeutic strategy for AD. This is the first study to explore this combined treatment in AD neurons.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5884714/.
Mo, Y., Deng, S., Zhang, L., Huang, Y., Li, W., Peng, Q., Liu, Z., & Ai, Y. (2019). SS-31 reduces inflammation and oxidative stress through the inhibition of Fis1 expression in lipopolysaccharide-stimulated microglia. Biochemical and biophysical research communications, 520(1), 171–178. https://doi.org/10.1016/j.bbrc.2019.09.077.
SS-31 reduces inflammation and oxidative stress through the inhibition of Fis1 expression in lipopolysaccharide-stimulated microglia
SS-31, a mitochondrion-targeted peptide, has shown significant neuroprotective effects. This study demonstrated that SS-31 protects murine microglial cells (BV-2) from lipopolysaccharide (LPS)-induced inflammation and oxidative stress by stabilizing mitochondrial morphology. SS-31 preserved mitochondrial structure by reducing the expression of the fission protein 1 (Fis1) and defended BV-2 cells against inflammation and oxidative stress through Fis1 suppression.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0006291X19318042?via%3Dihub.
Nhu NT, Xiao SY, Liu Y, Kumar VB, Cui ZY, Lee SD. Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration. Front Integr Neurosci. 2022 Jan 17;15:747901. doi: 10.3389/fnint.2021.747901. PMID: 35111001; PMCID: PMC8801496.
Neuroprotective Effects of a Small Mitochondrially-Targeted Tetrapeptide Elamipretide in Neurodegeneration
Elamipretide, a mitochondrially-targeted tetrapeptide, has shown potential in treating neurodegeneration by enhancing mitochondrial respiration and biogenesis, promoting mitochondrial fusion, and inhibiting mitochondrial fission. It also reduces oxidative stress, neuroinflammation, and toxic protein accumulation, thereby preventing neural apoptosis and supporting neural survival. These properties suggest that elamipretide could slow the progression of neurodegenerative diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8801496/.
Smuder AJ, Roberts BM, Wiggs MP, Kwon OS, Yoo JK, Christou DD, Fuller DD, Szeto HH, Judge AR. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness. Oncotarget. 2020 Sep 22;11(38):3502-3514. doi: 10.18632/oncotarget.27748. PMID: 33014286; PMCID: PMC7517961.
Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness
Cancer cachexia leads to significant cardiac and diaphragm muscle wasting due to mitochondrial dysfunction and increased ROS production, resulting in muscle weakness and fatigue. In a study with C26 adenocarcinoma tumor-bearing mice, treatment with the mitochondria-targeting peptide SS-31 reduced ROS production and mitochondrial uncoupling, preserved left ventricular function, maintained diaphragm muscle fiber size and force production, and improved ventilatory function. These findings suggest that targeting mitochondrial dysfunction with SS-31 can prevent cancer cachexia-induced cardiorespiratory dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7517961/.
Wang B, Fu J, Yu T, Xu A, Qin W, Yang Z, Chen Y, Wang H. Contradictory effects of mitochondria- and non-mitochondria-targeted antioxidants on hepatocarcinogenesis by altering DNA repair in mice. Hepatology. 2018 Feb;67(2):623-635. doi: 10.1002/hep.29518. Epub 2018 Jan 7. PMID: 28898446.
Contradictory effects of mitochondria- and non-mitochondria-targeted antioxidants on hepatocarcinogenesis by altering DNA repair in mice
The effects of antioxidant supplementation on cancer prevention or promotion vary and are influenced by the type of antioxidant and tumor. In mouse models of chemical hepatocarcinogenesis, non-mitochondrial-targeted antioxidants like N-acetylcysteine (NAC) and Trolox prevented tumorigenesis, while mitochondrial-targeted antioxidants SS-31 and Mito-Q facilitated it. NAC and Trolox activated DNA repair mechanisms, whereas SS-31 and Mito-Q inactivated them, aggravating DNA damage. The study suggests that the type of antioxidant significantly affects hepatocarcinogenesis, highlighting the need for careful selection of antioxidants in cancer therapy.
You can read the abstract of the article at https://journals.lww.com/hep/abstract/2018/02000/contradictory_effects_of_mitochondria__and.20.aspx.
Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, Keller A, Zhang H, Rabinovitch PS, Marcinek DJ, Bruce JE. Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):15363-15373. doi: 10.1073/pnas.2002250117. Epub 2020 Jun 17. PMID: 32554501; PMCID: PMC7334473.
Mitochondrial protein interaction landscape of SS-31
Mitochondrial dysfunction contributes to various diseases and aging, and therapies restoring mitochondrial health are promising. The synthetic tetrapeptide elamipretide (SS-31) improves mitochondrial function, primarily by interacting with cardiolipin in the inner mitochondrial membrane. Using chemical cross-linking and mass spectrometry, this study identifies SS-31 protein interactors, revealing two groups involved in ATP production and 2-oxoglutarate metabolism. The binding regions of SS-31 are often near cardiolipin-protein interaction sites, providing mechanistic insights into SS-31’s therapeutic effects on mitochondria.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7334473/.
Ma W, Zhu X, Ding X, et al. Protective effects of SS31 on t‑BHP induced oxidative damage in 661W cells. Mol Med Rep. 2015;12(4):5026-5034. doi:10.3892/mmr.2015.4055.
Protective effects of SS31 on t‑BHP induced oxidative damage in 661W cells
The study demonstrated that the mitochondria-targeted peptide SS31 protects 661W cells from t-BHP-induced mitochondrial dysfunction and apoptosis by improving cell viability, reducing oxidative stress markers (nitrotyrosine and 8-OHdG), decreasing mitochondrial ROS, increasing mitochondrial membrane potential, and preventing cytochrome c release from mitochondria to the cytoplasm. By maintaining cytochrome c within the mitochondria, SS31 helps to prevent the initiation of the apoptotic cascade, thereby enhancing cell survival. Furthermore, the peptide’s ability to regulate cytochrome c release underscores its potential in mitigating mitochondrial-related oxidative damage and apoptosis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4581771/.
Ballarò, R., Lopalco, P., Audrito, V., Beltrà, M., Pin, F., Angelini, R., Costelli, P., Corcelli, A., Bonetto, A., Szeto, H. H., O’Connell, T. M., & Penna, F. (2021). Targeting Mitochondria by SS-31 Ameliorates the Whole Body Energy Status in Cancer- and Chemotherapy-Induced Cachexia. Cancers, 13(4), 850. https://doi.org/10.3390/cancers13040850.
Targeting Mitochondria by SS-31 Ameliorates the Whole Body Energy Status in Cancer- and Chemotherapy-Induced Cachexia
The study found that the mitochondria-targeted compound SS-31 can counteract muscle wasting and metabolic alterations in cancer cachexia, including those exacerbated by chemotherapy, by improving mitochondrial function, increasing ATP levels, and preventing muscle weight loss and mitochondrial loss in C26-bearing mice. SS-31 also modulated energy and protein metabolism in skeletal muscle and liver, suggesting its potential role in multi-modal therapies for cancer cachexia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7923037/.
Beltrà, M., Pin, F., Ballarò, R., Costelli, P., & Penna, F. (2021). Mitochondrial Dysfunction in Cancer Cachexia: Impact on Muscle Health and Regeneration. Cells, 10(11), 3150. https://doi.org/10.3390/cells10113150.
Mitochondrial Dysfunction in Cancer Cachexia: Impact on Muscle Health and Regeneration
Cancer cachexia is a debilitating syndrome that significantly affects patients’ quality of life and treatment outcomes. The multisystemic nature of its pathogenesis, involving multiple organs beyond skeletal muscle, complicates effective treatment. Mitochondrial dysfunction in skeletal muscle is emerging as a key trigger of metabolic derangements leading to hypercatabolism and tissue wasting. Enhancing mitochondrial function may improve energy status, chemotherapy tolerance, and muscle regeneration. This review discusses mitochondrial dysfunction in cancer cachexia and explores the potential of stimulating mitochondrial biogenesis via PGC-1α overexpression to mitigate muscle wasting.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8621344/.
Available from https://www.sciencedirect.com/science/article/pii/S0753332220307149.
Wyss J-C, Kumar R, Mikulic J, Schneider M, Mary J-L, Aebi JD, Juillerat-Jeanneret L and Golshayan D (2019) Differential Effects of the Mitochondria-Active Tetrapeptide SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2) and Its Peptidase-Targeted Prodrugs in Experimental Acute Kidney Injury. Front. Pharmacol. 10:1209. doi: 10.3389/fphar.2019.01209.
Differential Effects of the Mitochondria-Active Tetrapeptide SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2) and Its Peptidase-Targeted Prodrugs in Experimental Acute Kidney Injury
The mitochondria-active tetrapeptide SS-31 can mitigate oxidative tissue damage in kidney diseases. In murine models of acute tubular injury and glomerular damage, SS-31 reduced acute kidney injury and regulated inflammatory and oxidative stress responses. SS-31 also inhibited aminopeptidase A (APA) activity, which is involved in the renin-angiotensin system (RAS), selectively downregulated AT1R mRNA expression, and increased AT2R expression, potentially limiting renal damage. This opens new therapeutic possibilities for SS-31 in treating kidney diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6857474/.
Available from https://journals.physiology.org/doi/full/10.1152/ajprenal.00395.2007.
Mizuguchi, Y., Chen, J., Seshan, S. V., Poppas, D. P., Szeto, H. H., & Felsen, D. (2008). A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. American journal of physiology. Renal physiology, 295(5), F1545–F1553. https://doi.org/10.1152/ajprenal.00395.2007.
A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. American journal of physiology
Unilateral ureteral obstruction (UUO) leads to renal dysfunction, fibrosis, apoptosis, and inflammation. The mitochondria-targeted peptide SS-31 was used in a 14-day UUO model and significantly reduced renal damage, oxidative stress, apoptosis, and fibrosis. SS-31 also modulated NF-κB and p38 MAPK signaling pathways. This study demonstrates that mitochondrial-protective peptides like SS-31 can mitigate renal damage in UUO, highlighting their therapeutic potential.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2584902/.
Saad, A., Herrmann, S., Eirin, A., Ferguson, C. M., Glockner, J. F., Bjarnason, H., McKusick, M. A., Misra, S., Lerman, L. O., & Textor, S. C. (2017). Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients With Atherosclerotic Renal Artery Stenosis. Circulation. Cardiovascular interventions, 10(9), e005487. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005487.
Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients With Atherosclerotic Renal Artery Stenosis
Elamipretide, a mitochondrial-targeted peptide, was tested in patients with atherosclerotic renal artery stenosis undergoing percutaneous transluminal renal angioplasty (PTRA). The study found that adjunctive elamipretide treatment reduced post-procedural hypoxia, increased renal blood flow (RBF), and improved kidney function compared to placebo. These results suggest that elamipretide can mitigate ischemia/reperfusion injury and enhance the outcomes of revascularization in these patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5659347/.
Miyamoto, S., Zhang, G., Hall, D., Oates, P. J., Maity, S., Madesh, M., Han, X., & Sharma, K. (2020). Restoring mitochondrial superoxide levels with elamipretide (MTP-131) protects db/db mice against progression of diabetic kidney disease. The Journal of biological chemistry, 295(21), 7249–7260. https://doi.org/10.1074/jbc.RA119.011110.
Restoring mitochondrial superoxide levels with elamipretide (MTP-131) protects db/db mice against progression of diabetic kidney disease
In a type 2 diabetes mouse model (db/db mice), diabetic kidney disease (DKD) is associated with reduced renal and cardiac superoxide levels, increased albuminuria, and mesangial matrix accumulation. Treatment with the mitochondrial protectant MTP-131 (elamipretide) restored renal superoxide production, reduced albuminuria and mesangial matrix accumulation, and preserved renal lysocardiolipin levels, suggesting its potential in ameliorating DKD by regulating cardiolipin remodeling.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7247302/.
Zhu, Y., Luo, M., Bai, X., Li, J., Nie, P., Li, B., & Luo, P. (2022). SS-31, a Mitochondria-Targeting Peptide, Ameliorates Kidney Disease. Oxidative medicine and cellular longevity, 2022, 1295509. https://doi.org/10.1155/2022/1295509.
SS-31, a Mitochondria-Targeting Peptide, Ameliorates Kidney Disease
Mitochondrial dysfunction plays a critical role in the progression of renal diseases, and recent developments in mitochondria-targeting drugs show promise in addressing this issue. Traditional mitochondrial drugs have limitations due to poor absorption and high toxicity. SS-31, a newer mitochondria-targeting antioxidant, has shown potential by reducing mitochondrial reactive oxygen species, preventing depolarization, and avoiding mitochondrial damage without affecting normal mitochondria. Although few studies have investigated SS-31’s effects on renal diseases, early results are promising, highlighting its potential for future preclinical and clinical applications in improving renal health.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9192202/.
Daneshgar, N., Liang, P. I., Lan, R. S., Horstmann, M. M., Pack, L., Bhardwaj, G., Penniman, C. M., O’Neill, B. T., & Dai, D. F. (2022). Elamipretide treatment during pregnancy ameliorates the progression of polycystic kidney disease in maternal and neonatal mice with PKD1 mutations. Kidney international, 101(5), 906–911. https://doi.org/10.1016/j.kint.2021.12.006.
Elamipretide treatment during pregnancy ameliorates the progression of polycystic kidney disease in maternal and neonatal mice with PKD1 mutations
Pregnancy may worsen cyst progression in autosomal dominant polycystic kidney disease (ADPKD), but the only FDA-approved drug, Tolvaptan, is not recommended for pregnant women due to potential fetal harm. Elamipretide, a mitochondrial-protective peptide, was tested for safety and efficacy in pregnant Pkd1RC/RC mice. It reduced kidney disease progression and improved mitochondrial function without teratogenic effects, passing safely through the placenta and breast milk. Given its excellent safety profile and ongoing phase II and III clinical trials, Elamipretide holds potential for treating ADPKD in patients who cannot use Tolvaptan.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9038630/.
Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, Feldman LC, Gustafsson T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD. J Am Soc Nephrol. 2017 May;28(5):1437-1449. doi: 10.1681/ASN.2016070761. Epub 2016 Nov 23. PMID: 27881606; PMCID: PMC5407729.
Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1β and IL-18 and Arrests CKD
Mitochondrial damage triggers chronic inflammasome activation in acute kidney injury (AKI) and chronic kidney disease (CKD). A 9-month study in rats revealed persistent inflammation and fibrosis following renal ischemia, marked by mitochondrial degeneration and increased expression of inflammatory markers. Treatment with the mitoprotective agent SS-31 (elamipretide), initiated one month post-ischemia, preserved mitochondrial integrity, reduced inflammation, and prevented fibrosis, with effects lasting over six months after treatment ended. These findings suggest mitochondrial protection as a potential therapeutic strategy to halt CKD progression, effective even when administered long after AKI.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5407729/.
Available from https://www.karger.com/Article/FullText/484919.
Zhu LL, Li MQ, He F, Zhou SB, Jiang W. Mitochondria Targeted Peptide Attenuates Mitochondrial Dysfunction, Controls Inflammation and Protects Against Spinal Cord Injury-Induced Lung Injury. Cell Physiol Biochem. 2017;44(1):388-400. doi: 10.1159/000484919. Epub 2017 Nov 13. PMID: 29132140.
Mitochondria Targeted Peptide Attenuates Mitochondrial Dysfunction, Controls Inflammation and Protects Against Spinal Cord Injury-Induced Lung Injury
Spinal cord injury (SCI) often leads to systemic inflammation and secondary lung injury, with mitochondrial dysfunction playing a significant role. In a mouse model, treatment with the mitochondrial-targeted peptide SS-31 immediately after SCI reduced lung tissue damage, apoptosis, inflammation, and mitochondrial dysfunction. SS-31 also decreased lung edema, macrophage and neutrophil infiltration, reactive oxygen species levels, and NLRP3 inflammasome activation, thereby alleviating the severity of lung injury.
You can read the full article at https://karger.com/cpb/article/44/1/388/153240/Mitochondria-Targeted-Peptide-Attenuates.
Available from http://www.lcgdbzz.org/en/article/doi/10.3969/j.issn.1001-5256.2022.02.025.
Influence of mitochondria-targeted antioxidant SS-31 on acute liver injury in a mouse model of sepsis
In a mouse model of sepsis-induced acute liver injury, treatment with the mitochondria-targeted antioxidant SS-31 significantly reduced serum levels of ALT, AST, ROS, TNFα, IL-1β, and IL-6, and alleviated histopathological damage such as hepatocyte swelling and inflammatory cell infiltration. These findings suggest that SS-31 can mitigate oxidative stress and inflammation, thereby protecting against sepsis-related acute liver injury.
You can read the abstract of the article at http://www.lcgdbzz.org/en/article/doi/10.3969/j.issn.1001-5256.2022.02.025.
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

