GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Benefits of CJC-1295
- What is CJC-1295?
- How CJC-1295 Works
- Chemical Structure of CJC-1295
- Research on CJC 1295 Benefits
- CJC-1295 and Human Growth Hormone
- CJC-1295 and the Benefits of Elevated Growth Hormone Production
- CJC 1295 Side Effects
- CJC 1295 Dosage
- CJC 1295 Price
- The Power of CJC 1295 and Ipamorelin Combination
- CJC 1295 Ipamorelin Before and After
- CJC 1295 Ipamorelin Dosage
- FAQ
- Blog
- Reference
Book a Free Consultation
Table of Contents
- Potential Benefits of CJC-1295
- What is CJC-1295?
- How CJC-1295 Works
- Chemical Structure of CJC-1295
- Research on CJC 1295 Benefits
- CJC-1295 and Human Growth Hormone
- CJC-1295 and the Benefits of Elevated Growth Hormone Production
- CJC 1295 Side Effects
- CJC 1295 Dosage
- CJC 1295 Price
- The Power of CJC 1295 and Ipamorelin Combination
- CJC 1295 Ipamorelin Before and After
- CJC 1295 Ipamorelin Dosage
- FAQ
- Blog
- Reference
Potential Benefits of CJC 1295
- Improves muscle mass and strength [1-38]
- Promotes weight loss [39-57]
- Improves sleep quality [58-84]
- Improves cognitive function [85-98]
- Maintains a healthy skeletal frame [99-142]
- Improves mood and energy levels [143-168]
- Improves sex drive and sexual function [169-206]
- Enhances tissue regeneration [207-233]
- Strengthens the immune system [234-260]
- Improves women’s fertility [263]
What is CJC 1295?
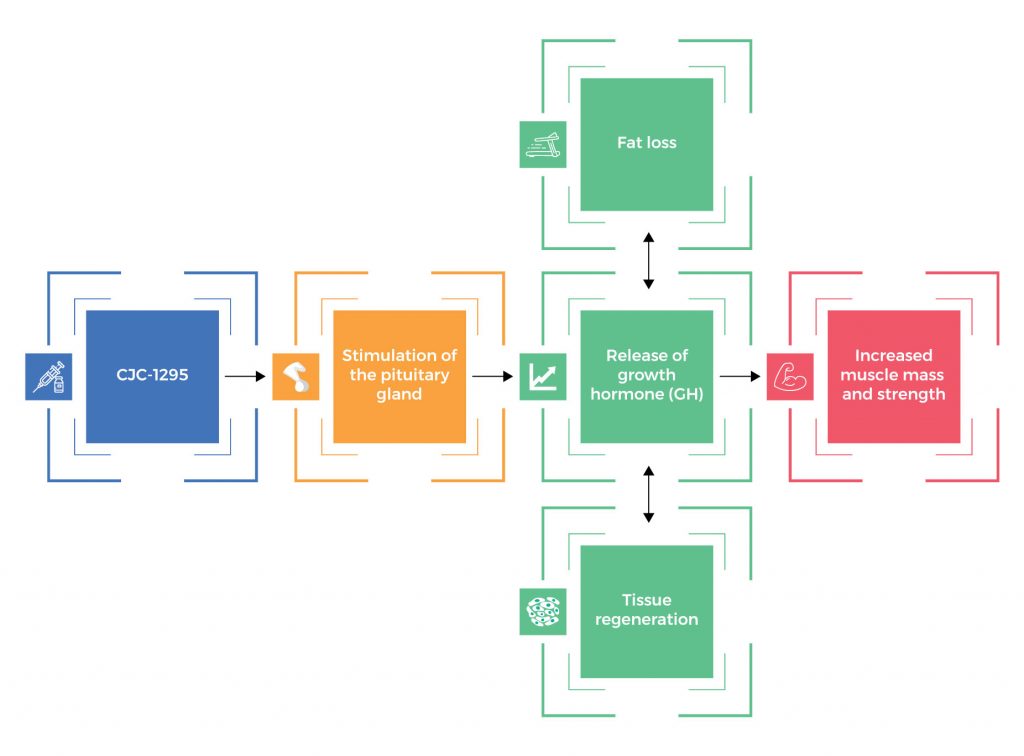
CJC-1295 is also known as drug affinity complex: growth hormone-releasing factor (DAC: GRF) or CJC 1295 DAC. It’s a synthetic analogue of growth hormone-releasing hormone (GHRH) and is primarily used to boost blood levels of human growth hormone by up to ten times its normal capacity. CJC-1295 can boost serum growth hormone levels by 200-1000% and the elevated growth hormone production can continue for up to 6 days.
Because CJC-1295 has a similar structure to GHRH, it has the ability to stimulate the pituitary gland to release growth hormone as well as other anabolic hormones such as insulin-like growth factor 1 or insulin growth factor 1. For this reason, many bodybuilders and athletes use CJC-1295 in order to improve muscle mass and strength, as well as performance. Originally, CJC-1295 and other synthetic peptides (compounds consisting of two or more amino acids linked in a chain) were commonly prescribed by medical professionals to treat patients suffering from muscle wasting, growth disorders, and burn injuries. Today, CJC-1295 is still widely prescribed in the treatment of these disorders because of its minimal side effects.
In general, patients who want to reap the potential health benefits of CJC-1295 may need 2 to 3 times daily injections (morning, before sleep, and after a workout). This method of administration is meant to mimic the actual secretion of growth hormones. Additionally, CJC-1295 has a long half-life. A longer half-life means its effects are not affected by food timing like other peptides.
How CJC 1295 Works
CJC-1295 stimulates the pituitary gland to release growth hormone (GH). In order to ensure balance (homeostasis), the human body still releases growth hormone in pulses. With increased GH levels, it leads to increased muscle mass and strength, fat loss, tissue regeneration, and other health benefits.
Chemical Structure of CJC 1295
Research on CJC 1295 Benefits
A. Improves Muscle Mass and Strength

CJC-1295 is a peptide known for its ability to increase muscle mass and strength by stimulating the release of growth hormone (GH) from the pituitary gland. This synthetic peptide enhances the pulsatile release of GH, which in turn promotes protein synthesis, muscle growth, and repair, making it a valuable tool for building muscle. Increased GH levels can lead to more efficient utilization of nutrients and improved muscle recovery, ultimately contributing to more muscle mass and enhanced muscle strength over time. Interestingly, an increase in muscle mass boosts allows the body’s resting metabolic rate to be boosted leading to more calories burned.
Studies show that this powerful peptide can boost the production of muscle cells:
- In normal adult subjects, CJC-1295 administration enhanced muscle mass and strength by increasing protein synthesis. [1]
- In healthy adult participants, subcutaneous administration of CJC-1295 safely and effectively increased plasma GH concentrations and IGF-1 levels, resulting in an increase in muscle mass and strength.[2]
- By increasing GH and IGF-1 levels, CJC-1295 significantly improved muscle mass and strength in both men and women subjects. [3-36]
- In mice, treatment with once-daily administration of CJC-1295 maintained normal body composition as well as muscle mass. [37-38]
B. Promotes Weight Loss

CJC-1295 promotes weight loss by stimulating the release of growth hormone from the pituitary gland, which in turn enhances the body’s ability to metabolize fat and build lean muscle tissue. This increase in growth hormone levels can lead to a higher metabolism, improved fat oxidation, and reduced body fat storage, ultimately contributing to weight loss and body composition improvements when combined with a healthy diet and exercise regimen.
Studies show that CJC-1295 can promote weight loss by burning more body fat and increasing lean muscle mass:
- In obese patients, CJC-1295 promoted fat loss by increasing the levels of growth hormone. [39-42]
- In healthy adults, subcutaneous administration of CJC-1295 safely and effectively increased GH and IGF-1 levels, resulting in a significant reduction in body fat percentage and body mass index (BMI). [43]
- In women, the use of synthetic growth hormone CJC-1295 reduced body weight. [44]
- In both men and women, CJC-1295 reduced body weight by boosting growth hormone levels. [45-56]
- In mice, treatment with once-daily administration of CJC-1295 improved body composition by preventing fat accumulation. [57]
C. Improves Sleep Quality

CJC-1295 can potentially improve sleep quality by stimulating the release of growth hormone (GH) during deep sleep stages. This peptide increases GH secretion, which is essential for restorative sleep, tissue repair, and overall sleep quality, offering a tremendous benefit. Improved sleep patterns and deeper sleep can contribute to a more refreshing and restful night’s sleep when CJC-1295 is used as part of a well-balanced peptide therapy under medical supervision.
Studies show that this powerful peptide can help you get a restful sleep:
- A study reported that CJC-1295 can improve sleep quality and quantity by promoting slow wave sleep, also known as the deep sleep stage. [58]
- In mice, once-daily administration of CJC-1295 normalized the GHRH response, which in turn induced significantly deeper sleep. [59]
- Studies found that by increasing GH levels, CJC-1295 can significantly improve the deep sleep stage. [60-65]
- In patients with insomnia, the administration of delta sleep-inducing peptides such as CJC-1295 was associated with higher sleep efficiency and shorter sleep latency (amount of time it takes to fall asleep). [66]
- In men, the administration of GHRH such as CJC-1295 promoted deep sleep by enhancing stage 2 sleep. [67]
- In normal men, the administration of GHRH such as CJC-1295 was associated with a 10-fold increase in slow wave sleep. [68]
- As a growth hormone-releasing factor, CJC-1295 induces sleep by activating sleep regulatory neurons in the brain. [69-70]
- In patients with schizophrenia, a chronic and severe mental disorder that alters cognition, emotion, and behavior, the administration of GHRH such as CJC-1295 improved sleep efficiency. [71]
- Studies showed that CJC-1295 can increase non-rapid eye movement (NREM) sleep and enhance EEG slow-wave activity (SWA). [72-83]
- In rats, the injection of GHRH such as CJC-1295 effectively promoted sleep. [84]
D. Improves Cognitive Function

CJC-1295, a very effective peptide and a growth hormone-releasing hormone (GHRH) analog, is believed to potentially treat diseases and enhance cognition and memory function by promoting the release of growth hormone in the body. Growth hormone plays a role in neurogenesis (the formation of new neurons), neuronal survival, and synaptic plasticity, all of which are essential for optimal brain function. By increasing growth hormone levels, CJC-1295 may support improved cognitive processes such as memory, learning, feeling mental clarity, and overall brain health, leading to a significant difference in the improved overall benefit for individuals seeking enhanced cognitive function.
There is increasing scientific evidence that GHRH like CJC-1295 can help combat cognitive impairment associated with advancing age and other medical conditions:
- In adults with mild cognitive impairment and healthy older adults, GHRH treatment significantly improved verbal memory. [85]
- In healthy older adults, GHRH supplementation ameliorated cognitive impairment associated with aging and Alzheimer’s disease. [86]
- In adults with mild cognitive impairment, GHRH supplementation improved cognitive function by boosting the levels of brain chemicals such as Gamma-Aminobutyric acid (GABA) and N-acetylaspartylglutamate (NAAG). [87]
- In older adults, GHRH supplementation improved various aspects of cognitive function. [88]
- In adults with mild cognitive impairment, GHRH supplementation slowed cognitive decline. [89-90]
- Studies reported that CJC-1295 improves cognitive function through its neuroprotective effects, allowing it to prevent damage to nerve cells in the brain. [91-92]
- In adults who are at increased risk of cognitive decline and dementia, GHRH administration had favorable effects on cognitive function. [93]
- In rats with traumatic brain injury, GHRH administration appears to improve cognitive function. [94-98]
E. Maintains a Healthy Skeletal Frame
Strong scientific evidence suggests that CJC-1295 plays an integral part in the maintenance of bone health and the prevention of bone disorders, such as osteoporosis and fractures, by stimulating the production of growth hormone, which in turn can enhance increased bone density and promote bone remodeling processes:
- In men with HIV-related fat accumulation, GHRH administration improved bone metabolism and bone density, suggesting that CJC-1295 can correct various bone disorders. [99]
- In elderly postmenopausal women with decreased bone mass, treatment with GHRH stimulated bone metabolism. [100]
- In healthy elderly women, GHRH administration prevented bone breakdown by increasing the blood levels of bone formation markers. [101]
- In patients with primary and secondary osteoporosis, GHRH administration increased bone formation and activity of bone cells (osteoblasts). [102]
- In women with postmenopausal osteoporosis, GHRH administration accelerated bone metabolism and activity of osteoblasts. [103-113]
- In men with osteoporosis of unknown cause, boosting GH levels through GHRH administration significantly improved bone mineral density (BMD). [114-115]
- In GH-deficient adults with osteoporosis, GHRH administration increased BMD and markers of bone formation and decreased markers of bone breakdown. [116-118]
- In patients with fractures, GHRH administration accelerated fracture healing. [119]
- In mice, CJC-1295 administration helped normalize bone growth in the legs. [120]
- Studies found that by increasing GH levels, CJC-1295 can significantly lower one’s risk of osteoporosis, fractures, and other bone disorders. [121-142]
F. Improves Mood and Energy Levels

Because of CJC-1295’s ability to boost GH levels by up to ten times its normal capacity, it can also help improve mood and energy levels in people suffering from depression, anxiety, and other mood disorders. There is compelling evidence supporting the beneficial effects of CJC-1295 on mood:
- Several studies showed that low blood levels of growth hormone were strongly linked with depression, anxiety, and other mood disorders, suggesting that the administration of GHRH such as CJC-1295 may help improve mood and energy levels by boosting GH production in the body. [143-156]
- In adults with mild cognitive impairment and healthy older adults, GHRH administration had favorable effects on cognition and mood. [157]
- In patients with major depression, GHRH administration increased GH levels, resulting in a reduction of depressive symptoms. [158]
- In patients with major depression, GHRH administration improved energy levels by decreasing sleepiness. [159]
- In patients with depression, GHRH administration significantly improved mood and energy levels by increasing the levels of neurotransmitters (brain chemicals) such as noradrenaline, dopamine, and acetylcholine. [160-168]
G. Improves Sex Drive and Sexual Function

Because CJC-1295 has a similar structure to GHRH, it can stimulate the pituitary gland to release various hormones that are involved in the regulation of sex drive and the ability to function properly sexually. Studies show that by boosting the levels of anabolic hormones such as growth hormone and insulin-like growth factor 1 (IGF-1), CJC 1295 may help ramp up sexual power and fight sexual dysfunction:
- Studies showed that growth hormone deficiency was strongly linked with low libido and erectile dysfunction, suggesting that increasing GH levels through CJC-1295 supplementation may have beneficial effects on sex drive and sexual function. [169-176]
- Studies also showed that IGF-1 deficiency was strongly linked with low libido and erectile dysfunction, suggesting that CJC-1295 supplementation may help improve sexual function. [177-183]
- In age-advanced men, GHRH supplementation significantly improved libido and general well-being. [184]
- Studies have shown that by increasing GH and IGF-1 levels, CJC-1295 also increases the levels of nitric oxide, a molecule naturally produced in the body which plays an integral part in penile erection. [185-193]
- Studies found that CJC-1295 administration improved sex drive and sexual function by increasing GH levels, which in turn boosts the levels of the primary male sex hormone testosterone and the primary female sex hormone estrogen. [194-195]
- Studies showed that peptides such as CJC-1295 were involved in the erection process and sexual arousal. [196-205]
- In animal models, GHRH administration increased testicular responsiveness and receptors. [206]
H. Enhances Tissue Regeneration
CJC-1295 can indirectly improve tissue regeneration of almost all body systems. Studies show that by stimulating the pituitary gland to release two vital hormones, GH and IGF-1, CJC-1295 may accelerate tissue repair after an injury:
- In the human tendon and skeletal muscle, GH repaired the damaged tissues by stimulating collagen synthesis. [207]
- A study reported that IGF-1 has a potential role in enhancing stem cell repair after a kidney injury. [208]
- A number of studies found that both GH and IGF-1 may play a role in brain repair after an injury. [209-218]
- Studies also showed that both GH and IGF-1 may help accelerate tissue repair by increasing collagen synthesis. [219-220]
- As a GHRH, CJC-1295 has been found to accelerate the wound healing process by promoting the formation of a protective covering of damaged skin tissues. [221-223]
- Recent evidence showed that CJC-1295 and other GHRHs can induce heart repair after myocardial infarction by mechanisms involving a direct action on the heart cells (cardiomyocytes). [224-225]
- A cell study found that GHRH promoted tissue regeneration by stimulating the proliferation of the cells of the pancreas while preventing programmed cell death (apoptosis). [226]
- Numerous studies found that GHRH administration accelerated the repair of tissue injury by increasing the number of cells needed for regeneration at the site of injury, suggesting that it may be a potential treatment for acute musculoskeletal injuries. [227-232]
- GHRH administration can also help improve the healing of debilitating injuries such as Achilles tendon rupture. [233]
I. Strengthens the Immune System
CJC-1295 can potentially contribute to a strong immune system by stimulating the production and release of growth hormone, which plays a vital role in supporting immune function. As a GHRH, CJC-1295 also possesses immune-modulating properties necessary for a strong immune system:
- In older men and women with compromised immune function, GHRH administration had profound immune-enhancing effects as evidenced by an increase in B and T cells of the immune system. [234]
- In animal models, GHRH injection increased the numbers of CD2, T-cells, and CD4 cells of the immune system. [235-237]
- Evidence suggests that GHRHs such as CJC-1295 strengthen the immune system by regulating the activities of natural killer cells and T cells. [238-241]
- In animal models, GHRH administration for 6 months increased the production of antibodies. [242]
- By increasing GH levels, CJC-1295 improved various aspects of the immune function in both human and animal subjects. [243-246]
- By boosting the levels of IGF-1, CJC-1295 also helps regulate immune function. [247-256]
- GHRH administration can also help fight inflammatory diseases by significantly reducing the levels of inflammatory substances in the body. [257-259]
- GHRH also has the ability to prevent the progression of tumors. [260]
J. Improves Women’s Fertility
There are also studies linking insulin-like growth factor-1 (IGF-1) and growth hormone to improved fertility in women:
- Strong scientific evidence suggests that insulin-like growth factor-1 (IGF-1) and growth hormone can improve fertility by regulating the ovarian follicular cycle. [261-262]
- A cell study investigated the effects of growth hormone releasing factor (GRF) on the ovulation cycle and found that cells treated with GRF had a significant increase in both follicular fluid IGF-1 levels and blood levels of GH, which in turn induced superovulation. [263]
CJC-1295 and Human Growth Hormone
CJC-1295 is a synthetic peptide that has garnered attention in the world of health and fitness due to its potential effects on growth hormone (GH) secretion. It is designed to mimic the action of the natural hormone growth hormone-releasing hormone (GHRH). GHRH stimulates the pituitary gland to produce growth hormone and release it into the bloodstream. CJC-1295, when administered, extends the half-life of GHRH in the body, making it more effective at stimulating the secretion of GH.
CJC-1295 and the Benefits of Elevated Growth Hormone Production
CJC-1295 is a synthetic peptide that mimics the action of GHRH, which stimulates the pituitary gland to produce growth hormone. When used responsibly and under medical supervision, CJC-1295 peptide therapy can offer several potential benefits associated with elevated GH production:
- Muscle growth and repair
- Fat loss
- Enhanced bone density
- Anti-aging effects (e.g. improved skin elasticity)
- Improved sleep and recovery
- Increased energy levels
- Enhanced immune function
- Connective tissue repair
- Mood and cognitive function
CJC 1295 Side Effects
CJC-1295 side effects are very uncommon and generally mild. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on CJC-1295. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of CJC-1295. Despite this, it was listed as a side effect associated with CJC-1295 even though these associated side effects are very uncommon.
Side effects associated with CJC-1295 may include the following:
- Difficulty swallowing
- Dizziness
- Drowsiness
- Flu-like symptoms
- Flushing of the skin
- Headache
- Hives or rashes
- Hyperactivity
- Injection site reactions (irritation, redness, pain, or itching)
- Nausea
CJC 1295 Dosage
The dosage of CJC-1295 can vary depending on the individual’s needs and goals. However, a typical starting dose for adults is 100-200 mcg (micrograms) administered via subcutaneous injection (just beneath the skin) once daily. The dosage can be adjusted up or down as needed.
CJC 1295 Price
The price of CJC-1295, a synthetic peptide used in the field of research and development, can vary depending on several factors such as the supplier, the quantity you’re purchasing, the purity of the product, and your location. Additionally, prices can change over time due to market fluctuations and changes in supply and demand.
The Power of CJC 1295 and Ipamorelin Combination
CJC-1295 and Ipamorelin are typically combined in peptide therapy due to their known compatibility and synergy. When combined, CJC 1295 and Ipamorelin can create a synergistic effect, amplifying their respective abilities to stimulate increased growth hormone secretion. This synergy is achieved by addressing two critical aspects of GH release:
- Increased Pulse Frequency: CJC 1295’s rapid onset extends the duration of GH-releasing hormone activity in the body, leading to more frequent GH pulses. These frequent pulses mimic the body’s natural GH release pattern during youth.
- Amplified GH Release: Ipamorelin, on the other hand, enhances the magnitude of GH release. By acting on pituitary receptors, it promotes a robust surge of GH, contributing to the overall elevations in GH levels.
CJC-1295 takes anywhere from 1-4 hours to reach peak serum levels in the blood, while Ipamorelin works much quicker. Ipamorelin is cleared from the body more rapidly with a half-life of about 2 hours. The combination of both peptides ensures a rapid onset with Ipamorelin and lasting effects with CJC-1295. Because of this, CJC 1295 and Ipamorelin are often incorporated into hormone replacement therapy regimens to stimulate natural growth hormone production and counter the effects of hormonal imbalances.
CJC 1295 Ipamorelin Before and After
CJC 1295-Ipamorelin combination is used for various purposes, including increasing muscle mass, fat loss, and anti-aging effects. In addition, CJC 1295 and Ipamorelin are commonly included in hormone replacement therapy regimens to optimize hormone levels and promote overall well-being. Moreover, ipamorelin improves insulin sensitivity, thus reducing high blood sugars in diabetic patients and the body’s triglycerides. However, it’s important to note that the effects of these peptides can vary from person to person, and results may not be as dramatic as with other substances like anabolic steroids. Additionally, the use of CJC 1295-Ipamorelin combination should be conducted under the guidance of a qualified healthcare professional and in compliance with all relevant laws and regulations.
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
CJC 1295 Ipamorelin Dosage
CJC 1295-Ipamorelin dosage can vary depending on the individual’s needs and goals. However, a typical starting dose for adults is 100-200 mcg (micrograms) of each peptide subcutaneously (under the skin) once daily. The dosage can be adjusted up or down as needed.
CJC-1295 is a long-acting growth hormone secretagogue, which means that it stimulates the release of growth hormone from the pituitary gland. Ipamorelin is a ghrelin receptor agonist, which means that it mimics the effect of the hormone ghrelin, which is responsible for stimulating appetite and growth hormone release.
CJC 1295-Ipamorelin combination has been shown to be more effective at increasing growth hormone levels than either peptide alone. It is also thought to be safer than other growth hormone-releasing peptides, such as GHRP-6 and GHRP-2, which can have side effects such as headaches and nausea.
Here are some additional things to keep in mind about CJC 1295-Ipamorelin dosage:
- The dosage may need to be adjusted based on the individual’s response.
- It is important to inject CJC-1295 and Ipamorelin at the same time each day.
- CJC 1295-Ipamorelin combination should not be used or taken together with other growth hormone secretagogues or growth hormone supplements.
- CJC 1295-Ipamorelin combination should not be taken by pregnant or breastfeeding women.
If you are considering taking CJC 1295-Ipamorelin to attain optimal health, it is important to do your research and talk to a doctor to make sure it is right for you.
FAQ
How many times a week should I take CJC-1295?
Most people take CJC-1295 once a day.
How long does it take for CJC-1295 to work?
It can take 2-4 weeks to see results from CJC-1295.
What are the long term benefits of CJC-1295?
It can help increase muscle mass, strength, and bone density. It can also help improve skin health, improve sleep quality, enhance insulin sensitivity, increase energy levels, and reduce wrinkles.
How long after injecting CJC-1295 can you eat?
You can eat immediately after injecting CJC-1295.
How long can you stay on CJC-1295 Ipamorelin?
It is generally recommended to stay on CJC-1295 Ipamorelin for 12-24 weeks.
Does CJC-1295 increase muscle mass?
Yes, CJC-1295 can help increase muscle mass.
Does CJC-1295 make you hungry?
CJC-1295 can make you feel hungry, but this is not a universal side effect.
Does CJC-1295 increase collagen?
Yes, CJC-1295 can help increase collagen production.
Does CJC-1295 burn fat?
CJC-1295 can help burn fat, but this is not its primary function.
How long does CJC-1295 stay in your system?
CJC-1295 has a half-life of 36 hours, meaning it takes 36 hours for half of the dose to be eliminated from the body.
Does CJC-1295 really work?
Yes, CJC-1295 has been shown to be effective in increasing muscle mass, strength, and bone density.
How much CJC-1295 should I take a day?
The dosage of CJC-1295 depends on the individual and their goals. A typical dosage is 1-2 mg per day.
What does CJC-1295 feel like?
Most people do not feel any effects from CJC-1295.
Does CJC-1295 help with sleep?
CJC-1295 can help improve sleep quality.
How long does CJC-1295 last?
CJC-1295 can last for up to 36 hours.
Can you take CJC-1295 on an empty stomach?
It is best to take CJC-1295 on an empty stomach.
What is the best combination with CJC 1295?
The best combination with CJC-1295 is Ipamorelin.
Why take CJC-1295 at night?
CJC-1295 can help improve sleep quality, so it is often taken at night.
How long does it take to see results from CJC-1295 Ipamorelin?
It can take 2-4 weeks to see results from CJC-1295 Ipamorelin.
Is CJC-1295 natural?
CJC-1295 is not a natural substance. It is a synthetic peptide that is designed to mimic the effects of growth hormone.
Does CJC-1295 increase cortisol?
CJC-1295 does not increase cortisol levels.
Does CJC 1295 affect testosterone?
CJC-1295 typically does not directly affect testosterone levels as its primary action is related to stimulating the release of growth hormone, but it may have indirect effects on testosterone through its influence on hormonal balance.
Reference
Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2009;19(6):471-477. doi:10.1016/j.ghir.2009.03.001.
Activation of the GH/IGF-1 axis by CJC-1295, a long acting GHRH analog, results in serum protein profile changes in normal adult subjects
The objective of this study was to discover serum biomarkers for growth hormone (GH) and insulin-like growth factor 1 (IGF-1) activity. Current biomarkers for GH function have limitations, and this research aimed to find more reliable indicators. They analyzed serum samples from 11 healthy young men before and after CJC-1295 injection using proteomics. They identified several proteins that changed significantly after treatment, including apolipoprotein A1, transthyretin, beta-hemoglobin, and albumin fragments. One of these proteins, a mix of immunoglobulin and albumin fragments, showed a linear relationship with IGF-1 levels, suggesting it could be a potential biomarker for GH and IGF-1 activity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2787983/.
Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91(3):799-805.
Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults
In this study, the aim was to assess the pharmacokinetics, pharmacodynamic effects, and safety of CJC-1295, a long-acting analog of growth hormone-releasing hormone (GHRH). The study consisted of two randomized, double-blind, placebo-controlled trials with varying durations. Healthy participants aged 21-61 received CJC-1295 or a placebo via subcutaneous injection. Results showed dose-dependent increases in GH and IGF-I levels lasting for several days, with an estimated half-life of 5.8-8.1 days. Multiple doses also demonstrated a cumulative effect. Importantly, the treatment was well-tolerated and safe, especially at certain doses (30 or 60 microg/kg), suggesting the potential therapeutic value of CJC-1295.
You can read the full article at https://academic.oup.com/jcem/article/91/3/799/2843281?login=true.
Tavares ABW, Micmacher E, Biesek S, et al. Effects of Growth Hormone Administration on Muscle Strength in Men over 50 Years Old. International Journal of Endocrinology. 2013;2013:942030. doi:10.1155/2013/942030.
Effects of Growth Hormone Administration on Muscle Strength in Men over 50 Years Old
The potential use of growth hormone (GH) to enhance physical capacity in individuals without GH deficiency has been linked to improved collagen synthesis in tendons and muscles, leading to better exercise training and increased strength. This study aimed to assess the impact of GH therapy on muscle strength in healthy men aged 50-70. Fourteen participants were divided into GH therapy (7) and placebo (7) groups, evaluated initially and after 6 months. Muscle strength increased significantly in leg press-responsive muscles of the GH group, while bench press-responsive muscles showed no change in either group. This highlights the potential for GH therapy to boost lower body strength in healthy men, suggesting implications for elderly populations dealing with muscle weakness. (Trial registered under NCT01853566.)
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3870652/.
Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79(5):1361-6.
Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men
The aging process involves unfavorable shifts in body composition, muscle strength, and somatotropic function. Declines in muscle strength contribute to frailty and susceptibility to fractures in older adults. While resistance exercise training initially boosts muscle strength, the improvements taper off, despite ongoing training. To explore whether age-related declines in the somatotropic axis hinder muscle strength gains from resistance training, a double-blind, placebo-controlled trial was conducted. Healthy elderly men (65-82 years) underwent 14 weeks of weight training, followed by 10 weeks of strength training with either recombinant human growth hormone (rhGH) or placebo. Both groups experienced strength gains (P = 0.0001) during the first 14 weeks, with marginal progress afterward. Muscle strength increased by 24-62% across muscle groups. Baseline insulin-like growth factor-I (IGF-I) levels were similar between groups. In the rhGH group, IGF-I levels rose significantly by week 15 and 24, with slight changes in the placebo group. Despite these changes, rhGH did not impact muscle strength, disproving its ergogenic potential. Lean mass increased and fat mass decreased with rhGH, but body weight remained unchanged. Overall, rhGH supplementation did not enhance strength training outcomes in elderly men, indicating that GH deficits don’t explain the leveling off of muscle strength with age and training.
You can read the abstract of this article at https://academic.oup.com/jcem/article-abstract/79/5/1361/2649268?redirectedFrom=fulltext&login=true.
Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and Growth Hormone Improve Body Composition and Muscle Performance in Older Men. The Journal of Clinical Endocrinology and Metabolism. 2009;94(6):1991-2001. doi:10.1210/jc.2008-2338.
Testosterone and Growth Hormone Improve Body Composition and Muscle Performance in Older Men
In this study’s context, age-related pituitary-gonadal axis decline is linked to muscle loss, function reduction, and upper body fat accumulation. The objective was to examine if combined testosterone and GH supplementation could enhance body composition and muscle performance in older men. A total of 122 men (70.8 +/- 4.2 years) with low testosterone and IGF-I levels were randomized to receive testosterone (5 or 10 g/d) and GH (0, 3, or 5 microg/kg . d) over 16 weeks. Results indicated increased lean mass, reduced fat mass, and improved muscle strength and endurance across treatment groups, with notable benefits in higher dose groups. Blood pressure showed slight increases, and other adverse effects were manageable. These findings suggest that testosterone supplementation, coupled with GH, led to substantial gains in lean mass, muscle strength, and endurance, potentially boosted further by GH.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2690426/.
Rennie M. Claims for the anabolic effects of growth hormone: a case of the Emperor’s new clothes? British Journal of Sports Medicine. 2003;37(2):100-105. doi:10.1136/bjsm.37.2.100.
Claims for the anabolic effects of growth hormone: a case of the Emperor’s new clothes
This review investigates the impact of growth hormone on the metabolism of adult humans. It finds that growth hormone significantly influences fat and carbohydrate metabolism, particularly promoting the use of stored fat. However, there’s insufficient evidence to support increased protein retention in adults, except potentially in connective tissue. The exaggerated claims of growth hormone’s muscle-building effects are driving its misuse, risking the health of athletes and elderly men without substantial benefits.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724606/.
Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology. 2008;154(3):557-568. doi:10.1038/bjp.2008.153.
Regulation of muscle mass by growth hormone and IGF-I
Growth hormone (GH) is commonly used as a performance-enhancing substance, mainly known for elevating insulin-like growth factor I (IGF-I) levels, primarily produced in the liver but also induced in various non-hepatic tissues. While GH plays a crucial role in postnatal body growth through IGF-I, it’s now being recognized for IGF-I-independent functions. Supraphysiological GH or IGF-I levels in skeletal muscle of healthy individuals lack substantial evidence for an anabolic role, though other performance-enhancing GH effects may exist. Conversely, muscle-specific IGF-I infusion shows clear hypertrophic effects in animal models and cell cultures. The systemic and local effects of GH and IGF-I may distinctly influence muscle mass regulation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2439518/.
Adams GR. Insulin-like growth factor in muscle growth and its potential abuse by athletes. Western Journal of Medicine. 2001;175(1):7-9.
Welle S, Thornton C, Statt M, Mchenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81(9):3239-43.
Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy sugbjects over 60 years old
In individuals over 60 years old, the synthesis rate of myofibrillar proteins in muscle is slower compared to young adults, potentially due to reduced activity in the GH/insulin-like growth factor-I system. To investigate if GH could rejuvenate myofibrillar protein synthesis, a study was conducted with healthy subjects over 60 years old. They received a single injection of recombinant human GH or a placebo, and another group underwent three months of GH or placebo treatment. While GH reduced whole-body leucine oxidation, it had no acute or long-term effect on myofibrillar protein synthesis in the quadriceps. However, GH treatment for three months did increase lean body mass, muscle mass, and thigh strength in these individuals, indicating its ability to enhance muscle mass and strength but not restore a youthful myofibrillar protein synthesis rate.
You can read the abstract of the article at https://academic.oup.com/jcem/article/81/9/3239/2651047?login=false.
Harman SM, Blackman MR. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm Res. 2003;60(Suppl 1):121-4.
The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men
In a 26-week study involving healthy older women and men aged 65 to 88, the effects of recombinant human growth hormone (GH) and/or sex steroids on various factors were examined. Results showed that GH, with or without sex steroids, increased lean body mass and decreased fat mass in both genders. However, men experienced a marginal increase in muscle strength and maximum oxygen uptake with sex steroid + GH, while women did not show significant changes in strength or cardiovascular endurance. Adverse effects, including diabetes and glucose intolerance, were observed in GH-treated men, suggesting that GH interventions in the elderly should be limited to controlled studies.
You can read the full article at https://jamanetwork.com/journals/jama/fullarticle/1108358.
Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87(12):5649-57.
Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men
We conducted a study on the effects of growth hormone (GH) and/or testosterone (T) administration in healthy older men, examining body composition, performance, mood, sexual function, bone turnover, and muscle-gene expression. Ten men completed one-month interventions of transdermal T patch, recombinant human GH, and combined hormones in a randomized order with a washout period. GH and combined hormone treatments raised serum GH and IGF-I concentrations, while combined hormones also increased estradiol and free T levels. Notably, fat-free mass increased with combined hormone exposure, and improvements were observed in balance, 30-meter walk time, and stair climb time during different interventions. Muscle IGF-I gene expression increased with both GH and combined hormone treatments, and serum osteocalcin levels also rose. No significant adverse events occurred during the study, suggesting that short-term GH and/or T administration can enhance certain aspects of physical performance in older men and boost muscle IGF-I gene expression.
You can read the full article at https://academic.oup.com/jcem/article/87/12/5649/2823629?login=false.
Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87(12):5649-57.
Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men
We investigated the impact of growth hormone (GH) and/or testosterone (T) administration in healthy older men, examining body composition, performance, mood, sexual function, bone turnover, and muscle gene expression. Ten men underwent one-month interventions of transdermal T patch, recombinant human GH, or combined hormones in a randomized order with a 3-month washout period. GH and combined hormones similarly elevated serum GH and IGF-I concentrations. Combined hormone treatment increased total estradiol and both T and combined hormone interventions raised free T levels. While there were no significant changes in strength, flexibility, body fat percentage, or sexual function and mood, fat-free mass increased with combined hormone exposure, and balance improved with GH. Muscle IGF-I gene expression increased during GH and combined hormone administration. Serum osteocalcin also increased in response to GH and combined hormone interventions. No significant adverse events were observed during the study, suggesting that one month of GH and/or T administration improves specific aspects of balance and physical performance in older men while increasing muscle IGF-I gene expression.
You can read the full article at https://academic.oup.com/jcem/article/87/12/5649/2823629?login=false.
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2011;96(6):1587–1609.
Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline
The objective was to update The Endocrine Society Clinical Practice Guideline on the Evaluation and Treatment of Adult Growth Hormone Deficiency (GHD), originally published in 2006. The consensus process involved systematic reviews of evidence, discussions through conference calls and emails, and multiple stages of review by members of The Endocrine Society. The key findings indicate that GHD can persist from childhood or develop later in life, often requiring confirmation through stimulation testing. Growth hormone (GH) therapy can offer various benefits, including improvements in body composition, exercise capacity, skeletal integrity, and quality of life, with minimal associated risks. GH dosing should be personalized, and the decision to treat adults with GHD should consider individual circumstances, weighing the benefits and risks.
You can read the full article at https://academic.oup.com/jcem/article/96/6/1587/2833853?login=false.
Lanfranco R, Pellegrino M, Maccario M, Arvat E. Ageing, growth hormone and physical performance. Journal of Endocrinological Investigation. 2003;26(9):861–872.
Ageing, growth hormone and physical performance
Aging is linked to a decrease in GH/IGF-I axis activity, leading to changes in body composition, function, and metabolism similar to those in younger adults with GH deficiency. These age-related changes primarily result from variations in hypothalamic control of somatotroph function, influenced by peripheral hormones and metabolism. The term “somatopause” connects declining GH and IGF-I levels with age-related shifts in body composition and metabolism. Physical exercise plays a vital role in regulating this axis, with increased fitness and training boosting GH production in adults. Regular exercise in older individuals can enhance overall fitness, quality of life, and longevity, similar to the effects of GH therapy. Clinical trials have explored interventions like rhGH, rhlGF-I, GHRH, and GHS to restore GH/IGF-I activity for anti-aging purposes, but there’s currently no definitive evidence of benefit in frail elderly individuals. This article reviews GH/IGF-I axis changes during aging, considering physical activity’s role and the effects of restoring GH and IGF-I levels on the body and performance.
You can read the abstract of the article at https://link.springer.com/article/10.1007/BF03345237.
Lange KHW, Andersen JL, Beyer N, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. Journal of Clinical Endocrinology and Metabolism. 2002;87(2):513–523.
GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men
The study investigated the effects of GH administration, with or without resistance exercise training, on muscle mass and strength in elderly men. Thirty-one participants were assigned to different groups, and various measurements were taken before and after 12 weeks. GH alone did not significantly impact muscle strength, power, size, or composition, although it influenced myosin heavy chain composition. Resistance exercise training, on the other hand, led to substantial improvements in muscle strength, power, and size. When GH was combined with resistance training, it did not further enhance these gains. Overall, the study suggests that GH may not be effective in increasing muscle strength or mass in healthy elderly men, whether used alone or in conjunction with resistance training, although it may influence myosin heavy chain composition.
You can read the full article at https://academic.oup.com/jcem/article/87/2/513/2846630?login=false.
Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. Journal of Clinical Endocrinology and Metabolism. 1994;79(5):1361–1366.
Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men
Normal aging involves adverse changes in body composition, muscle strength, and somatotropic function. Muscle strength decline in older adults contributes to frailty and fracture risk, despite resistance exercise training. To investigate whether age-related somatotropic deficits limit the benefits of resistance training in muscle strength, a 24-week double-blind, placebo-controlled trial was conducted with healthy elderly men. Initially, participants underwent 14 weeks of weight training, followed by 10 weeks of either recombinant human GH (rhGH) or placebo, along with further strength training. Muscle strength improved significantly in both groups during the first 14 weeks but showed limited gains thereafter. Baseline IGF-I levels were lower in both groups compared to young adults. While rhGH supplementation increased IGF-I levels and improved body composition, it had no impact on muscle strength, indicating that GH does not enhance the effects of strength training in elderly men. These results challenge the notion of GH as an ergogenic aid for aging individuals.
You can read the full article at https://academic.oup.com/jcem/article-abstract/79/5/1361/2649268?redirectedFrom=fulltext&login=false.
Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Annals of Internal Medicine. 1996;124(8):708–716.
Growth hormone replacement in healthy older men improves body composition but not functional ability
In a randomized, controlled, double-blind trial involving 52 healthy men aged over 69 with good functional ability but low insulin-like growth factor 1 levels, growth hormone (GH) replacement or a placebo was administered three times a week for six months. Results showed that GH increased lean mass by 4.3% while reducing fat mass by 13.1%, compared to minimal changes in the placebo group. However, GH did not significantly improve muscle strength, systemic endurance, or cognitive function. There was a slight improvement in the Trails B score but no substantial change in the Mini-Mental Status Examination or the Digit Symbol Substitution Test. GH use was also associated with a higher incidence of side effects and required dose reduction. Ultimately, while GH affected body composition positively, it did not enhance functional abilities in these older men.
You can read the full article at https://www.acpjournals.org/doi/10.7326/0003-4819-124-8-199604150-00002?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. American Journal of Physiology-Endocrinology and Metabolism. 1995;268(2):E268–E276.
Effect of growth hormone and resistance exercise on muscle growth and strength in older men
This study aimed to assess whether growth hormone (GH) administration would enhance muscle protein anabolism during heavy-resistance exercise training in older men. After a 16-week progressive resistance exercise program, participants were randomly assigned to either a GH or placebo group. While the GH group experienced greater increases in fat-free mass and total body water, the improvements in muscle protein synthesis rate, muscle strength, and other muscle-related parameters were similar in both groups. These findings suggest that resistance exercise training benefits muscle strength and anabolism in older men, but adding daily GH administration did not enhance these improvements. The increased fat-free mass with GH may have been due to noncontractile protein and fluid retention.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1995.268.2.E268?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Weltman A, Weltman JY, Schurrer R, Evans WS, Veldhuis JD, Rogol AD. Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. Journal of Applied Physiology. 1992;72(6):2188–2196.
Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity
This study examined the impact of run training intensity on the pulsatile release of growth hormone (GH) in 21 untrained women with regular menstrual cycles. Measurements included O2 consumption at the lactate threshold (LT), fixed blood lactate concentrations (FBLC) at 2.0, 2.5, and 4.0 mM, peak VO2, maximal VO2, body composition, and GH release. After one year of run training, both the at-lactate threshold (/LT) and above-lactate threshold (greater than LT) training groups showed increased VO2 at LT, FBLC levels, and VO2max, with a greater increase observed in the greater than LT group. Body weight remained stable, but there were trends toward reduced body fat and increased fat-free weight in the greater than LT group. These findings suggest that higher-intensity run training can lead to greater improvements in fitness and body composition compared to lower-intensity training or no training.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/jappl.1992.72.6.2188?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Poehlman ET, Copeland KC. Influence of physical activity on insulin-like growth factor-I in healthy younger and older men. Journal of Clinical Endocrinology and Metabolism. 1990;71(6):1468–1473.
Influence of physical activity on insulin-like growth factor-I in healthy younger and older men
We investigated the hypothesis that reduced physical activity contributes to the decline in insulin-like growth factor-I (IGF-I) during aging in healthy nonobese men. We assessed IGF-I levels in both younger and older men, considering factors such as maximal aerobic capacity (VO2 max), leisure time physical activity, fat-free weight, body fat percentage, body fat distribution, and daily caloric intake. IGF-I was 33% lower in older men than younger men, negatively correlated with body fat and upper body fat distribution, and positively correlated with VO2 max and leisure time physical activity. After accounting for age, VO2 max and leisure time physical activity were the only independent factors associated with IGF-I. These findings suggest that reduced physical activity contributes, in part, to the age-related decline in IGF-I in men.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/71/6/1468/2652646?redirectedFrom=fulltext&login=false.
Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St. Clair C, Pabst KM, Harman SM 2002 Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292.
Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial
In a 26-week randomized, double-blind, placebo-controlled trial involving healthy, ambulatory, elderly women and men aged 65 to 88 years, the study aimed to assess the effects of recombinant human growth hormone (GH) and/or sex steroids on body composition, strength, endurance, and adverse outcomes. Participants were assigned to different treatment groups, including GH and sex steroids, GH alone, sex steroids alone, or placebos. The results showed that GH, with or without sex steroids, increased lean body mass and reduced fat mass in both women and men. However, the effects on muscle strength and cardiovascular endurance were marginal and varied between genders. Adverse effects, including diabetes and glucose intolerance, were more common in GH-treated individuals, emphasizing the need for caution when using GH interventions in the elderly.
You can read the full article at https://jamanetwork.com/journals/jama/fullarticle/1108358.
Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD 2002 Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab 87:5649–5657.
Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men
We conducted a study to assess the effects of growth hormone (GH) and/or testosterone (T) administration in healthy older men. Participants completed various one-month interventions, including transdermal T patch, recombinant human GH, and a combination of both (GHT), with a washout period in between. While GH and GHT significantly increased serum GH and IGF-I concentrations, there were no major changes in strength, flexibility, body fat percentage, or sexual function and mood. However, GHT led to an increase in fat-free mass and improved balance, and both T and GHT reduced 30-meter walk time and stair climb time compared to baseline. Muscle IGF-I gene expression increased during GH and GHT administration, while serum osteocalcin levels also rose in response to GH and GHT. No significant adverse events were reported during the study, indicating that GH and/or T administration can have positive effects on physical performance and muscle gene expression in older men.
You can read the full article at https://academic.oup.com/jcem/article/87/12/5649/2823629?login=false.
Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6.
Effects of human growth hormone in men over 60 years old.
In a study involving 21 healthy men aged 61 to 81 with low plasma IGF-I levels, we aimed to investigate the impact of growth hormone (GH) treatment. Group 1 received subcutaneous injections of biosynthetic human GH three times a week, while group 2 received no treatment. Group 1 experienced a rise in plasma IGF-I levels to a more youthful range, resulting in an 8.8 percent increase in lean body mass, a 14.4 percent reduction in adipose tissue mass, a 1.6 percent rise in lumbar vertebral bone density, and a 7.1 percent increase in skin thickness. Group 2, however, showed no significant changes in these parameters. These findings suggest that declining GH secretion contributes to age-related decreases in lean body mass, increases in adipose tissue mass, and skin thinning.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJM199007053230101?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov.
Welle S, Thornton C, Statt M, McHenry B 1996 Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81:3239–3243.
Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old
In a study involving healthy individuals aged over 60, we investigated the effects of recombinant human GH on myofibrillar protein synthesis. Participants received either a single GH injection or GH treatment for three months. GH reduced whole-body leucine oxidation and increased lean body mass, muscle mass, and thigh strength after three months. However, GH did not rejuvenate the rate of myofibrillar protein synthesis in the quadriceps. These findings suggest that GH can enhance muscle mass and strength in individuals over 60 but does not restore a youthful myofibrillar protein synthesis rate.
You can read the abstract of the article at https://academic.oup.com/jcem/article/81/9/3239/2651047?login=false.
Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C 1996 Growth hormone replacement in healthy older men improves body composition but not functional ability [see comments]. Ann Intern Med 124:708–716.
Growth hormone replacement in healthy older men improves body composition but not functional ability
In a randomized, controlled, double-blind trial involving 52 healthy men over 69 years old with low baseline insulin-like growth factor 1 levels, the effects of growth hormone replacement were assessed. After 6 months, the growth hormone group showed a 4.4 percentage point increase in lean mass and a 12.8 percentage point decrease in fat mass compared to the placebo group. However, no significant improvements were observed in muscle strength, systemic endurance, or cognitive function. While the Trails B score improved in the growth hormone group, Mini-Mental Status Examination scores did not show significant differences. Side effects were more frequent in the growth hormone group, leading to dose reductions. In conclusion, growth hormone increased lean tissue mass and reduced fat mass in healthy older men but did not enhance functional ability, and side effects were common.
You can read the full article at https://www.acpjournals.org/doi/10.7326/0003-4819-124-8-199604150-00002?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Lange KH, Larsson B, Flyvbjerg A, Dall R, Bennekou M, Rasmussen MH, Orskov H, Kjaer M 2002 Acute growth hormone administration causes exaggerated increases in plasma lactate and glycerol during moderate to high intensity bicycling in trained young men. J Clin Endocrinol Metab 87:4966–4975.
Acute growth hormone administration causes exaggerated increases in plasma lactate and glycerol during moderate to high intensity bicycling in trained young men
In a randomized, double-blinded, cross-over trial, seven highly trained men received a single subcutaneous dose of 7.5 IU (2.5 mg) of GH or placebo 4 hours before a 90-minute bicycling exercise. While all subjects completed the exercise protocol in the placebo trial, two subjects could not complete it, and one barely managed to in the GH trial. GH administration led to exaggerated increases in plasma lactate concentrations during exercise, and the combined lipolytic effect of GH and exercise significantly increased plasma glycerol and serum nonesterified fatty acids (NEFA) concentrations, but this didn’t result in increased whole-body fat oxidation. Additionally, plasma glucose was higher during exercise after GH administration. These findings suggest that a single GH dose can negatively impact exercise performance and metabolism during bicycling.
You can read the full article at https://academic.oup.com/jcem/article/87/11/4966/2823155?login=false.
Hennessey JV, Chromiak JA, DellaVentura S, Reinert SE, Puhl J, Kiel DP, Rosen CJ, Vandenburgh H, MacLean DB 2001 Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. J Am Geriatr Soc 49:852–858.
Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people.
Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people
In a double-blind trial conducted at an outpatient clinical research center, 31 frail older subjects (mean age 71.3 +/- 4.5 years) participated in a study examining the impact of recombinant human growth hormone (rhGH) treatment, with or without a structured resistance exercise program, on muscle strength, fiber type, and cross-sectional area. Participants were randomly assigned to one of four protocols: rhGH alone, rhGH with exercise, exercise with placebo injections, or placebo injections only. Muscle biopsies were taken from the vastus lateralis muscle, and isokinetic dynamometry strength tests were performed. RhGH administration significantly increased circulating insulin-like growth factor-I (IGF-I) levels, and both the rhGH/exercise and exercise-only groups experienced notable increases in muscle strength. Moreover, the combined rhGH-treated subjects exhibited a significant rise in the proportion of type 2 muscle fibers compared to those not receiving rhGH. These findings suggest a potential benefit of growth hormone in addressing age-related muscle deficits, though further research is needed to explore changes in fiber cross-sectional area and absolute number with long-term growth hormone use.
You can read the abstract of the article at https://agsjournals.onlinelibrary.wiley.com/doi/abs/10.1046/j.1532-5415.2001.49173.x?sid=nlm%3Apubmed.
Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, Marcus R 1994 Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab 79:1361–1366.
Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men
A double-blind, placebo-controlled exercise trial investigated the impact of recombinant human growth hormone (rhGH) on muscle strength improvement in healthy elderly men (65-82 years) undergoing strength training. Initially, all participants underwent 14 weeks of progressive weight training to establish a trained state, after which they were randomly assigned to receive either rhGH or placebo while continuing strength training for an additional 10 weeks. Muscle strength improved significantly for both groups during the first 14 weeks of training (24-62% increase), with minimal gains thereafter. Baseline insulin-like growth factor-I (IGF-I) levels were similar between groups, but rhGH significantly elevated IGF-I levels. However, rhGH did not enhance muscle strength at any point during the study, indicating that GH supplementation did not augment the response to strength training in elderly men and did not support the idea of GH as an ergogenic aid for this purpose.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/79/5/1361/2649268?redirectedFrom=fulltext&login=false.
Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM 1995 Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 268:E268–E276.
Effect of growth hormone and resistance exercise on muscle growth and strength in older men.
This study aimed to assess whether growth hormone (GH) supplementation enhances muscle protein anabolism in response to heavy-resistance exercise training in older men. Sedentary older men underwent a 16-week progressive resistance exercise program and were randomly assigned to receive either GH or a placebo. The GH group experienced greater increases in fat-free mass (FFM) and total body water. While whole-body protein synthesis and breakdown rates increased in the GH group, muscle protein synthesis rate, urinary creatinine excretion, and muscle strength improvements were similar in both groups. The study suggests that resistance exercise training effectively enhances muscle strength and anabolism in older men, but GH supplementation did not further enhance these benefits, potentially contributing to increased noncontractile protein and fluid retention in the GH group.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1995.268.2.E268?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
D’Ercole J, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA. 1984;81:935–939.
Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action
We have successfully validated a method to extract and measure somatomedin C (Sm-C)/insulin-like growth factor I (IGF-I) tissue content, a growth-promoting peptide regulated by growth hormone. The Sm-C content in tissue extracts displayed strong growth hormone dependence, as tissues from hypophysectomized rats contained significantly less Sm-C than normal tissues. Administering ovine growth hormone (oGH) intraperitoneally to hypophysectomized rats increased tissue extractable Sm-C in various organs, with maximal responses occurring 12 hours after treatment, preceding the peak in serum levels. In liver and lung, Sm-C responses to oGH followed linear regression models, with doses of oGH required for Sm-C increase falling within the range needed for protein synthesis enhancement. These findings support the idea that somatomedins may primarily act through autocrine or paracrine mechanisms, produced at multiple sites and influencing nearby tissues.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC344954/.
Gostelli-Peter M, Winterhalter KH, Schmid C, Froesch ER, Zapf J. Expression and regulation of insulin like growth factor I (IGF-I) and IGF binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology. 1994;135:2558–2567.
Expression and regulation of insulin like growth factor I (IGF-I) and IGF binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone.
We examined the expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins (IGFBP-2, -3, -4, and -5) mRNA in various tissues of hypophysectomized rats following saline, recombinant human (rh) IGF-I, or rhGH infusion, comparing them to age-matched normal rats. IGF-I mRNA was present in all tissues, with higher levels in the liver and white adipose tissue (WAT) but low levels in the kidney, brain, and thymus. The liver, skeletal muscle, and WAT showed the greatest GH dependence. Tissue-specific expression patterns were observed for IGFBPs. Hypophysectomy led to reduced IGFBP expression in several tissues, except for IGFBP-2 in the liver. GH infusion had distinct effects on IGF-I and IGFBP expression, differing from infused rhIGF-I, possibly due to GH’s direct tissue-level actions and local IGF-I production.
You can read the abstract of the article at https://academic.oup.com/endo/article-abstract/135/6/2558/3036385?redirectedFrom=fulltext&login=false.
Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533.
The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH
GH’s activation of the GH receptor (GHR)-associated tyrosine kinase JAK2 and the transcription factors Stats (signal transducers and activators of transcription) 1, 3, and 5 is investigated in this study. GH was found to stimulate the tyrosyl phosphorylation of Stats 1, 3, and 5 in CHO cells expressing GHRs that bind JAK2 but not in cells expressing GHR that does not bind JAK2. When tyrosines 333 and 338 were mutated to phenylalanine in the GHR1-454 receptor, GH-dependent phosphorylation of Stats 1, 3, and 5 was severely reduced. Additionally, GH was observed to stimulate tyrosyl phosphorylation of JAK2 and JAK1, with JAK2 having a more substantial response, suggesting the importance of JAK2 in GH signaling and potential Stat-JAK2 binding.
You can read the abstract of the article at https://academic.oup.com/mend/article/10/5/519/2713379?login=false.
Adams GR. The role of IGF-I in the regulation of skeletal muscle adaptation. Exerc Sport Sci Rev 1998;26: 31-60.
The role of IGF-I in the regulation of skeletal muscle adaptation
Muscle adaptations in response to changes in loading involve adjustments in protein synthesis and degradation within the muscle fibers and a regulation of the number of myonuclei to maintain a consistent ratio with myofiber size. Figure 2.6 illustrates that when muscle experiences increased loading, it increases production and secretion of IGF-I. This growth factor not only promotes muscle growth processes but also prompts nearby satellite cells to proliferate. Eventually, these satellite cells combine with the muscle fibers, introducing more myonuclei to maintain the balance with the enlarged muscle fibers.
Taaffe DR, Jin IH, Vu TH, et al. Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression. J Clin Endocrinol Metab 1996;81: 421-425.
Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression
Muscle samples were collected from 18 healthy elderly men who participated in a study involving recombinant human GH (rhGH) and exercise. After an initial 14-week resistance training period, the subjects were randomly assigned to receive either rhGH or a placebo while continuing their training for another 10 weeks. Cross-sectional areas of both type I and type II muscle fibers increased significantly during the initial training period. However, during the subsequent treatment period (weeks 14-24), there were no significant differences between the groups in terms of muscle fiber size or the expression of GH and insulin-like growth factors. This suggests that rhGH administration in elderly men engaged in exercise does not enhance muscle fiber hypertrophy or the expression of GH-IGF and indicates that age-related deficits in the GH-IGF-I axis do not hinder the muscle tissue response to training.
You can read the abstract of the article at https://academic.oup.com/jcem/article/81/1/421/2649610?login=false.
Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab 1994;79: 1361-1366.
Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men
Aging typically leads to negative changes in body composition and muscle strength, contributing to frailty and fractures in older individuals. Although resistance exercise can enhance muscle strength in older adults, these gains tend to plateau with continued training. To explore if age-related deficiencies in the somatotropic axis impact the potential improvement in muscle strength through resistance training, we conducted a double-blind, placebo-controlled exercise trial with 18 healthy elderly men (aged 65-82 years). They initially underwent 14 weeks of progressive weight training, followed by random assignment to receive either recombinant human GH (rhGH) or a placebo for another 10 weeks of strength training. Both groups showed significant strength gains during the first 14 weeks (ranging from 24-62% depending on muscle group), but these improvements did not significantly differ between them. Despite rhGH increasing insulin-like growth factor-I (IGF-I) levels, it did not enhance muscle strength at any point, suggesting that GH deficiencies do not explain the observed plateau in muscle strength during training in older adults, challenging the idea of GH as an ergogenic aid.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/79/5/1361/2649268?redirectedFrom=fulltext&login=false.
Yarasheski KE, Zachwieja JJ, Campbell JA, et al. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 1995;268: E268-E276.
Effect of growth hormone and resistance exercise on muscle growth and strength in older men
This study aimed to assess whether growth hormone (GH) administration could enhance muscle growth in response to heavy-resistance exercise. Sixteen men (aged 21-34) were divided into two groups, one receiving resistance training plus GH (7 participants) and the other receiving resistance training plus a placebo (9 participants) for 12 weeks. Both groups underwent the same training regimen. Results showed that both groups experienced increases in fat-free mass (FFM) and total body water, with the GH group showing greater FFM gains. While whole-body protein synthesis rate and balance improved more in the GH group, muscle size, strength, and protein synthesis in the quadriceps did not show significant differences between the two groups. These findings suggest that in young men, resistance exercise alone or with GH supplementation leads to similar gains in muscle size and strength, indicating that GH primarily increases lean tissue other than skeletal muscle and does not further enhance muscle growth and function when combined with resistance training.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1992.262.3.E261?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Alba M, Fintini D, Sagazio A, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006;291(6):E1290-4.
Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse
In children with isolated growth hormone (GH) deficiency, GH-releasing hormone (GHRH) can be effective for promoting growth. However, its short half-life limits its therapeutic use. To address this limitation, CJC-1295, a synthetic GHRH analog, was tested in mice lacking the GHRH gene. Three groups of 1-week-old GHRHKO mice were treated with CJC-1295 at intervals of 24, 48, and 72 hours for 5 weeks. Daily CJC-1295 treatment normalized body weight and length, while mice treated every 48 and 72 hours showed improved growth but not complete normalization. Bone length remained normal in animals treated every 24 and 48 hours. CJC-1295 also increased pituitary RNA and GH mRNA levels, indicating somatotroph cell proliferation. Overall, once-daily CJC-1295 administration effectively maintained normal body composition and growth in GHRHKO mice, with less frequent dosing being less effective.
You can read the abstract of the article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00201.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Gautam D, Jeon J, Starost MF, et al. Neuronal M3 muscarinic acetylcholine receptors are essential for somatotroph proliferation and normal somatic growth. Proc Natl Acad Sci USA. 2009;106(15):6398-403.
Neuronal M3 muscarinic acetylcholine receptors are essential for somatotroph proliferation and normal somatic growth
Our study delves into the molecular mechanisms governing the proliferation and maintenance of pituitary somatotrophs and other anterior pituitary cell types. While the exact pathways are not well understood, this knowledge could lead to innovative treatments for growth disorders. We discovered that mutant mice lacking the M(3) muscarinic acetylcholine receptor subtype in the brain exhibited dwarfism, smaller anterior pituitary glands, and reduced growth hormone (GH) and prolactin levels. Surprisingly, treatment with CJC-1295, a synthetic GH-releasing hormone (GHRH) analog, restored normal growth in these mice by normalizing pituitary size and GH and IGF-1 levels. These findings suggest a crucial role for central M(3) receptors in regulating longitudinal growth by promoting pituitary somatotroph cell proliferation through their influence on hypothalamic GHRH neurons.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2662962/.
Engelson ES, Glesby MJ, Mendez D, Albu JB, Wang J, Heymsfield SB, Kotler DP 2002 Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection. J Acquired Immune Defic Syndr 30:379–391.
Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection
In individuals with HIV-associated lipodystrophy marked by excess visceral fat, two prospective open-label trials were conducted involving recombinant human growth hormone (rhGH) treatment. One group received 6 mg/d of rhGH, while the other received 4 mg every other day, with a 12-week washout period in between. The trials included 30 HIV-positive participants. Notably, despite stable body weight, those on the higher rhGH dose experienced an average 42% reduction in visceral adipose tissue (VAT) after 12 weeks, with trends toward further decreases. Subcutaneous adipose tissue also decreased but to a lesser extent. Skeletal muscle increased. However, the effects on lipids were inconsistent, and adverse events, including joint pain and decreased insulin sensitivity, were observed. These findings highlight rhGH’s potential for reducing excess visceral fat in HIV lipodystrophy but emphasize the need for controlled studies and careful monitoring due to adverse effects.
You can read the abstract of the article at https://journals.lww.com/jaids/abstract/2002/08010/effect_of_recombinant_human_growth_hormone_in_the.2.aspx.
Falutz J, Allas S, Kotler D, Thompson M, Koutkia P, Albu J, Trottier B, Routy J-P, Cote P, Abribat T, Grinspoon S 2005 A placebo-controlled, dose-ranging study of a growth hormone releasing factor in HIV-infected patients with abdominal fat accumulation. AIDS 19:1279–1287.
A placebo-controlled, dose-ranging study of a growth hormone releasing factor in HIV-infected patients with abdominal fat accumulation
In a randomized, double-blind, placebo-controlled trial involving 61 HIV-infected patients with central fat accumulation, the effects of TH9507, a novel growth hormone releasing factor, were investigated. Participants received subcutaneous doses of placebo, 1 mg, or 2 mg TH9507 once daily for 12 weeks. TH9507 led to dose-related increases in insulin-like growth factor-I (IGF-I) and resulted in a significant reduction in trunk fat in the 2 mg group compared to placebo. Visceral fat decreased the most in the 2 mg group, although this change wasn’t statistically significant. Subcutaneous fat was preserved. Lean body mass improved, and triglyceride levels decreased significantly in the 2 mg group. TH9507 treatment was generally well-tolerated without affecting glucose levels. These findings suggest TH9507 could be a beneficial treatment for central fat accumulation in HIV-infected patients, but further studies are needed to assess its effects on visceral fat, treatment durability, and safety in the long term.
You can read the full article at https://journals.lww.com/aidsonline/fulltext/2005/08120/a_placebo_controlled,_dose_ranging_study_of_a.6.aspx.
Teichman, Sam L.; Neale, Ann; Lawrence, Betty; Gagnon, Catherine; Castaigne, Jean-Paul; Frohman, Lawrence A. (2006). “Prolonged Stimulation of Growth Hormone (GH) and Insulin-Like Growth Factor I Secretion by CJC-1295, a Long-Acting Analog of GH-Releasing Hormone, in Healthy Adults”. The Journal of Clinical Endocrinology & Metabolism. 91 (3): 799–805. doi:10.1210/jc.2005-1536. ISSN 0021-972X. PMID 16352683.
“Prolonged Stimulation of Growth Hormone (GH) and Insulin-Like Growth Factor I Secretion by CJC-1295, a Long-Acting Analog of GH-Releasing Hormone, in Healthy Adults”
In two randomized, placebo-controlled, double-blind trials lasting 28 and 49 days, the pharmacokinetic profile, pharmacodynamic effects, and safety of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, were examined in healthy subjects aged 21-61 years. After a single subcutaneous injection of CJC-1295, there were dose-dependent increases in plasma growth hormone (GH) concentrations by 2- to 10-fold for 6 days or more and in plasma insulin-like growth factor-I (IGF-I) concentrations by 1.5- to 3-fold for 9-11 days. The estimated half-life of CJC-1295 was 5.8-8.1 days. With multiple CJC-1295 doses, IGF-I levels remained elevated for up to 28 days. No serious adverse reactions were reported, suggesting that CJC-1295 has the potential to be a safe and effective therapeutic agent.
You can read the full article at https://academic.oup.com/jcem/article/91/3/799/2843281?login=false.
Ionescu, Madalina; Frohman, Lawrence A. (2006). “Pulsatile Secretion of Growth Hormone (GH) Persists during Continuous Stimulation by CJC-1295, a Long-Acting GH-Releasing Hormone Analog”. The Journal of Clinical Endocrinology & Metabolism. 91 (12): 4792–4797. doi:10.1210/jc.2006-1702. ISSN 0021-972X.
“Pulsatile Secretion of Growth Hormone (GH) Persists during Continuous Stimulation by CJC-1295, a Long-Acting GH-Releasing Hormone Analog”
In healthy men aged 20 to 40 years, GH pulsatility was assessed through 20-minute blood sampling over a 12-hour period before and after a single injection of either 60 or 90 microg/kg CJC-1295, a synthetic GHRH analog with an extended half-life. While CJC-1295 increased GH secretion, it preserved GH pulsatility, with no significant changes in the frequency and magnitude of GH secretory pulses. However, basal GH levels saw a substantial increase (7.5-fold), contributing to overall elevated GH secretion (46%) and IGF-I levels (45%). These findings highlight the importance of increased trough GH levels in enhancing IGF-I production, suggesting potential clinical utility for long-acting GHRH preparations in individuals with intact pituitary GH secretion capabilities.
You can read the full article at https://academic.oup.com/jcem/article/91/12/4792/2656274?login=false.
Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91(3):799-805.
Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults
This study aimed to assess the pharmacokinetics, pharmacodynamics, and safety of CJC-1295, a long-acting GHRH analog, in healthy participants aged 21 to 61 years. The research comprised two randomized, double-blind, placebo-controlled trials, lasting 28 and 49 days, conducted at two sites. Following subcutaneous injections of CJC-1295 in escalating single doses or multiple weekly or biweekly doses, there were dose-dependent increases in mean plasma GH levels (2- to 10-fold) lasting for over 6 days and in mean plasma IGF-I levels (1.5- to 3-fold) for 9-11 days after a single injection. The estimated half-life of CJC-1295 ranged from 5.8 to 8.1 days. After multiple doses, IGF-I levels remained elevated for up to 28 days, with no reported serious adverse reactions. These findings suggest that CJC-1295 holds promise as a therapeutic agent due to its sustained GH and IGF-I elevations and favorable safety profile, particularly at doses of 30 or 60 microg/kg.
You can read the full article at https://academic.oup.com/jcem/article/91/3/799/2843281?login=false.
Van hout MC, Hearne E. Netnography of Female Use of the Synthetic Growth Hormone CJC-1295: Pulses and Potions. Subst Use Misuse. 2016;51(1):73-84.
Netnography of Female Use of the Synthetic Growth Hormone CJC-1295: Pulses and Potions
This study employed “netnography” to investigate female usage of CJC-1295, a synthetic growth hormone analog, as discussed in online forums. Through a systematic internet search focusing on bodybuilding websites and female forum activity related to CJC-1295, 23 discussion threads were identified and analyzed using the Empirical Phenomenological Psychological method. Forum participants exhibited a strong understanding of performance and image-enhancing drug supplementation. Their motivations for using CJC-1295 included weight loss, muscle enhancement, youthful skin, improved sleep, and injury healing. Concerns were expressed regarding the gender-specific aspects of growth hormone pulsatility, impacting dosage estimation, cycling, and long-term effects. These findings underscore the need for public health interventions to address female self-medication practices involving synthetic growth hormones and potential associated health risks.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.3109/10826084.2015.1082595?journalCode=isum20.
Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol. 2010;316(2):147-53.
Obesity, growth hormone and weight loss
Growth hormone (GH) plays a crucial role in postnatal longitudinal growth but becomes less important in adults. Adults with GH deficiency (GHD) experience various disturbances in body composition, lipid metabolism, cardiovascular risk factors, and bone mineral density. Notably, GHD in adults is associated with increased fat accumulation, especially abdominal fat. GH replacement in adult GHD patients has been shown to reduce fat mass, particularly abdominal fat. Obesity, especially abdominal obesity, can lead to reduced GH secretion and lower insulin-like growth factor-I (IGF-I) levels. However, the exact role of GH in obesity remains unclear. Clinical studies have demonstrated limited or no significant weight loss effects with GH treatment in obese individuals, although it can reduce overall and abdominal fat mass to a modest degree, similar to the effects achievable through diet or exercise interventions.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0303720709004377?via%3Dihub.
Gertner JM. Effects of growth hormone on body fat in adults. Horm Res. 1993;40(1-3):10-5.
Effects of growth hormone on body fat in adults
The relationship between growth hormone (GH) and adipose tissue forms a cycle: GH has lipolytic properties, reducing and redistributing body fat, while obesity is associated with decreased GH production. Studies on GH’s impact on adipose tissue have been conducted in obese, elderly, and nonobese GH-deficient adults and children. In GH-deficient adults, GH treatment appears to result in a net reduction of fat tissue, but substantial weight loss in obese individuals through GH treatment is supported by limited evidence. GH treatment in elderly individuals leads to increased lean body mass and modest decreases in fat mass, though interpreting data is challenging due to measurement methods and the lack of significant weight loss effects in the obese population.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/40/1-3/10/370285/Effects-of-Growth-Hormone-on-Body-Fat-in-Adults?redirectedFrom=fulltext.
Shadid S, Jensen MD. Effects of growth hormone administration in human obesity. Obes Res. 2003;11(2):170-5.
Effects of growth hormone administration in human obesity
This review aimed to summarize literature reports on the impact of growth hormone (GH) treatment for obesity in adults. Clinical trials were examined, focusing on GH’s effects on body fat, body fat distribution, glucose tolerance/insulin resistance, and treatment-related adverse outcomes. The findings revealed that GH, when administered alongside hypocaloric diets, did not significantly enhance fat loss or preserve lean tissue mass. There was limited evidence supporting an independent positive effect of GH on visceral fat. Most studies indicated that glucose tolerance worsened during GH treatment compared to placebo. Overall, despite low serum GH levels in obese individuals, the majority of studies suggested that GH treatment had little to no beneficial effects on obesity.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2003.27.
Kim KR, Nam SY, Song YD, Lim SK, Lee HC, Huh KB. Low-dose growth hormone treatment with diet restriction accelerates body fat loss, exerts anabolic effect and improves growth hormone secretory dysfunction in obese adults. Horm Res. 1999;51(2):78-84.
Low-dose growth hormone treatment with diet restriction accelerates body fat loss, exerts anabolic effect and improves growth hormone secretory dysfunction in obese adults
Growth hormone (GH) can accelerate lipolysis, and impaired GH secretion in obesity leads to a loss of this effect. To address obesity, dietary restriction is often challenging due to compliance issues and slow weight loss. A study involving 24 obese individuals explored the effects of GH treatment and dietary restriction on lipolysis, anabolism, and changes in insulin and GH secretion. GH treatment resulted in a greater loss of body weight as fat and a more substantial reduction in visceral fat compared to a placebo. In contrast, the placebo group experienced a loss in lean body mass and negative nitrogen balance, while the GH group saw an increase in lean body mass and positive nitrogen balance. GH treatment also increased insulin-like growth factor-I (IGF-I) levels and improved GH secretion. These findings suggest that low-dose GH with caloric restriction may have a therapeutic role in managing obesity.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/51/2/78/371457/Low-Dose-Growth-Hormone-Treatment-with-Diet?redirectedFrom=fulltext.
Thompson JL, Butterfield GE, Gylfadottir UK, et al. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab. 1998;83(5):1477-84.
Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women
In a 12-week randomized, double-blind study involving 33 moderately obese postmenopausal women, the effects of GH and insulin-like growth factor I (IGF-I) administration, combined with diet and exercise, were investigated. Participants followed a calorie-restricted diet and exercised regularly. They self-administered GH, IGF-I, a combination of both, or a placebo. While all groups experienced weight loss, the GH plus IGF-I group achieved the greatest reduction in weight and fat mass. Basal metabolic rate (BMR) remained stable in all groups, and muscle strength improved with training. Additionally, women receiving IGF-I reported reduced depression and anxiety scores. These findings demonstrate that obese postmenopausal women can lose weight and fat while preserving fat-free mass, BMR, and muscle strength. The combination of GH and IGF-I may enhance fat loss compared to either treatment alone.
You can read the full article at https://academic.oup.com/jcem/article/83/5/1477/2865247?login=false.
Devesa J, Almengló C, Devesa P. Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clinical Medicine Insights Endocrinology and Diabetes. 2016;9:47-71. doi:10.4137/CMED.S38201.
Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clinical Medicine Insights Endocrinology and Diabetes
This review examines the impact of growth hormone (GH) on various tissues and organs and its potential role in an organism’s longitudinal growth. It is concluded that GH plays a vital role in maintaining tissue and organ homogeneity during normal human development and after injury. Growth effects do not appear to occur during fetal or early infancy stages but are mediated by insulin-like growth factor I (IGF-I) during childhood and puberty. Proper GH secretion is crucial for IGF-I transcription, although in many tissues, IGF-I production can be GH-independent. The review also suggests that GH may function as a prohormone, as it is proteolytically cleaved into shorter forms with unknown activity in many tissues and organs.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5063841/.
Skaggs SR, Crist DM. Exogenous human growth hormone reduces body fat in obese women. Horm Res. 1991;35(1):19-24.
Exogenous human growth hormone reduces body fat in obese women
The effects of biosynthetic methionyl human growth hormone (met-hGH) on body composition and endogenous insulin-like growth factor I (IGF-I) secretion were studied in obese women with a range of 138 to 226% of ideal body weight. In a double-blind study, 12 subjects received met-hGH or placebo for 27-28 days, with no significant dietary calorie restriction. Met-hGH led to a significant reduction in body fat and a near-significant increase in fat-free mass (FFM), regardless of initial body fat levels or endogenous GH status. Circulating IGF-I levels showed similar changes to FFM, approaching significance. No significant changes were observed in the placebo group, indicating that exogenous GH can reduce body fat in obese women without significant calorie restriction, independent of endogenous GH secretion or initial body composition.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/35/1/19/365537/Exogenous-Human-Growth-Hormone-Reduces-Body-Fat-in?redirectedFrom=fulltext.
Chaves VE, Júnior FM, Bertolini GL. The metabolic effects of growth hormone in adipose tissue. Endocrine. 2013;44(2):293-302.
The metabolic effects of growth hormone in adipose tissue
The physiological effects of growth hormone (GH) go beyond promoting linear growth and extend to significant metabolic impacts on adipose tissue. GH influences both the growth and specialization of preadipocytes, although the extent of this effect varies among different cell lines and preadipocyte cultures. Specific GH receptors are present on both preadipocytes and mature adipocytes. GH may exert its actions through these receptors, but some effects are indirectly mediated by GH-induced secretion of insulin-like growth factor-I (IGF-I) within adipose tissue. GH stimulates lipolysis by inhibiting lipoprotein lipase, which breaks down triglycerides in the blood for storage in adipose tissue, and it also activates hormone-sensitive lipase (HSL), a key enzyme for releasing stored triglycerides from adipocytes (lipolysis). As GH is used for various non-growth purposes, understanding its metabolic effects on adipocytes is crucial for comprehending the clinical outcomes of GH therapy.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/11086655/#:~:text=GH%20promotes%20lipolysis%20via%20inhibition,triglyceride%20accumulation%20in%20adipose%20tissue..
Mekala KC, Tritos NA. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab. 2009;94(1):130-7.
Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis.
Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis
A meta-analysis of human studies aimed to assess the efficacy and safety of recombinant human growth hormone (rhGH) therapy for obesity in adults. The analysis found that rhGH treatment resulted in significant alterations in body composition, including reduced fat mass, lower percent body fat, increased lean body mass, and decreased visceral adipose area. Beneficial changes in lipid profile, such as reduced total cholesterol and low-density lipoprotein-cholesterol, were observed. However, there were increases in fasting plasma glucose and insulin levels, particularly in shorter-term studies. Adverse effects included arthralgias, peripheral edema, and paresthesias. While rhGH therapy did not induce weight loss, it contributed to decreased visceral adiposity. Future studies using carefully titrated rhGH protocols of longer duration are needed to fully understand its effects on obesity, including its impact on cardiovascular health.
You can read the full article at https://academic.oup.com/jcem/article/94/1/130/2597868?login=false.
Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–141.
Obesity and growth during childhood and puberty
Growth during childhood and adolescence is influenced by genetic and environmental factors, with nutritional status playing a vital role. Childhood obesity, a concerning and growing issue, has both short-term and long-term metabolic and cardiovascular consequences. It’s been observed that obese children tend to have accelerated growth during prepubertal years, characterized by higher height velocity and advanced bone age compared to lean counterparts. However, as they enter puberty, this growth advantage diminishes, with reduced growth spurts. This accelerated growth appears to be independent of growth hormone (GH) and is linked to factors like increased leptin, insulin, adrenal androgens, insulin-like growth factor (IGF)-1, IGF-binding protein-1, and GH-binding proteins. Childhood obesity also affects pubertal development, leading to early onset of puberty in girls and potential impacts on hormonal levels. Preventing childhood obesity is crucial not only for overall health but also for maintaining normal growth and pubertal patterns.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/23428692/#:~:text=Nutritional%20status%20plays%20an%20important,term%20metabolic%20and%20cardiovascular%20complications.
De Leonibus C, Marcovecchio ML, Chiarelli F. Update on statural growth and pubertal development in obese children. Pediatr Rep. 2012;4:e35.
Update on statural growth and pubertal development in obese children
Childhood obesity is a concerning and escalating issue, linked to various short-term and long-term metabolic and cardiovascular problems. It’s also been proposed that excessive weight during childhood can impact growth and pubertal development. Studies reveal that obese children often experience higher growth rates during pre-pubertal years, but this advantage diminishes during puberty, resulting in similar final heights to non-obese peers. Obesity can also influence the timing of puberty, leading to earlier onset in girls, who may face an elevated risk of hyperandrogenism and polycystic ovary syndrome. For boys, the evidence is mixed, with some studies suggesting earlier puberty in obese individuals and others indicating delayed onset. Overall, the link between obesity and alterations in growth and puberty patterns underscores the importance of combating childhood obesity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3555205/.
Louvi A, Accili D, Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol. 1997;189:33–48.
Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development
Genetic investigations of dwarfing traits resulting from targeted mutations in the genes responsible for insulin-like growth factors (IGF-I and IGF-II) and their type 1 IGF receptor (IGF1R) have revealed the critical role of this signaling system in mouse embryonic growth. Among the two IGF ligands, IGF-I exclusively interacts with IGF1R, while IGF-II engages an additional receptor (XR). The absence of both IGR1R and IGF-II results in severe growth retardation (30% of normal birth weight), surpassing the effects observed in single mutants lacking either Igf1r or Igf2 (45% and 60% of normal, respectively). To investigate whether XR is the insulin receptor (IR), embryos lacking both Igf1r and Insr were examined. While the absence of IR alone had a minor impact on growth, the simultaneous absence of IGF1R and IR led to a severe growth-deficient phenotype (30% of normal size at birth), evident from Embryonic Day 13.5 and characterized by various developmental abnormalities. These findings indicate that IGF-II’s growth-promoting function during mouse embryogenesis involves, in part, signaling through the insulin receptor.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/pii/S0012160697986668?via%3Dihub.
Alba M, Fintini D, Sagazio A, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006;291(6):E1290-4.
Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse
While most children with isolated growth hormone (GH) deficiency respond well to GH-releasing hormone (GHRH), its short half-life limits its therapeutic use. A synthetic GHRH analog called CJC-1295, with an extended half-life due to its binding to endogenous albumin, was tested in GHRH-deficient mice. When administered daily, CJC-1295 normalized body weight and length, and when given every 48 or 72 hours, it still improved growth, although not fully normalized. Bone length remained normal in mice treated every 24 and 48 hours, and body composition was maintained. CJC-1295 also stimulated pituitary cell proliferation and GH production. These findings demonstrate that once-daily CJC-1295 treatment can maintain normal growth and body composition in GHRH-deficient mice, with less efficacy when administered every 48 or 72 hours.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00201.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91(3):799-805.
Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults
In two double-blind, placebo-controlled trials, the pharmacokinetic profile, pharmacodynamic effects, and safety of CJC-1295, a long-acting GHRH analog, were assessed in healthy subjects aged 21-61 years. After a single subcutaneous injection of CJC-1295, there were dose-dependent increases in plasma GH concentrations lasting 6 days or more and in plasma IGF-I concentrations for 9-11 days. Multiple doses of CJC-1295 sustained elevated IGF-I levels for up to 28 days. The estimated half-life of CJC-1295 ranged from 5.8 to 8.1 days, and the treatment was safe and generally well-tolerated, especially at doses of 30 or 60 microg/kg, suggesting its potential as a therapeutic agent.
You can read the full article at https://academic.oup.com/jcem/article/91/3/799/2843281?login=false.
Alba M, Fintini D, Sagazio A, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006;291(6):E1290-4.
Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse
In this study, the effects of CJC-1295 administration in mice lacking the GHRH gene (GHRH knockout; GHRHKO) were investigated. CJC-1295, a synthetic GHRH analog with an extended half-life, was administered to three groups of 1-week-old GHRHKO mice at varying intervals. Daily doses of CJC-1295 allowed these mice to maintain normal body weight and length. Treatment every 48 and 72 hours also resulted in increased body weight and length compared to placebo-treated mice, although full growth normalization was not achieved. Additionally, CJC-1295 stimulated pituitary RNA and GH mRNA, indicating somatotroph cell proliferation, as confirmed by immunohistochemistry. This study highlights the effectiveness of once-daily CJC-1295 administration in maintaining normal body composition and growth in GHRHKO mice, with less efficacy observed in less frequent dosing regimens.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00201.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Morselli LL, Nedeltcheva A, Leproult R, et al. Impact of growth hormone replacement therapy on sleep in adult patients with growth hormone deficiency of pituitary origin. European journal of endocrinology/European Federation of Endocrine Societies. 2013;168(5):10.1530/EJE-12-1037. doi:10.1530/EJE-12-1037.
Impact of growth hormone replacement therapy on sleep in adult patients with growth hormone deficiency of pituitary origin
In a single-blind, randomized, crossover study involving 14 adult patients with confirmed or pituitary growth hormone deficiency (GHD), the impact of recombinant human GH (rhGH) therapy versus a placebo on objective sleep quality was investigated. Compared to the placebo period, after 4 months of rhGH treatment, patients experienced a shorter sleep period duration, primarily due to an earlier wake-up time, and a reduction in the intensity of slow-wave sleep (delta activity). These findings suggest that rhGH replacement therapy partially reversed sleep disturbances previously observed in untreated patients, providing further support for the hypothesis that excess high-intensity slow-wave sleep in untreated pituitary GHD patients may result from overactivity of the hypothalamic GHRH system due to the absence of negative feedback inhibition by GH.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3832204/.
Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, Lafranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88(5):2206-12.
Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome
This 12-month study aimed to assess the impact of GH administration on various aspects in children with Prader-Willi syndrome using a randomized, double-blind, placebo-controlled crossover design. GH intervention resulted in improvements in pulmonary function, with increased peak flow rate, vital capacity, and forced expiratory flow rate. While some improvements in sleep, such as reduced apnea events, were observed, GH also led to an increase in hyperactivity. Additionally, it enhanced linear growth velocity, increased resting energy expenditure, and improved body composition by reducing fat mass and percentage of body fat. However, fasting ghrelin concentration remained unaffected by GH administration.
You can read the full article at https://academic.oup.com/jcem/article/88/5/2206/2845458?login=false.
Van cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Horm IGF Res. 2000;10 Suppl B:S57-62.
Interrelationships between growth hormone and sleep
In healthy young adults, the typical 24-hour plasma growth hormone (GH) p0attern involves sustained low levels occasionally punctuated by bursts of secretion. While women typically experience frequent daytime GH pulses, men often have a significant GH pulse associated with sleep onset. Strong evidence indicates a direct link between slow-wave (SW) sleep and heightened GH secretion, with the amount of SW sleep correlating linearly with GH release, regardless of how it’s measured. As individuals age, both SW sleep and GH secretion diminish in a similar chronological fashion. Enhancing SW sleep pharmacologically can lead to increased GH release, suggesting that compounds promoting SW sleep might offer a new avenue for GH stimulation.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637400800118?via%3Dihub.
Copinschi G, Nedeltcheva A, Leproult R, et al. Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiency. J Clin Endocrinol Metab. 2010;95(5):2195-202.
Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiency
In individuals with untreated growth hormone deficiency (GHD), low energy and fatigue are common complaints, potentially linked to sleep quality disruptions due to the established connection between sleep and GH regulation. This study included 30 GHD patients with primary pituitary or hypothalamic defects and 30 gender, age, and BMI-matched healthy controls. GHD patients, regardless of the underlying cause, exhibited poorer sleep quality based on the Pittsburgh Sleep Quality Index and lower scores on the Quality of Life Assessment for GHD in Adults, with tiredness being the most affected aspect. Pituitary GHD patients spent more time in slow-wave sleep (SWS) with higher SWS intensity than controls, while hypothalamic GHD patients had lower SWS intensity. These findings suggest that GHD is associated with sleep disturbances and reduced subjective sleep quality and daytime alertness, potentially contributing to increased tiredness, a significant aspect of quality of life in GHD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2869538/.
Davidson JR, Moldofsky H, Lue FA. Growth hormone and cortisol secretion in relation to sleep and wakefulness. Journal of Psychiatry and Neuroscience. 1991;16(2):96-102.
Growth hormone and cortisol secretion in relation to sleep and wakefulness
This study examined the secretion patterns of growth hormone (GH) and cortisol in relation to sleep and wakefulness in 10 young men. During baseline conditions, GH displayed a normal nocturnal surge during sleep, which disappeared with sleep deprivation and intensified afterward. GH levels were highest during slow-wave sleep (SWS). After sleep deprivation, GH secretion was prolonged, with some subjects experiencing additional GH peaks unrelated to SWS. Cortisol levels across 24 hours remained unchanged with sleep deprivation, but the timing of the nocturnal cortisol rise shifted earlier after sleep deprivation and later after resumed sleep. This suggests that the nocturnal GH surge is primarily influenced by sleep, and although cortisol rhythms are not sleep-dependent, the timing of the cortisol rise may be affected by abrupt changes in sleep-wake patterns.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1188300/.
Moreno-reyes R, Kerkhofs M, L’hermite-balériaux M, Thorner MO, Van cauter E, Copinschi G. Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulation. Am J Physiol. 1998;274(5 Pt 1):E779-84.
Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulation
In this study involving seven healthy young men, the potential sleep-enhancing effects of GH-releasing peptide (GHRP) were explored. When GHRP-2 was administered intravenously during the third rapid-eye-movement period, it led to transient elevations in prolactin and GH levels within the physiological range. However, unlike growth hormone-releasing hormone (GHRH), GHRP-2 did not significantly impact sleep. There were no observable stimulatory effects on slow-wave (SW) sleep, indicating that late-night injections of GHRP-2, despite similar GH elevations, do not enhance SW sleep in contrast to GHRH. This suggests that the GHRP axis is not involved in regulating human SW sleep.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.1998.274.5.E779?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Bes F, Hofman W, Schuur J, Van boxtel C. Effects of delta sleep-inducing peptide on sleep of chronic insomniac patients. A double-blind study. Neuropsychobiology. 1992;26(4):193-7.
Effects of delta sleep-inducing peptide on sleep of chronic insomniac patients. A double-blind study
The impact of delta sleep-inducing peptide (DSIP) on sleep was examined in 16 chronic insomniac patients in a double-blind study. Patients spent five consecutive nights in a laboratory, with the first night for adaptation and the second night for baseline measurements. On the afternoons of the 3rd, 4th, and 5th nights, half of the patients received DSIP intravenously at a dose of 25 nmol/kg body weight, while the other half received a placebo (glucose solution). The results showed that DSIP led to slightly higher sleep efficiency and shorter sleep latency compared to the placebo group, along with a slight reduction in subjectively estimated tiredness within the DSIP group. However, the statistically significant effects were relatively weak, and some could be attributed to chance variations in the placebo group. Since other measures, including subjective sleep quality, remained unchanged, it was concluded that short-term DSIP treatment for chronic insomnia may not provide significant therapeutic benefits.
You can read the abstract of the article at https://karger.com/nps/article-abstract/26/4/193/231036/Effects-of-Delta-Sleep-Inducing-Peptide-on-Sleep?redirectedFrom=fulltext.
Frieboes RM, Murck H, Maier P, Schier T, Holsboer F, Steiger A. Growth hormone-releasing peptide-6 stimulates sleep, growth hormone, ACTH and cortisol release in normal man. Neuroendocrinology. 1995;61(5):584-9.
Growth hormone-releasing peptide-6 stimulates sleep, growth hormone, ACTH and cortisol release in normal man
The synthetic hexapeptide growth hormone-releasing peptide (GHRP-6) and growth hormone-releasing hormone (GHRH) both stimulate growth hormone (GH) release and have effects on sleep. A study compared the impact of repetitive intravenous boluses of GHRP and a placebo on sleep and hormone secretion in normal male controls. After GHRP administration, GH and adrenocorticotropic hormone (ACTH) levels increased during the early night, and cortisol secretion was enhanced throughout the night, particularly during the first half. Stage 2 sleep increased, but slow-wave sleep (SWS) remained unchanged. These findings suggest that GHRP stimulates GH release and hypothalamic-pituitary-adrenocortical hormone secretion, while promoting stage 2 sleep without affecting SWS.
You can read the abstract of the article at https://karger.com/nen/article-abstract/61/5/584/224521/Growth-Hormone-Releasing-Peptide-6-Stimulates?redirectedFrom=fulltext.
Kerkhofs M, Van cauter E, Van onderbergen A, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol. 1993;264(4 Pt 1):E594-8.
Sleep-promoting effects of growth hormone-releasing hormone in normal men
Growth hormone-releasing hormone (GHRH) is known to promote both rapid-eye-movement (REM) and non-REM sleep in animals, and this study aimed to investigate its somnogenic effects in humans. Injections of GHRH in healthy young men were given during various sleep periods. When administered in early sleep without sleep deprivation, GHRH increased REM sleep but didn’t affect slow-wave (SW) sleep. When given during the third REM period after sleep deprivation, it significantly decreased wakefulness and notably increased SW sleep. GHRH was found to have sleep-promoting effects in young adults, especially when administered during periods of decreased sleep propensity.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1993.264.4.E594?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2010;298(1):R147-R156. doi:10.1152/ajpregu.00494.2009.
Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus
We investigated whether growth hormone-releasing hormone (GHRH) could enhance non-rapid eye movement (NREM) sleep by activating GABAergic neurons in the preoptic area. In male Sprague-Dawley rats, intracerebroventricular injections of GHRH were administered, promoting increased NREM sleep. Sleep-deprived rats experienced a rise in double-labeled Fos+GAD cell counts in the median preoptic nucleus (MnPN) and ventrolateral preoptic nucleus (VLPO) after GHRH injection. Intracerebroventricular octreotide (somatostatin analog OCT) and GHRH antagonist reduced NREM sleep, with single c-Fos-labeled cell counts in the MnPN increasing with OCT. These results suggest that GHRH affects NREM sleep by targeting GABAergic neurons in the MnPN and VLPO.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2806209/.
Obal F, Jr, Krueger JM. GHRH and sleep. Sleep Med Rev 8: 367– 377, 2004.
GHRH and sleep
A significant portion of daily growth hormone (GH) secretion occurs during deep non-REM sleep (NREMS), which is stimulated by the hypothalamic hormone GH-releasing hormone (GHRH). Exogenous GHRH promotes NREMS in various species, while suppressing endogenous GHRH leads to a simultaneous inhibition of NREMS. Mutant and transgenic animals with GHRHergic defects exhibit permanently reduced NREMS, not reversible with GH supplementation. Hypothalamic GHRH levels correlate with sleep-wake activity and sleep-related changes. GABAergic neurons in the anterior hypothalamus/preoptic region may mediate GHRH’s promotion of NREMS, while GH mediates the stimulation of REM sleep. Simultaneous stimulation of NREMS and GH secretion by GHRH may help adjust tissue anabolism during sleep.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S1087079204000279?via%3Dihub.
Künzel H, Held K, Schmidt D, Ziegenbein M, Murck H, Steiger A. Sleep-endocrine effects of growth hormone-releasing hormone (GHRH) in patients with schizophrenia. J Psychiatr Res. 2018;101:1-4.
Sleep-endocrine effects of growth hormone-releasing hormone (GHRH) in patients with schizophrenia
Changes in sleep-EEG patterns in patients with schizophrenia after endocrine stimulation tests include reduced sleep efficiency, longer sleep latency, and increased awakenings after sleep onset, with ambiguous findings regarding sleep-associated growth hormone (GH) secretion. This study aimed to clarify sleep-endocrine activity, particularly in the GH system, in schizophrenia patients following repeated GHRH administration. Nine patients underwent four injections of 50 μg GHRH between 22:00 and 01:00 h after a week without medication. Results showed a non-significant increase in sleep onset latency and wakefulness compared to controls, along with a significant reduction in sleep stage 2. No significant differences were observed in ACTH and cortisol, but GH secretion in patients after GHRH stimulation was significantly higher than in controls. These findings suggest differences in the regulatory sensitivity of the GH system between daytime and nighttime in drug-free patients with schizophrenia.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0022395617310622?via%3Dihub.
Ehlers C, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology 42: 467– 474, 1986.
Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats
The impact of intracerebroventricular (ICV) administration of corticotropin-releasing factor (CRF) and growth hormone-releasing factor (GRF) on electroencephalographic (EEG) and behavioral sleep-wake patterns was examined in adult male rats. CRF (0.0015-0.015 nmol) was found to reduce slow-wave sleep while decreasing spectral power in lower frequencies (1-6 Hz) and increasing power in higher frequencies (32-64 Hz). Conversely, GRF (2.0 nmol) increased EEG and behavioral indicators of slow-wave sleep, associated with amplified spectral power in lower frequencies (1-2 Hz) and reduced power in higher frequencies (32-64 Hz). Additionally, ICV administration of GRF led to decreased locomotion when given during the active phase of the circadian cycle. These findings align with known circadian patterns of corticosteroid and growth hormone release during the sleep-wake cycle in both rats and humans.
You can read the full article at https://karger.com/nen/article-abstract/42/6/467/222711/Effects-of-Corticotropin-Releasing-Factor-and?redirectedFrom=fulltext.
Nistico G, DeSarro GB, Bagetta G, Müller EE. Behavioural and electrocortical spectrum power effects of growth hormone releasing factor in rats. Neuropharmacology 26: 75– 78, 1987.
Behavioural and electrocortical spectrum power effects of growth hormone releasing factor in rats
The study examined the impact of human pancreatic growth hormone-releasing Factor-40 (hpGRF) injections (10-100 ng) delivered into different brain regions (intracerebroventricular, intrahippocampal, and intracaudate) on behavior and electrocortical spectrum power in rats. When administered into the third cerebral ventricle or dorsal hippocampus (50-100 ng), hpGRF induced behavioral sedation accompanied by electrocortical synchronization, increasing total voltage power with a predominant boost in lower frequency bands. Conversely, unilateral injection of hpGRF (75 ng) into the head of the caudate nucleus heightened locomotor activity, triggered postural changes, contralateral circling episodes, and intense stereotyped movements. These results suggest that, apart from its known endocrinological effects, hpGRF in small doses possesses notable behavioral and electrocortical actions, the precise mechanisms of which require further investigation.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/3104815/.
Obal F, Jr, Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 255: R310– R316, 1988.
Growth hormone-releasing factor enhances sleep in rats and rabbits
In previous research, it was proposed that a hypothalamic mechanism connects the secretion of somatotropin (growth hormone or GH) to sleep regulation, potentially explaining the temporal correlation between GH release and nonrapid eye movement sleep (NREMS) at sleep onset. This study aimed to investigate whether growth hormone-releasing factor (GRF), a hypothalamic peptide responsible for stimulating GH secretion, could promote sleep in rats and rabbits. Rats received intracerebroventricular injections of artificial cerebrospinal fluid or GRF (human GRF-[1-40]) at varying doses, while rabbits received the same doses of GRF. The results showed that GRF promoted both NREMS and rapid eye movement sleep (REMS), increasing EEG slow-wave activity in both rats and rabbits. These findings suggest that GRF has sleep-promoting effects and may play a role in linking GH secretion and sleep regulation.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpregu.1988.255.2.R310?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Kerkhofs M, Van CE, Van OA, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol Endocrinol Metab 264: E594– E598, 1993.
Sleep-promoting effects of growth hormone-releasing hormone in normal men
In this study, researchers explored the potential sleep-promoting effects of growth hormone-releasing hormone (GHRH) in healthy young men. They administered GHRH via intravenous bolus injections at various times during the sleep-wake cycle, including early sleep, late sleep, and early sleep after sleep deprivation. The results indicated that GHRH had sleep-promoting effects, increasing rapid-eye-movement (REM) sleep and non-REM sleep (slow-wave sleep or SW sleep) in young adults, especially when administered during periods of reduced sleep readiness.
You can read the abstract of the article at https://journals.physiology.org/doi/abs/10.1152/ajpendo.1993.264.4.E594?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Marshall L, Molle M, Boschen G, Steiger A, Fehm HL, Born J. Greater efficacy of episodic than continuous growth hormone-releasing hormone (GHRH) administration in promoting slow-wave sleep (SWS). J Clin Endocrinol Metab 81: 1009– 1013, 1996.
Greater efficacy of episodic than continuous growth hormone-releasing hormone (GHRH) administration in promoting slow-wave sleep (SWS)
In this study involving healthy volunteers, researchers compared the effects of growth hormone-releasing hormone (GHRH) administration on nocturnal sleep using two different methods: episodic (four boluses of 50 micrograms each at 2200, 2300, 2400, and 0100 h) and continuous infusion (57 micrograms/h between 2130 and 0100 h). They found that episodic GHRH administration significantly increased the time spent in stage 4 of slow-wave sleep (SWS) compared to continuous infusion (P < 0.01). Episodic GHRH administration also enhanced SWS and rapid eye movement (REM) sleep while reducing wakefulness and sleep stage 1 compared to a placebo condition (P < 0.05). Both methods of GHRH administration increased plasma GH concentrations (P < 0.01), with episodic administration showing slightly greater efficacy in promoting sleep (P < 0.05). These results suggest that episodic GHRH stimulation has a more significant impact on sleep than continuous infusion.
You can read the abstract of the article at https://academic.oup.com/jcem/article/81/3/1009/2649589?login=false.
Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology 56: 566– 573, 1992.
Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls
In this study involving 7 male controls, the effects of intravenous administration of growth hormone-releasing hormone (GHRH) and somatostatin (SRIF) on sleep were compared using polysomnography. Four boluses of placebo, 50 micrograms GHRH, or 50 micrograms SRIF were given at 22.00, 23.00, 24.00, and 1.00 h. GHRH significantly increased plasma growth hormone (GH) concentration throughout the night, while SRIF had no substantial impact on GH release. GHRH also reduced nocturnal cortisol secretion, whereas SRIF had no effect. Quantitative sleep EEG staging revealed a significant increase in slow-wave sleep (SWS) after GHRH administration but no change after SRIF. Additionally, SRIF showed a trend toward increased REM density. These findings suggest an interaction between central GHRH and corticotropin-releasing hormone in the neuroregulation of human sleep, with opposite effects on SWS, GH, and cortisol secretion.
You can read the abstract of the article at https://karger.com/nen/article-abstract/56/4/566/223844/Effects-of-Growth-Hormone-Releasing-Hormone-and?redirectedFrom=fulltext.
Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest 47: 2079– 2090, 1968.
Growth hormone secretion during sleep
During this study involving eight young adults, plasma growth hormone (GH), insulin, cortisol, and glucose levels were monitored throughout the night on 38 occasions. Blood samples were collected at 30-minute intervals while EEG and electrooculogram recordings were made. In seven subjects, a substantial GH peak lasting 1.5-3.5 hours coincided with the onset of deep sleep, with occasional smaller GH peaks occurring during subsequent deep sleep phases. The timing of the peak GH secretion was delayed when sleep onset was delayed. In cases where subjects were awakened for 2-3 hours and then allowed to return to sleep, another peak of GH secretion was observed. Interestingly, this GH secretion pattern was not correlated with changes in plasma glucose, insulin, or cortisol levels. The study also explored the effects of various CNS-active drugs on sleep-related GH secretion, with imipramine eliminating GH peaks in two out of four subjects, while other drugs like chlorpromazine, phenobarbital, diphenylhydantoin, chlordiazepoxide, and isocarboxazid did not inhibit GH peaks. These results suggest that GH secretion during the onset of sleep is influenced by altered hypothalamic activity and is unrelated to hypoglycemia or changes in cortisol and insulin secretion.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC297368/.
Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98: 562– 570, 1976.
Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat
Sequential blood samples were obtained from undisturbed freely-behaving male rats bearing chronic intracardiac venous cannulae. Blood was withdrawn every 15 min for periods of 4-24 h; plasma was separated, and saline-resuspended red cells were reinjected. Plasma GH was determined by radioimmunoassay. Pulsatile GH secretion was evident in each animal with most peak values greater than 200 ng/ml and most trough values less than ng/ml. The GH secretory episodes occurred at approximately 3 h intervals, and this rhythmic pattern of GH secretion persisted unchanged across all phases of a 12-h light-dark (L-D) cycle. Seven major episodes of GH secretion were observed during a single 24-h period. The mean period, or time interval between episodes, in 24 animals was 3.32 +/- 0.07 (SEM)h. The timing of the pulses with respect to the L-D cycle was similar in most animals, indicating that the rhythm may be entrained to the L-D cycle. The role of environmental lighting was further assessed in 14 animals exposed to constant light for 7 weeks. The results show that the basic rhythm was unchanged (mean period 3.18 +/- 0.06 h, peaks greater than 200 ng/ml, troughs less than 1 ng/ml), although entrainment to time of day was not evident. Subsequent exposure to the 12-h L-D cycle resulted in reversion to an entrained rhythm. These results suggest 1) that GH secretion in the rat is governed by an endogenous ultradian rhythm, with a periodicity of approximately 3.3 h, and 2) that the alternation of light and darkness probably serves as a Zeitgeber which sets the biological “clock” for GH secretion, but is not necessary for maintenance of the basic rhythm.
You can read the abstract of the article at https://academic.oup.com/endo/article-abstract/98/3/562/2619430?redirectedFrom=fulltext&login=false.
Kimura F, Tsai CW. Ultradian rhythm of growth hormone secretion and sleep in the adult male rat. J Physiol (Lond) 353: 305– 315, 1984.
Ultradian rhythm of growth hormone secretion and sleep in the adult male rat
In this study involving adult male rats, the relationship between growth hormone (GH) secretion and the sleep-wakefulness cycle was examined through serial blood sampling at 10-minute intervals, alongside continuous electroencephalogram (EEG) recordings. The amount of sleep within each 10-minute interval was plotted against the corresponding GH values measured by radioimmunoassay. Analysis of GH concentration patterns in control rats revealed a mean periodicity of 2.93 +/- 0.10 hours from 00.00 to 12.00 h in five rats and 2.85 +/- 0.06 hours from 12.00 to 24.00 h in eight rats. When EEG recordings were synchronized with blood sampling from 12.00 to 24.00 h, cross-correlation analysis demonstrated a significant positive correlation between sleep and GH levels, with short time lags (0-10 min) in six out of fourteen rats and longer time lags (20-50 min) in four. Interestingly, most rats exhibited a consistent relationship, with GH peaks consistently occurring approximately 40 to 70 minutes following the onset of the sleep cycle. Furthermore, sleep deprivation conducted from 13.00 to 16.00 h effectively prevented the occurrence of high-level GH pulses that would typically appear during this time of day. Corticosterone concentrations measured concurrently during the sleep-deprivation experiment remained unaffected by sleep deprivation. These findings suggest that GH secretion in adult rats is correlated with the sleep-wakefulness cycle, although the pattern differs somewhat from that observed in immature rats.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1193308/.
Mitsugi N, Kimura F. Simultaneous determination of blood levels of corticosterone and growth hormone in the male rat: relation to sleep-wakefulness cycle. Neuroendocrinology 41: 125– 130, 1985.
Simultaneous determination of blood levels of corticosterone and growth hormone in the male rat: relation to sleep-wakefulness cycle
In this study involving adult male rats, the temporal relationship between the secretion of corticosterone (CS) and growth hormone (GH) and the sleep-wakefulness cycle was investigated through 10-minute interval blood sampling over an 11-hour period from 11.00 to 22.00 h. Concurrently, cortical EEGs were continuously recorded and categorized into wakefulness and sleep, with the amounts of sleep plotted against CS and GH values. The results revealed that all 11-hour time series of CS, GH, and sleep exhibited three major ultradian rhythms with periodicities of 1.5 hours and its multiples. Notably, a significant negative cross-correlation was observed between the amounts of sleep and CS secretion. While the most prominent CS rhythm had a 1.5-hour period, CS secretion with a 3.0-hour period was synchronized with the sleep rhythm, beginning during the latter stage of the sleep cycle and peaking around the transition from sleep to wakefulness between cycles. In contrast, GH secretory bursts occurred early in the sleep cycle, also following a 3.0-hour period but with a distinct time lag after the onset of the sleep cycle. This study highlights the presence of common ultradian rhythms with similar periodicities in CS, GH secretion, and the sleep-wakefulness cycle, each regulated by its own pacemaker and maintaining specific phase relationships with one another.
You can read the abstract of the article at https://karger.com/nen/article-abstract/41/2/125/222507/Simultaneous-Determination-of-Blood-Levels-of?redirectedFrom=fulltext.
Bruhn TO, Anthony EL, Wu P, Jackson IM. GRF immunoreactive neurons in the paraventricular nucleus of the rat: an immunohistochemical study with monoclonal and polyclonal antibodies. Brain Res 424: 290– 298, 1987.
GRF immunoreactive neurons in the paraventricular nucleus of the rat: an immunohistochemical study with monoclonal and polyclonal antibodies
Our study reveals the presence of a intricate growth hormone-releasing factor (GRF) neuronal network in the rat hypothalamus. Utilizing both high-affinity polyclonal and highly specific monoclonal antibodies targeted at rat GRF, we have confirmed the existence of immunoreactive GRF (GRF-i) cell bodies in the parvocellular region of the paraventricular nucleus. Additionally, we observed GRF-i neuronal terminals in various hypothalamic areas, including the lateral arcuate nucleus, lateral hypothalamus, perifornical area, dorsomedial nucleus, external zone of the median eminence, periventricular nucleus, suprachiasmatic nucleus, and ventral premammillary nucleus.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0006899387914739?via%3Dihub.
Steiger A. Sleep and endocrine regulation. Front Biosci 8: s358– s376, 2003.
Sleep and endocrine regulation
A bidirectional interaction between sleep electroencephalogram (EEG) and endocrine activity is observed across various species, including humans. Various hormones, including peptides and steroids, play roles in regulating sleep. Notably, the reciprocal interplay between sleep-promoting growth hormone-releasing hormone (GHRH) and sleep-disrupting corticotropin-releasing hormone (CRH) is crucial, with changes in the GHRH:CRH ratio impacting sleep-endocrine activity. Altered ratios favoring CRH are associated with sleep disturbances in aging and depression. Other factors such as ghrelin, galanin, somatostatin, and neuropeptide Y (NPY) influence sleep, with NPY acting as a CRH antagonist. Prolactin enhances rapid eye movement sleep (REMS), while vasocactive intestinal polypeptide (VIP) affects the temporal organization of sleep cycles. Neuroactive steroids like pregnenolone, progesterone, allopregnanolone, and dehydroepiandrosterone modulate sleep EEG through GABAA receptors. Cortisol appears to promote REMS, and gonadal hormones also contribute to sleep regulation, with clinical applications seen in estrogen replacement therapy and CRH-1 receptor antagonism for depression.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/12700062/.
Zhang J, Obal F, Jr, Zheng T, Fang J, Taishi P, Krueger JM. Intrapreoptic microinjection of GHRH or its antagonist alters sleep in rats. J Neurosci 19: 2187– 2194, 1999.
Intrapreoptic microinjection of GHRH or its antagonist alters sleep in rats
Prior findings suggest that growth hormone-releasing hormone (GHRH) plays a role in regulating sleep, particularly nonrapid eye movement sleep (NREMS), independently of the pituitary. In experiments with rats, microinjections of GHRH into the preoptic area increased NREMS duration and intensity in a dose-dependent manner, while a GHRH antagonist decreased NREMS and prolonged sleep latency. These effects were not observed in rapid eye movement sleep (REMS) or brain temperature. Additionally, the antagonist mitigated the NREMS enhancements caused by sleep deprivation. The study concluded that the preoptic area is the site where GHRH exerts its sleep-promoting effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6782549/.
Baker LD, Barsness SM, Borson S, et al. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69(11):1420-9.
Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial
This study investigated the impact of tesamorelin, a stabilized analog of human growth hormone-releasing hormone (GHRH), on cognitive function in 152 older adults, including 66 with mild cognitive impairment (MCI). Administered via daily subcutaneous injections for 20 weeks, the treatment showed favorable cognitive effects (P=.03) that were consistent between adults with MCI and healthy older adults. The completer analysis further supported these findings (P=.002), with GHRH positively influencing executive function (P=.005) and exhibiting a trend towards improving verbal memory (P=.08). Additionally, GHRH treatment increased insulin-like growth factor 1 levels and reduced body fat. Mild adverse events were reported in 68% of GHRH-treated adults and 36% of the placebo group. Further, longer-duration trials are warranted to explore the therapeutic potential of GHRH in aging and Alzheimer’s disease.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764914/.
Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006;27(2):318-23.
Growth hormone releasing hormone improves the cognition of healthy older adults
The study investigated the impact of six months of daily growth hormone releasing hormone (GHRH) treatment on the cognitive function of 89 healthy older adults. GHRH demonstrated significant improvements in various cognitive measures, including WAIS-R performance IQ, picture arrangement, finding A’s, verbal sets, and single-dual task performance. These improvements were consistent across gender, estrogen status, and baseline cognitive capacity, suggesting that the decline in the somatotrophic axis with age may be linked to cognitive decline, and GHRH supplementation could potentially mitigate such declines in both healthy older adults and those with cognitive impairments like mild cognitive impairment and Alzheimer’s disease.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0197458005000631?via%3Dihub.
Friedman SD, Baker LD, Borson S, et al. Growth Hormone–Releasing Hormone Effects on Brain γ-Aminobutyric Acid Levels in Mild Cognitive Impairment and Healthy Aging. JAMA neurology. 2013;70(7):883-890. doi:10.1001/jamaneurol.2013.1425.
Growth Hormone–Releasing Hormone Effects on Brain γ-Aminobutyric Acid Levels in Mild Cognitive Impairment and Healthy Aging
This study explored the impact of growth hormone-releasing hormone (GHRH) on brain neurochemistry in a subgroup of participants from a larger trial. Over 20 weeks, GHRH administration increased gamma-aminobutyric acid (GABA) levels in all three brain regions studied, raised N-acetylaspartylglutamate (NAAG) levels in the frontal cortex, and lowered myo-inositol (MI) levels in the posterior cingulate. These effects were consistent in adults with mild cognitive impairment (MCI) and those with normal cognitive function. While no significant correlations were found between neurochemical changes and cognitive outcomes, this research offers preliminary evidence that GHRH may influence cognition by modulating inhibitory neurotransmitter and brain metabolite levels.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764915/.
Baker LD, Vitiello MV. Growth hormone-releasing hormone improves cognitive function in older adults: sleep on it–reply. JAMA Neurol. 2013;70(4):529-30.
Growth hormone-releasing hormone improves cognitive function in older adults: sleep on it–reply
We found the study by Baker and colleagues1 on the cognitive effects of 20 weeks of subcutaneous growth hormone-releasing hormone (GHRH) administration in adults with mild cognitive impairment and healthy older adults intriguing. While it demonstrates the potential of GHRH in mitigating age-related cognitive decline, the underlying mechanism warrants further exploration. Age-related cognitive deficits often coincide with a decrease in slow-wave sleep (SWS) and somatotropic axis activity. SWS plays a crucial role in memory formation, and pharmacological enhancement of SWS has been linked to improved cognitive functions. Given that exogenous GHRH promotes sleep, particularly SWS, it’s plausible that the observed cognitive benefits of GHRH were related to improved sleep patterns. Notably, the study relied on self-reported sleep quality rather than objective sleep measures like polysomnography, leaving room for further investigation into the connection between GHRH, sleep, and cognition.
You can read the abstract of the article at https://jamanetwork.com/journals/jamaneurology/article-abstract/1676654.
Available at https://www.alzforum.org/news/research-news/growth-hormone-releasing-hormone-slows-cognitive-decline-mci.
Growth hormone-releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006 Feb;27(2):318-23.
Growth hormone-releasing hormone improves the cognition of healthy older adults
Age-related declines in the somatotrophic axis activity are associated with various physiological changes, including cognitive function. This study investigated the impact of six months of daily growth hormone releasing hormone (GHRH) treatment on 89 healthy older adults’ cognition. GHRH significantly improved performance in multiple cognitive measures, including WAIS-R performance IQ, picture arrangement, finding A’s, verbal sets, and single-dual task, with these improvements unaffected by gender, estrogen status, or baseline cognitive ability. These findings suggest that the age-related decline in the somatotrophic axis may contribute to cognitive decline and that GHRH supplementation could potentially mitigate such declines in healthy older adults and those with cognitive impairments like mild cognitive impairment and Alzheimer’s disease.
You can read the full article at https://linkinghub.elsevier.com/retrieve/pii/S0197-4580(05)00063-1.
Dou Y, Zhang Q, Zhang X, Dong JY, Tang JJ, et al. (2009) Effect of different immunomodulation on inflammatory response in burn rats with sepsis. Zhonghua Shao Shang Za Zhi CHN 25: 275–280.
Effect of different immunomodulation on inflammatory response in burn rats with sepsis
The study aimed to explore the impact of Thymosin and growth hormone (GH) on inflammatory responses in burn rats and burn rats with sepsis. Rats were divided into various groups based on treatments, and measurements were taken on factors related to inflammation. The results showed that both Thymosin and GH had similar inhibitory effects on extensive inflammatory reactions, regardless of the presence of trauma. However, GH demonstrated a superior effect compared to Thymosin in the presence of trauma. This suggests that GH may be more effective in modulating inflammatory responses, particularly in the context of traumatic injuries.
You can read the abstract of the article article at https://rs.yiigle.com/cmaid/53353.
Isgaard J, Aberg D, Nilsson M (2007) Protective and regenerative effects of the GH/IGF-I axis on the brain. Minerva Endocrinol ITA 32: 103–113.
Protective and regenerative effects of the GH/IGF-I axis on the brain
In addition to its role in regulating somatic growth and metabolism, there is growing evidence of the GH/IGF-I axis’s involvement in brain growth, development, and myelination. Both growth hormone (GH) and IGF-I have been linked to neuroprotective effects in various experimental models. These effects extend to cognition and brain biochemistry in adults, with some mediated by circulating IGF-I and others potentially resulting from locally produced IGF-I in the brain. It’s also possible that GH may directly impact the central nervous system (CNS) independently of IGF-I. These factors influence the functional dynamics between neurons, astrocytes, and oligodendrocytes, with GH and IGF-I affecting these cell types in various ways. Besides neuroprotection, GH and IGF-I have shown promise in promoting brain regeneration and plasticity, with specific effects on cell proliferation, oligodendrogenesis, and vascular density in different brain regions. Additionally, this review will delve into recent findings concerning neuroprotective effects and their impact on synaptic plasticity by GH secretagogues.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/17557036/.
Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006;27(2):318–323.
Growth hormone releasing hormone improves the cognition of healthy older adults
The study investigated the impact of 6 months of daily growth hormone releasing hormone (GHRH) treatment on the cognition of 89 healthy older adults (average age 68.0+/-0.7). GHRH significantly improved performance in cognitive tests, including WAIS-R performance IQ, picture arrangement, finding A’s, verbal sets, and single-dual task, with these improvements unaffected by gender, estrogen status, or baseline cognitive capacity. These findings suggest that the age-related decline in the somatotrophic axis may be linked to cognitive decline, and GHRH supplementation could potentially alleviate such cognitive declines in both healthy older adults and individuals with impaired cognitive function, such as mild cognitive impairment and Alzheimer’s disease.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0197458005000631?via%3Dihub.
Zhang H, Han M, Zhang X, Sun X, Ling F. The Effect and Mechanism of Growth Hormone Replacement on Cognitive Function in Rats with Traumatic Brain Injury. Ferreira ST, ed. PLoS ONE. 2014;9(9):e108518. doi:10.1371/journal.pone.0108518.
The Effect and Mechanism of Growth Hormone Replacement on Cognitive Function in Rats with Traumatic Brain Injury
The study aimed to assess the impact of growth hormone (GH) on cognitive dysfunction using a controlled cortical impact (CCI) rat model and investigate the underlying mechanisms. Three-month-old male SD rats were divided into different groups, and cognitive function was evaluated using tests. Eight weeks post-injury, CCI rats displayed cognitive dysfunction regardless of GH levels, but treatment with recombinant human GH (rhGH) improved cognitive function, especially in GH-deficient rats. The study also found correlations between the expression of certain brain-related genes like BDNF, TrkB, and SYN mRNA and cognitive test scores in various brain regions, suggesting that rhGH therapy may enhance cognitive function through these gene expressions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4182486/.
Pathipati P, Surus A, Williams CE, Scheepens A (2009) Delayed and chronic treatment with growth hormone after endothelin-induced stroke in the adult rat. Behav Brain Res NED 204: 93–101.
Delayed and chronic treatment with growth hormone after endothelin-induced stroke in the adult rat
In this study, researchers explored the effects of a neurorestorative treatment involving prolonged central delivery of growth hormone (GH) initiated four days after a stroke. They developed a buffer to maintain GH activity over two weeks at body temperature and used implanted minipumps to continuously deliver GH into the lateral ventricle of stroke-injured rats. The study involved a dose-ranging pilot study to understand GH distribution and neuroendocrine effects, followed by a six-week treatment trial starting four days post-stroke, with a six-week recovery period. GH localized to specific brain regions, and the treatment led to accelerated recovery in motor function and improved spatial memory, without affecting learning. Additionally, GH treatment temporarily increased body weight while halving circulating IGF-1 levels. This suggests that delayed and chronic central GH treatment may enhance certain aspects of functional recovery and long-term spatial memory after a stroke.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0166432809003374?via%3Dihub.
Ying XG, Wu BC, Zhang YM, Shao JH (2004) Recombinant human growth hormone on acute effects of coronary collateral circulation in rats with myocardial infarction. J Clin Cardiovascu Dis CHN 10: 613–615.
Recombinant human growth hormone on acute effects of coronary collateral circulation in rats with myocardial infarction
This study explored a novel approach for delivering recombinant human growth hormone (rhGH) using genetically modified bioartificial muscles (BAMs) and examined its effects on left ventricular (LV) function in rats with chronic heart failure (CHF). Primary rat myoblasts were engineered to secrete rhGH and incorporated into BAMs. When implanted in rats with CHF, these GH-secreting BAMs improved LV ejection fraction (EF), fractional shortening (FS), and reduced LV end-diastolic dimension (LVEDD) compared to rats receiving non-rhGH-secreting BAMs. The treatment also elevated serum GH and insulin-like growth factor-1 (IGF-1) levels while decreasing TNF-alpha, suggesting that rhGH delivery through genetically modified bioartificial muscles could effectively enhance LV function and prevent cardiac remodeling in CHF rats.
You can read the full article at https://journals.lww.com/cmj/fulltext/2009/10010/recombinant_human_growth_hormone_secreted_from.29.aspx.
Christophidis LJ, Gorba T, Gustavsson M, Williams CE, Werther GA, et al. (2009) Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: a potential role for growth hormone in injury-induced neurogenesis. Growth Horm Igf Res SCO 19: 497–506.
Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: a potential role for growth hormone in injury-induced neurogenesis
In the context of recovery from ischemic brain injury, researchers investigated the activation of a cerebral growth hormone (GH) axis and its potential role in neuro-restorative processes. They examined GH receptor (GH-R) immunoreactivity within the subventricular zone (SVZ) of juvenile rats after brain injury and assessed GH’s impact on the proliferation of embryonic mouse neural stem cells. The study found increased GH-R immunoreactivity in the ipsilateral SVZ, coinciding with injury-induced neurogenesis, and identified proliferating cells, neural progenitor cells, and migratory neuroblasts among GH-R immunopositive cells. Stimulation of neural stem cells with GH led to a dose-dependent proliferative response. These findings suggest that GH and its receptor play a novel role in injury-induced neurogenesis, potentially enhancing endogenous neuro-restorative processes post-brain injury.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S109663740900080X?via%3Dihub
Koutkia P, Canavan B, Breu J, Grinspoon S. Effects of growth hormone-releasing hormone on bone turnover in human immunodeficiency virus-infected men with fat accumulation. J Clin Endocrinol Metab. 2005;90(4):2154-60.
Effects of growth hormone-releasing hormone on bone turnover in human immunodeficiency virus-infected men with fat accumulation
Growth hormone-releasing hormone (GHRH) was investigated as a potential strategy to simultaneously address fat distribution and bone turnover in HIV-infected individuals with abdominal fat accumulation. In a randomized, double-blind, placebo-controlled study involving 31 HIV-infected men, GHRH treatment over 12 weeks showed significant effects on fat distribution and bone metabolism. Specifically, GHRH correlated with markers of bone turnover, including N-terminal telopeptide (NTx) and C-terminal telopeptide (CTx). Notably, GHRH led to increased bone resorption markers (CTx and NTx) and bone formation markers (N-terminal propeptide of type 1 procollagen and osteocalcin), indicating improvements in bone metabolism. These findings suggest that GHRH can positively influence both fat distribution and bone health in HIV-infected individuals with fat accumulation, with the potential for long-term benefits on bone density.
You can read the full article at https://academic.oup.com/jcem/article/90/4/2154/2836791?login=false.
Clemmesen B, Overgaard K, Riis B, Christiansen C. Human growth hormone and growth hormone releasing hormone: a double-masked, placebo-controlled study of their effects on bone metabolism in elderly women. Osteoporos Int. 1993;3(6):330-6.
Human growth hormone and growth hormone releasing hormone: a double-masked, placebo-controlled study of their effects on bone metabolism in elderly women
In a 12-week study involving 42 postmenopausal women with reduced bone mass, three groups received either human growth hormone, growth hormone releasing hormone, or a placebo. The group treated with growth hormone experienced significant increases in biochemical markers related to bone formation and resorption, while the other groups showed no changes. Upon discontinuation of therapy, the bone markers declined but did not return to baseline levels. However, bone density in the forearm, spine, and proximal femur remained unchanged in all groups. This suggests that growth hormone treatment stimulates bone metabolism in elderly postmenopausal women with reduced bone mass.
You can read the abstract of the article at https://link.springer.com/article/10.1007/BF01637319.
Holloway L, Butterfield G, Hintz RL, Gesundheit N, Marcus R. Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women. J Clin Endocrinol Metab. 1994;79:470–479. doi: 10.1210/jcem.79.2.7519191.
Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women
In a controlled trial involving 27 healthy elderly women, with 8 taking replacement estrogen, recombinant human GH (rhGH) was administered via daily injections. Initially, a daily dose of 0.043 mg/kg BW was given, but side effects necessitated 50% dose reductions for most participants. After 6 months, 13 women in the rhGH group and 14 in the placebo group completed the study. RhGH significantly increased insulin-like growth factor-I (IGF-I) and IGF-I-binding protein-3 (IGFBP-3) levels, with greater effects in women not receiving estrogen. It also led to an 11% reduction in fat mass and a 9% decrease in percent fat. Bone turnover markers were significantly elevated with rhGH, especially in women not on estrogen. Other metabolic parameters showed minimal changes.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/79/2/470/2650681?redirectedFrom=fulltext&login=false.
Kruse HP, Kuhlencordt F. On an attempt to treat primary and secondary osteoporosis with human growth hormone. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme. 1975;7:488–491. doi: 10.1055/s-0028-1093710.
On an attempt to treat primary and secondary osteoporosis with human growth hormone
Three male patients suffering from severe osteoporosis, with one having primary osteoporosis and the other two having osteogenesis imperfecta, received human growth hormone treatment for 8 to 15 months at daily doses ranging from 1.45 to 2.3 mg. Clinical and calcium kinetic data did not reveal significant treatment effects, but histomorphometry of bone biopsies unmistakably showed changes. These changes included increased periosteal new bone formation and intracortical bone resorption, along with a notable rise in the relative activity of osteoblasts on endosteal surfaces.
You can read the abstract of the article at https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0028-1093710.
Brixen K, Kassem M, Nielsen HK, Loft AG, Flyvbjerg A, Mosekilde L. Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study. J Bone Miner Res Off J Am Soc Bone Miner Res. 1995;10:1865–1874. doi: 10.1002/jbmr.5650101205.
Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study
In a study investigating the potential use of growth hormone (GH) in the treatment of postmenopausal osteoporosis, 40 postmenopausal women with osteopenia (ages 52-73 years) were treated with GH (0.05, 0.10, or 0.20 IU/kg/day) or a placebo for 7 days. GH treatment led to dose-dependent increases in serum osteocalcin, C-terminal type-I procollagen propeptide, and various bone turnover markers, including type-I collagen telopeptide and urinary hydroxyproline/creatinine. These effects were transient, lasting 1-2 weeks, except for serum osteocalcin in the highest dose group, which showed a somewhat longer-lasting effect. GH also elevated serum levels of IGF-I, insulin, and tri-iodothyronin, with no impact on other calciotropic hormones. Adverse effects, mainly fluid retention, were dose-dependent and reversible. In summary, short-term GH treatment stimulated both bone formation and resorption in postmenopausal women with osteopenia.
You can read the abstract of the article at https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.5650101205.
Clemmesen B, Overgaard K, Riis B, Christiansen C. Human growth hormone and growth hormone releasing hormone: a double-masked, placebo-controlled study of their effects on bone metabolism in elderly women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 1993;3:330–336.
Human growth hormone and growth hormone releasing hormone: a double-masked, placebo-controlled study of their effects on bone metabolism in elderly women
In a 12-week study involving 42 postmenopausal women with reduced bone mass, we administered human growth hormone, growth hormone releasing hormone, or a placebo. We assessed bone density and biochemical markers before, during, and 4 weeks after treatment cessation. Notably, the group receiving growth hormone exhibited significant increases in bone formation and resorption markers, while the other groups remained unchanged. Upon treatment withdrawal, the bone markers decreased but did not return to baseline levels. Surprisingly, bone density in the forearm, spine, and proximal femur remained unaffected across all groups. In summary, our findings indicate that growth hormone treatment stimulates bone metabolism in elderly postmenopausal women with decreased bone mass
You can read the abstract of the article at https://link.springer.com/article/10.1007/BF01637319.
Erdtsieck RJ, Pols HA, Valk NK, van Ouwerkerk BM, Lamberts SW, Mulder P, Birkenhäger JC. Treatment of post-menopausal osteoporosis with a combination of growth hormone and pamidronate: a placebo controlled trial. Clin Endocrinol (Oxf) 1995;43:557–565.
Treatment of post-menopausal osteoporosis with a combination of growth hormone and pamidronate: a placebo controlled trial
In this study, we investigated the impact of recombinant human GH (rhGH) on bone mineral mass and turnover in post-menopausal osteoporotic women receiving the bone resorption inhibiting agent, pamidronate, over a 12-month period. The rhGH group exhibited a significant increase in serum IGF-I levels but showed no changes in bone mineral mass at the lumbar spine or forearm, unlike the pamidronate-treated group, which displayed a consistent increase in bone mass at the lumbar spine and forearm after 6 months. However, no changes were observed at the femoral neck or forearm in either group. Biochemical markers of bone turnover decreased by approximately 50% in the pamidronate group, but this effect was attenuated in the rhGH-treated group. While rhGH had a temporary impact on body composition, decreasing fat mass by about 5% and increasing lean body mass by about 3%, these effects disappeared after rhGH treatment cessation. In summary, this study suggests that rhGH treatment blunted the effects of pamidronate on bone mass accumulation and bone turnover markers, and the positive impact on body composition was not sustained after rhGH treatment discontinuation.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2265.1995.tb02920.x?sid=nlm%3Apubmed.
Holloway L, Kohlmeier L, Kent K, Marcus R. Skeletal effects of cyclic recombinant human growth hormone and salmon calcitonin in osteopenic postmenopausal women. J Clin Endocrinol Metab. 1997;82:1111–1117. doi: 10.1210/jcem.82.4.3901.
Skeletal effects of cyclic recombinant human growth hormone and salmon calcitonin in osteopenic postmenopausal women
In this study, we aimed to determine if cyclic administration of recombinant human GH, alone or in combination with salmon calcitonin (CT), could significantly increase bone mineral density (BMD) at the lumbar spine and proximal femur in postmenopausal osteopenic women. The study involved 12 treatment cycles over 56 days, with each cycle comprising 12 days of hormone administration followed by 44 days of supplemental calcium alone. Recombinant GH was given via daily injections, and salmon CT was administered for five days within each cycle. The results showed that GH treatment increased insulin-like growth factor I levels and led to statistically significant increases in lumbar spine BMD after two years. However, these gains were relatively modest compared to other osteoporosis treatments, and adverse effects were relatively common. Therefore, cyclic GH therapy, with or without CT, may not be a clinically useful approach for treating postmenopausal osteoporosis.
You can read the full article at https://academic.oup.com/jcem/article/82/4/1111/2866207?login=false.
Joseph F, Ahmad AM, Ul-Haq M, Durham BH, Whittingham P, Fraser WD, Vora JP. Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2008;23:721–729. doi: 10.1359/jbmr.071117.
Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis
In this study, we investigated the effects of recombinant human GH (GH) on bone mineral metabolism in postmenopausal women with osteoporosis. Compared to healthy premenopausal controls, the osteoporotic women had lower IGF-1 levels and higher PTH concentrations. GH administration over 12 months significantly increased IGF-1, reduced PTH levels, and improved target organ sensitivity to PTH. It also positively influenced bone mineral metabolism, including increased bone formation, higher calcium and phosphate levels, and improved bone balance. These findings shed light on the mechanism behind the increased bone mineral density observed in previous long-term GH studies for postmenopausal osteoporosis and suggest a potential role for GH in addressing age-related postmenopausal osteoporosis.
You can read the full article at https://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.071117.
Kassem M, Brixen K, Mosekilde L, Blum WF, Flyvbjerg A. Effects of growth hormone treatment on serum levels of insulin-like growth factors (IGFs) and IGF binding proteins 1-4 in postmenopausal women. Clin Endocrinol (Oxf) 1998;49:747–756.
Effects of growth hormone treatment on serum levels of insulin-like growth factors (IGFs) and IGF binding proteins 1-4 in postmenopausal women
In this double-blind, placebo-controlled study involving post-menopausal women with low bone mass, we investigated the impact of different doses of growth hormone (GH) on circulating levels of insulin-like growth factors (IGFs) and insulin-like growth factor binding proteins (IGFBPs). The results demonstrated that GH treatment led to significant increases in both IGF-I and IGF-II, while IGFBP-1 and IGFBP-2 decreased significantly, and IGFBP-3 and IGFBP-4 increased, all in a dose-dependent manner. Multiple regression analyses revealed various correlations between GH dosage, IGFs, and specific IGFBPs. In summary, GH administration had significant effects on IGFs and IGFBPs in post-menopausal women, influencing their levels in a dose-dependent manner.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2265.1998.00606.x?sid=nlm%3Apubmed.
Kassem M, Brixen K, Blum WF, Mosekilde L, Eriksen EF. Normal osteoclastic and osteoblastic responses to exogenous growth hormone in patients with postmenopausal spinal osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994a;9:1365–1370. doi: 10.1002/jbmr.5650090907.
Normal osteoclastic and osteoblastic responses to exogenous growth hormone in patients with postmenopausal spinal osteoporosis
The cause of bone loss in osteoporosis patients is not fully understood, but it may involve increased bone resorption and decreased bone formation. To investigate how bone cells respond to hormonal stimuli, we conducted a study comparing 15 postmenopausal osteoporosis patients with 15 healthy postmenopausal women. During a 3-day GH stimulation test, both groups showed increased levels of serum insulin-like growth factor I and biochemical markers related to bone resorption and bone formation. GH treatment reduced alkaline phosphatase levels in both groups. Importantly, the maximal response and the area under the curve for IGF-I, bone resorption markers, and bone formation markers were similar between the two groups. These findings suggest that osteoporotic patients do not exhibit major disturbances in their responsiveness to GH.
You can read the abstract of the article at https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.5650090907.
Kassem M, Brixen K, Blum W, Mosekilde L, Eriksen EF. No evidence for reduced spontaneous or growth-hormone-stimulated serum levels of insulin-like growth factor (IGF)-I, IGF-II or IGF binding protein 3 in women with spinal osteoporosis. Eur J Endocrinol Eur Fed Endocr Soc. 1994b;131:150–155.
No evidence for reduced spontaneous or growth-hormone-stimulated serum levels of insulin-like growth factor (IGF)-I, IGF-II or IGF binding protein 3 in women with spinal osteoporosis
To investigate the potential role of a dysfunctional GH-insulin-like growth factor (IGF) axis in osteoporosis, we compared IGF-I, IGF-II, and IGFBP-3 levels in 15 women with spinal osteoporosis and 15 age-matched healthy women. Baseline levels of IGF-I, IGF-II, and IGFBP-3 were similar between the two groups. Following treatment with recombinant human GH (r-hGH) for 3 days, both groups exhibited increased IGF-I, IGF-II, and IGFBP-3 levels. The maximal responses and area under the response curves did not differ significantly between osteoporotic patients and controls. These findings do not support the hypothesis of a dysfunctional GH-IGF axis in women with spinal osteoporosis.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/131/2/150/6750801?redirectedFrom=fulltext&login=false.
Landin-Wilhelmsen K, Nilsson A, Bosaeus I, Bengtsson BA. Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:393–405. doi: 10.1359/jbmr.2003.18.3.393.
Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial
In an 18-month double-blinded study, eighty postmenopausal women with osteoporosis and ongoing estrogen therapy (HRT) were randomly assigned to receive subcutaneous recombinant human growth hormone (GH) at either 1.0 U/day or 2.5 U/day, or a placebo, with all groups also receiving calcium and vitamin D. At 18 months, total body bone mineral content was highest in the GH 2.5 U group. After 3 years of GH treatment, both GH-treated groups showed increased total body and femoral neck bone mineral content. At the 4-year follow-up, the GH 2.5 U group exhibited significant increases in total body and lumbar spine bone mineral content. However, no differences were observed between groups at the 5-year follow-up. Bone markers indicated increased turnover, and a few fractures occurred in the GH 1.0 U group, but side effects were rare. In conclusion, GH treatment, when added to HRT and calcium/vitamin D, led to significant increases in bone mineral content in postmenopausal women with osteoporosis, suggesting a delayed, extended, and dose-dependent anabolic effect of GH on bone.
You can read the abstract of the article at https://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.2003.18.3.393.
Sugimoto T, Nakaoka D, Nasu M, Kanzawa M, Sugishita T, Chihara K. Effect of recombinant human growth hormone in elderly osteoporotic women. Clin Endocrinol (Oxf) 1999;51:715–724.
Effect of recombinant human growth hormone in elderly osteoporotic women
In an open study involving eight elderly osteoporotic women (mean age 71 years), we assessed the impact of recombinant human GH treatment administered via a single daily subcutaneous injection for 48 weeks. GH treatment led to rapid and sustained increases in IGF-I and IGFBP-3 levels. Bone turnover markers exhibited gradual increases, with bone formation markers remaining elevated and the bone resorption marker showing a tendency to return to baseline after 24 weeks of GH therapy. GH treatment resulted in improved hand grip strength and reduced waist/hip ratio. Although the changes in bone mineral density (BMD) of the mid-radius and lumbar spine were not statistically significant over 48 weeks of treatment, further monitoring for an additional 48 weeks post-GH treatment showed significant increases in radial and lumbar BMD. Overall, GH treatment appeared to mitigate the decline in muscle strength, bone mass, and abdominal fat gain associated with aging in elderly women, suggesting its potential application in this population.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2265.1999.00867.x?sid=nlm%3Apubmed.
Kruse HP, Kuhlencordt F. On an attempt to treat primary and secondary osteoporosis with human growth hormone. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme. 1975;7:488–491. doi: 10.1055/s-0028-1093710.
On an attempt to treat primary and secondary osteoporosis with human growth hormone
Three male patients, one with primary osteoporosis and two with osteogenesis imperfecta, underwent treatment with human growth hormone for 8 to 15 months at average daily doses of 1.45 to 2.3 mg. Although clinical observations and 47calcium kinetic data did not reveal significant treatment effects, histomorphometric analysis of bone biopsies demonstrated clear changes. Notably, there was an increase in periosteal new bone formation and intracortical bone resorption, coupled with a significant rise in the relative activity of osteoblasts on endosteal surfaces.
You can read the abstract of the article at https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0028-1093710.
Krantz E, Trimpou P, Landin-Wilhelmsen K. Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study. The Journal of Clinical Endocrinology and Metabolism. 2015;100(9):3251-3259. doi:10.1210/jc.2015-1757.
Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study
In a 10-year follow-up of women with postmenopausal osteoporosis who received growth hormone (GH) treatment for 3 years, bone data, fracture rates, and quality of life (QoL) were evaluated and compared to controls. GH treatment, administered as either 1.0 U or 2.5 U of recombinant human GH or placebo daily for 3 years, resulted in dose-dependent increases in bone mineral density (BMD) and bone mineral content in all regions. Over the 10-year period, fractures decreased from 56% to 28% among GH-treated patients (P = .0003), while controls experienced an increase from 8% to 32% (P = .0008). However, QoL remained unchanged during GH treatment and the 10-year follow-up, with no significant differences compared to controls. This suggests that GH treatment had a positive impact on bone and fracture outcomes but did not affect QoL in women with postmenopausal osteoporosis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4570174/.
Gillberg P, Mallmin H, Petrén-Mallmin M, Ljunghall S, Nilsson AG. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 2002;87:4900–4906. doi: 10.1210/jc.2002-020231.
Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis
We examined the impact of GH therapy on bone turnover, bone size, bone mineral density (BMD), and bone mineral content (BMC) in 29 men with idiopathic osteoporosis aged 27-62 years. These men were divided into two groups: one receiving continuous GH treatment with daily 0.4 mg GH injections (group A, n = 14), and the other receiving intermittent GH treatment with 0.8 mg GH daily for 14 days every 3 months (group B, n = 15). All participants received GH treatment for 24 months, followed by a 12-month follow-up period, alongside daily intake of 500 mg calcium and 400 U vitamin D3 throughout the entire 36 months. Results showed that after 2 years, there was an increase in BMD, particularly in the lumbar spine (4.1% in group A), as well as in total body BMD (2.6% in group A and 2.7% in group B). BMC in both the total body and lean body mass increased, while fat mass decreased in both treatment groups. After 36 months, BMD and BMC in the lumbar spine and total body continued to increase in both groups. In conclusion, 2 years of intermittent or continuous GH treatment in men with idiopathic osteoporosis led to sustained increases in BMD and BMC, even up to 1 year after treatment cessation.
You can read the full article at https://academic.oup.com/jcem/article/87/11/4900/2823079?login=false.
Johansson AG, Lindh E, Blum WF, Kollerup G, Sørensen OH, Ljunghall S. Effects of growth hormone and insulin-like growth factor I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1996;81:44–48. doi: 10.1210/jcem.81.1.8550792.
Effects of growth hormone and insulin-like growth factor I in men with idiopathic osteoporosis
In a crossover study, 12 men with idiopathic osteoporosis received daily subcutaneous injections of GH (2 IU/m2) or IGF-I (80 micrograms/kg) for 7 days with a 12-week washout period. GH treatment led to a 29% increase in serum procollagen type I levels (P < 0.001), while IGF-I treatment resulted in a 43% increase (P < 0.001 compared to baseline; P < 0.05 compared to GH injections), indicating enhanced bone formation. Both treatments increased osteocalcin concentrations by 20% (P < 0.001), reflecting increased bone formation. Additionally, evidence of stimulated bone resorption was observed, with urinary deoxypyridinoline levels increasing by 44% following GH injections (P < 0.001) and by 29% following IGF-I injections (P < 0.001). Serum IGF-I concentrations were 28% higher after GH compared to IGF-I injections. While both treatments increased bone metabolism markers, IGF-I appeared to enhance collagen type I formation more than GH. Furthermore, GH injections initiated bone resorption stimulation as early as 4 days into the treatment. These differences may be dose-dependent but could also suggest distinct cellular-level mechanisms.
You can read the abstract of the article at https://academic.oup.com/jcem/article/81/1/44/2649285?login=false.
Tzanela M. Adult growth hormone deficiency: to treat or not to treat. Expert Opin Pharmacother. 2007;8:787–795. doi: 10.1517/14656566.8.6.787.
Adult growth hormone deficiency: to treat or not to treat
Over the past 15 years, it has become evident that adult growth hormone deficiency (GHD) is linked to increased health challenges such as metabolic syndrome, osteoporosis, muscle loss, and reduced quality of life. Additionally, adults with GHD experience a higher incidence of cardiovascular events, contributing to elevated mortality rates within this population. The primary cause of adult GHD is pituitary adenomas and their associated treatments like surgery and radiation. Individuals with a confirmed severe GHD diagnosis should consider growth hormone replacement therapy only if they have GHD-related health issues. While this therapy can alleviate morbidity, its impact on mortality remains uncertain. The decision to continue treatment for GHD patients transitioning from late adolescence to early adulthood, who have already achieved their final height through growth hormone replacement, should be made judiciously based on clinical assessment.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.1517/14656566.8.6.787?journalCode=ieop20.
Kužma M, Kužmová Z, Zelinková Z, Killinger Z, Vaňuga P, Lazurová I, Tomková S, Payer J. Impact of the growth hormone replacement on bone status in growth hormone deficient adults. Growth Horm IGF Res Off J Growth Horm Res Soc Int IGF Res Soc. 2014;24:22–28. doi: 10.1016/j.ghir.2013.12.001.
Impact of the growth hormone replacement on bone status in growth hormone deficient adults
In this study, we aimed to investigate the impact of growth hormone replacement therapy on bone mineral density (BMD) and explore its effects on bone turnover and microarchitecture in adults with growth hormone deficiency (GHD). Over a 2-year period, we followed adult GHD patients, both those with childhood onset GHD (CO-GHD) and those with adult onset GHD (AO-GHD), who underwent GH replacement therapy to normalize IGF-I levels. We found that lumbar spine and femur BMD significantly increased by 14% and 7%, respectively, after 2 years of treatment. Bone turnover markers initially increased during the first 12 months and subsequently declined. Furthermore, trabecular bone score (TBS) showed a significant 4% increase at month 24. Interestingly, the BMD improvements were more pronounced in male patients and those with childhood onset GHD. These findings highlight the positive effects of GH supplementation on bone health, with variations influenced by gender and the age of onset of GHD.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637413001093?via%3Dihub.
Mo D, Fleseriu M, Qi R, Jia N, Child CJ, Bouillon R, Hardin DS. Fracture risk in adult patients treated with growth hormone replacement therapy for growth hormone deficiency: a prospective observational cohort study. Lancet Diabetes Endocrinol. 2015;3:331–338. doi: 10.1016/S2213-8587(15)00098-4.
Fracture risk in adult patients treated with growth hormone replacement therapy for growth hormone deficiency: a prospective observational cohort study
In this study, we aimed to assess the impact of long-term growth hormone replacement therapy on fracture risk in adult patients with growth hormone deficiency, utilizing data from the international Hypopituitary Control and Complications Study (HypoCCS) surveillance database. Over a mean follow-up period of 4.6 years, involving 9,641 patients, we found that patients who received growth hormone treatment had a lower annual fracture incidence rate compared to those who did not receive it (1.19% vs. 1.91%). This suggests that growth hormone replacement therapy may provide protection against fractures in adult patients with growth hormone deficiency who do not have pre-existing osteoporosis. Initiating growth hormone therapy before the development of osteoporosis could be beneficial for the bone health of these individuals.
You can read the abstract of the article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(15)00098-4/fulltext.
Schmidmaier G, Wildemann B, Heeger J, et al. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31(1):165-72.
Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1
Fracture healing is a complex process influenced by various factors like hormones and growth factors. This study aimed to understand the mechanisms behind improved bone regeneration. In a rat model with midshaft tibia fractures, growth factors IGF-1 and TGF-beta1 were locally applied through a coating on titanium K-wires. Systemic GH was administered through subcutaneous injections and compared with a placebo group. Both GH and local growth factor application significantly improved fracture healing, with the local approach showing stronger effects. Combining both methods did not provide additional benefits, suggesting that local growth factor application may be more effective than systemic GH for accelerating fracture healing in rats.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S8756328202007986?via%3Dihub.
Alba M, Fintini D, Sagazio A, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006;291(6):E1290-4.
Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse
In children with isolated growth hormone (GH) deficiency, GH-releasing hormone (GHRH) therapy has limitations due to its short half-life. A synthetic GHRH analog called CJC-1295, which extends its action by binding to endogenous albumin after injection, was tested in GHRH knockout (GHRHKO) mice. Different dosing intervals (24, 48, and 72 hours) were administered for 5 weeks to three groups of GHRHKO mice. Daily CJC-1295 treatment resulted in normal body weight and length, while treatment every 48 and 72 hours improved these parameters but didn’t fully normalize growth. Femur and tibia length remained normal with 24 and 48-hour dosing. CJC-1295 also increased pituitary RNA and GH mRNA, suggesting somatotroph cell proliferation. These findings indicate that once-daily CJC-1295 can maintain normal body composition and growth in GHRHKO mice, with varying effectiveness at longer dosing intervals.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00201.2006?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Barake M, Arabi A, Nakhoul N, et al. Effects of growth hormone therapy on bone density and fracture risk in age-related osteoporosis in the absence of growth hormone deficiency: a systematic review and meta-analysis. Endocrine. 2018;59(1):39-49.
Effects of growth hormone therapy on bone density and fracture risk in age-related osteoporosis in the absence of growth hormone deficiency: a systematic review and meta-analysis
In adults, the relationship between growth hormone deficiency (GHD) and bone health has been studied extensively. A systematic review of eight studies involving post-menopausal women aged 61-69 years investigated the impact of growth hormone (GH) on bone mineral density (BMD), bone mineral content (BMC), bone biomarkers, and fracture risk. The results showed that GH did not significantly affect BMD at various skeletal sites but did increase the bone formation marker procollagen type-I carboxy-terminal propeptide (PICP). While GH showed a trend of increasing other bone biomarkers and resulted in minor adverse events related to fluid retention, it significantly reduced fracture risk compared to the control group. Further research, including larger and longer-duration studies, is needed to better understand the effects of GH on bone quality in both genders and different populations.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s12020-017-1440-0.
Gillberg P, Mallmin H, Petrén-mallmin M, Ljunghall S, Nilsson AG. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 2002;87(11):4900-6.
Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis
In a study involving 29 men with idiopathic osteoporosis aged 27-62 years, the effects of growth hormone (GH) treatment were investigated. The patients were divided into two groups: one received continuous GH treatment with daily injections of 0.4 mg GH/day (group A), while the other received intermittent treatment with 0.8 mg GH/day for 14 days every 3 months (group B). All patients were treated with GH for 24 months, followed by a 12-month follow-up period, alongside daily calcium and vitamin D3 supplements. Results showed that after 2 years, there was a significant increase in bone mineral density (BMD), particularly in the lumbar spine (4.1% increase in group A) and total body (2.6% increase in group A and 2.7% in group B). Total body bone mineral content (BMC) and lean body mass increased, while fat mass decreased in both treatment groups. Importantly, these positive effects on BMD and BMC were sustained for at least 1 year post-treatment.
You can read the full article at https://academic.oup.com/jcem/article/87/11/4900/2823079?login=false.
Krantz E, Trimpou P, Landin-wilhelmsen K. Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study. J Clin Endocrinol Metab. 2015;100(9):3251-9.
Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study
In a 10-year follow-up of women who had received growth hormone (GH) treatment for 3 years for postmenopausal osteoporosis, it was found that GH increased bone mineral density (BMD) and reduced fractures in a dose-dependent manner. The study involved 80 women compared to an age-matched control group. After a decade, fractures decreased from 56% to 28% in GH-treated patients but increased from 8% to 32% in controls. Quality of life (QoL) remained unchanged throughout GH treatment and the follow-up, with no significant difference from the control group. In summary, GH treatment improved bone health and reduced fractures over a 10-year period, without impacting QoL in postmenopausal osteoporotic women.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4570174/.
Kuzma M, Payer J. [Growth hormone deficiency, its influence on bone mineral density and risk of osteoporotic fractures]. Cas Lek Cesk. 2010;149(5):211-6.
Growth hormone deficiency, its influence on bone mineral density and risk of osteoporotic fractures
Growth hormone (GH) is the most abundant hormone among all pituitary hormones and plays a crucial role in linear bone growth, primarily through the action of insulin-like growth factor I (IGF I). GH is pivotal for longitudinal bone growth and achieving peak bone mass (PBM) during childhood and adolescence, a critical predictor of osteoporotic fractures. Even after the closure of growth plates, GH and IGF continue to impact bone turnover, mass, density, and strength by regulating bone remodeling. In individuals with hypopituitarism, especially those with growth hormone deficiency (GHD), low bone mineral density (BMD) has been observed, initially decreasing after 6-12 months of GH therapy but later normalizing or even increasing. Reduced BMD contributes to an increased risk of fractures in older patients. Studies have indicated that GH therapy slightly increases BMD in adult men with adult-onset GHD (AO-GHD) but has less significant effects in women. Previous research has shown an elevated fracture risk in GHD patients, particularly in women with childhood-onset GHD (CO GHD). Men, on the other hand, exhibit a lower incidence of fractures compared to control groups. Additionally, sexual differences in the effects of GH on bone exist, with women generally having higher GH secretion levels, while the normal range for serum IGF-I is similar in both genders. In a placebo-controlled double-blind study where men and women with GHD received the same GH dose per unit of body mass, men experienced a greater increase in serum IGF-I levels. These differences in response to GH therapy may be influenced by sex hormones’ impact on GH secretion.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/20629339/.
Vittorio Locatelli and Vittorio E. Bianchi, “Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis,” International Journal of Endocrinology, vol. 2014, Article ID 235060, 25 pages, 2014.
Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis
In the context of skeletal growth during puberty and lifelong bone health, growth hormone (GH) and insulin-like growth factor (IGF-1) play pivotal roles. GH promotes tissue formation directly and indirectly in target cells, while IGF-1 is a crucial mediator of bone growth. This study summarizes 39 clinical studies retrieved from PubMed, focusing on the impact of GH and IGF-1 on bone metabolism in individuals with osteopenia and osteoporosis, as well as their influence on bone healing in patients with normal GH secretion who underwent surgery. Of these studies, 18 examined the effects of GH treatment, 14 reported clinical outcomes with IGF-1 administration, and seven explored the GH/IGF-1 effect on bone healing. The findings indicate that both GH and IGF-1 administration significantly increase bone resorption and formation in most cases, with potential benefits for bone healing in patients with hip or tibial fractures, although some conflicting results exist. Overall, GH and IGF-1 therapy demonstrate significant anabolic effects, and GH treatment for osteoporosis and bone fractures holds promise for improving clinical outcomes, with consideration of the interaction between GH and sex steroids in the anabolic process and the role of GH resistance.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4132406/.
Capozzi A, Casa SD, Altieri B, Pontecorvi A. Low bone mineral density in a growth hormone deficient (GHD) adolescent. Clinical Cases in Mineral and Bone Metabolism. 2013;10(3):203-205.
Low bone mineral density in a growth hormone deficient (GHD) adolescent
Growth Hormone (GH) is well-known for its crucial role in childhood growth and achieving optimal height in early adulthood. It also significantly impacts various metabolic processes, cardiovascular health, and quality of life in adults. GH is instrumental in attaining optimal Bone Mineral Density (BMD), a key predictor of osteoporotic fractures, during the transition from childhood to adulthood. This period is critical for assessing the persistence of Growth Hormone Deficiency (GHD) in individuals with childhood-onset GHD (COGHD) and determining the need for continued treatment with recombinant human Growth Hormone (rhGH), even after growth plate closure. COGHD patients have a higher risk of fractures, and GH therapy aims to minimize or prevent osteoporosis in adulthood. This case report highlights a young man with COGHD who received rhGH treatment until reaching his final height but experienced a wrist fracture and early bone density loss.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3917585/.
Aloia JF, Zanzi I, Ellis K, et al. Effects of growth hormone in osteoporosis. J Clin Endocrinol Metab. 1976;43(5):992-9.
Effects of growth hormone in osteoporosis
The study investigated the impact of long-term growth hormone (GH) administration in osteoporotic patients using various analytical methods. Two dosage regimens were tested over six months: 2 units daily and 0.2 units of GH daily per kilogram of body weight. The lower dose showed no significant changes in the measured parameters. With the higher dose, there was no clear anabolic effect observed in most patients, as total body levels of key elements remained unchanged, and urinary calcium and hydroxyproline excretion rates increased while bone mineral content decreased. Bone biopsies indicated some alterations in bone formation and resorption surfaces, but these were not statistically significant. Additionally, several side effects resembling acromegaly, including hyperglycemia, hypertension, arthralgia, and carpal tunnel syndrome, were observed. Given the absence of demonstrated benefits and the associated complications, GH administration does not appear to be a valuable treatment for osteoporosis under the conditions of this study.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/43/5/992/2684707?redirectedFrom=fulltext&login=false.
Bolanowski M, Halupczok J, Jawiarczyk-Przybyłowska A. Pituitary Disorders and Osteoporosis. International Journal of Endocrinology. 2015;2015:206853. doi:10.1155/2015/206853.
Pituitary Disorders and Osteoporosis
Hormonal disorders can disrupt bone metabolism and lead to secondary osteoporosis, substantially elevating the risk of fractures. Within pituitary disorders, such effects are evident in conditions like Cushing’s disease, hyperprolactinemia, acromegaly, and hypopituitarism. Severe osteoporosis can arise from the concurrent presence of these disorders and hypogonadism, a situation that occurs quite frequently.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4383139/.
Sugimoto T, Kaji H, Nakaoka D, et al. Effect of low-dose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis. Eur J Endocrinol. 2002;147(3):339-48.
Effect of low-dose of recombinant human growth hormone on bone metabolism in elderly women with osteoporosis
In this study, the researchers aimed to explore the impact of low-dose growth hormone (GH) treatment on various aspects, including body composition, bone turnover markers, serum levels of IGF-I and IGF-binding proteins (IGFBPs), and bone mineral density (BMD) in eight elderly Japanese women diagnosed with osteoporosis. The participants received GH as a single daily subcutaneous injection over 48 weeks. The results showed that GH treatment initially increased markers of both bone formation and resorption, but bone resorption markers returned to baseline levels after 24 weeks. GH treatment led to a sustained rise in serum IGF-I concentration and gradual increases in IGFBP-5 levels. While radial BMD showed a potential increase later in the treatment, it was not statistically significant, and lumbar BMD remained unchanged. GH treatment also improved hand grip strength, and no new fractures or side effects were observed. Notably, radial BMD significantly increased after discontinuing GH treatment for another 48 weeks, with a similar trend observed in the lumbar spine. Overall, the study suggests that low-dose GH treatment can help mitigate muscle strength and bone mass decline in elderly women without adverse effects, though dietary and exercise factors may influence BMD changes. These findings offer valuable insights into the potential use of low-dose GH treatment for elderly women with osteoporosis.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/147/3/339/6753569?redirectedFrom=fulltext&login=false.
The Endocrine Society. “Growth hormone reduces risk of osteoporosis fractures in older women: Long-term follow-up study shows benefits lasted for years after randomized trial.” ScienceDaily. ScienceDaily, 27 August 2015. <www.sciencedaily.com/releases/2015/08/150827141905.htm>.
Holmes D. Bone: Antifracture efficacy of growth hormone-confirmation at long last. Nat Rev Endocrinol. 2015;11(11):631.
Bone: Antifracture efficacy of growth hormone-confirmation at long last
A recent study led by Emily Krantz suggests that growth hormone (GH) treatment can reduce the long-term fracture risk in postmenopausal women with osteoporosis. In this 10-year follow-up of a double-blind, placebo-controlled trial at Sahlgrenska University Hospital, 80 women with postmenopausal osteoporosis received GH (1.0 IU or 2.5 IU daily) or a placebo for 3 years, alongside calcium and vitamin D supplementation. In comparison to a control group, the osteoporosis group saw a decrease in fracture incidence from 56% to 28% at the 10-year mark, accompanied by positive effects on bone mineral density and content. While this study highlights the potential of GH therapy for osteoporosis, its cost, daily injections, and specialist clinic monitoring make the development of a long-acting preparation crucial for practical use alongside existing treatments like calcium, vitamin D, and bisphosphonates.
You can read the abstract of the article at https://www.nature.com/articles/nrendo.2015.160.
Balercia G, Giovannini L, Paggi F, Spaziani M, Tahani N, Boscaro M, Lenzi A, Radicioni A. GH deficiency in the transition period: body composition and gonad function. J Endocrinol Invest. 2011 Jun 21.
GH deficiency in the transition period: body composition and gonad function
Recombinant GH therapy is typically administered to children with GH deficiency to attain satisfactory height during childhood and adolescence. However, GH’s role extends beyond reaching final height into the transition period, where the body undergoes physical and psychological changes leading to adulthood. During this phase, GH influences various metabolic functions, including improving the lipid profile by increasing HDL and reducing LDL, thereby providing cardiovascular protection. It also affects body composition, enhancing muscle strength, lean body mass, and reducing body fat, aids in achieving peak bone density, and supports gonad maturation. Retesting during this transition phase, involving IGF-I measurement along with a provocative test, is crucial for identifying persistent GH deficiency necessitating continued replacement therapy. Collaboration between pediatric and adult endocrinologists is essential for a smooth transition, and this review highlights GH’s impact on body composition, metabolic functions, and gonadal development during this critical period.
You can read the abstract of the article at https://link.springer.com/article/10.3275/7804.
Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998 Feb;19(1):55–79.
Growth hormone and bone
While the role of growth hormone (GH) in longitudinal bone growth is well-established, its influence on bone metabolism in humans has become clearer recently. Studies in both living organisms and cell cultures have demonstrated that GH plays a significant role in regulating both bone formation and resorption. GH promotes bone formation directly by interacting with osteoblasts via growth hormone receptors (GHRs) and indirectly through the induction of endocrine and autocrine/paracrine insulin-like growth factor-I (IGF-I). Its effect mediated by IGFs versus IGF-independent effects remains unclear. GH treatment also leads to increased bone resorption, potentially by regulating osteoclast formation. Modulations of the GH/IGF axis by glucocorticoids and estrogens are considered. In individuals with GH deficiency (GHD), GH deficiency leads to decreased bone mass, while long-term GH treatment (>18 months) increases bone mass. Interestingly, GH treatment initially stimulates bone resorption in GHD adults, followed by a phase of increased bone formation, leading to a net gain in bone mass. This “biphasic model” suggests that GH treatment in GHD adults results in an overall increase in bone mass after approximately 12-18 months. However, it remains uncertain whether this model applies to individuals with normal GH secretion. Approaches to enhance the GH/IGF-I axis in bone metabolism might include GH, IGF, factors increasing local IGF-I production, and GH-releasing factors. GH-releasing factors have advantages over GH due to their potential oral administration and more physiological GH secretion patterns.
You can read the full article at https://academic.oup.com/edrv/article/19/1/55/2530792?login=false.
Bex M, Bouillon R. Growth hormone and bone health. Horm Res. 2003;60( Suppl 3):80–6.
Growth hormone and bone health
Both growth hormone (GH) and insulin-like growth factor-I profoundly influence growth plate chondrocytes and all bone cells. Childhood-onset GH deficiency (GHD) significantly impedes linear growth and bone size, resulting in adult peak bone mass that’s approximately 50% of those with normal height. While true bone mineral density (BMD) remains nearly normal, untreated childhood-onset GHD adults experience a notably increased prevalence of fractures. Adequate GH treatment can largely correct bone size and, in several studies, bone mass, but typically necessitates more than 5 years of continuous therapy. In adult-onset GHD, there’s a modest deficit in bone mineral content and BMD, along with increased fracture incidence. GH replacement therapy boosts bone turnover and may moderately increase BMD in male adults with adult-onset GHD, while its effect on women appears less significant. GHD, whether childhood or adult-onset, diminishes bone mass and strength, and appropriate long-term substitution therapy can address these deficiencies. Further research is needed to explore GH therapy for other non-primary GHD-related bone disorders, as its favorable effects on the bone remodeling cycle may offer benefits.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/60/Suppl.%203/80/372414/Growth-Hormone-and-Bone-Health?redirectedFrom=fulltext.
Groban L, Lin M, Kassik KA, Ingram RL, Sonntag WE. Early-onset growth hormone deficiency results in diastolic dysfunction in adult-life and is prevented by growth hormone supplementation. Growth Horm IGF Res. 2011 Apr;21(2):81–8. Epub 2011 Mar 2.
Early-onset growth hormone deficiency results in diastolic dysfunction in adult-life and is prevented by growth hormone supplementation
The primary objective of growth hormone (GH) replacement is to stimulate linear growth in children with GH deficiency (GHD). GH and insulin-like growth factor-1 (IGF-1) also play roles in cardiac development and adult heart function. However, little is known about cardiac diastolic function in young adults with childhood-onset GHD who discontinued GH treatment post-puberty. This study used dwarf rats as models for GHD, some with GH replacement initiated at 4 weeks of age and either continued or discontinued after 10 weeks. Results showed that early-onset GHD led to diastolic dysfunction in early adulthood, associated with reduced cardiac sarcoplasmic reticulum Ca(2+) ATPase (SERCA2) content. Peri-adolescent GH supplementation partially mitigated diastolic deficits, while continual GH replacement preserved diastolic function. Thus, GH treatment into adulthood may be beneficial in preventing diastolic dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3085723/.
Isaksson OG, Ohlsson C, Bengtsson BA, Johannsson G. GH and bone-experimental and clinical studies. Endocr J. 2000 Mar;47( Suppl):S9–16.
GH and bone-experimental and clinical studies
Growth hormone (GH) enhances bone formation through direct interactions with GH receptors on osteoblasts and locally produced IGF-I. GH deficiency results in reduced bone mass in both humans and animals, and GH treatment in GHD patients increases bone mass over several months. In rats with normal GH secretion, GH treatment also boosts bone mass and mechanical strength. However, the effects of short-term GH treatment in individuals with normal GH secretion on bone mass remain inconclusive. In GHD adults, GH acts in a biphasic manner, initially leading to bone mass loss and subsequently, a net gain, with the transition point occurring around six months of treatment. This “Biphasic model” is primarily based on GHD adults’ findings, and its applicability to individuals with normal GH secretion requires further investigation.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/10890175/.
Li Y, Chen LQ, Liang L. Effects of recombinant human growth hormone (GH) replacement therapy on bone metabolism in children with GH deficiency. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2005 Jul;34(4):312–5.
Effects of recombinant human growth hormone (GH) replacement therapy on bone metabolism in children with GH deficiency
The objective of this study was to investigate changes in bone turnover markers and bone mass in children with growth hormone (GH) deficiency before and after recombinant human GH replacement therapy. The study involved 37 cases with complete GH deficiency (CGHD), 31 with partial GH deficiency (PGHD), and 31 age- and sex-matched healthy controls. Baseline levels of bone turnover markers were lower in the CGHD group, and IGF1 was lower in both CGHD and PGHD groups compared to controls. After 3 months of GH replacement therapy, all bone turnover markers and IGF1 significantly increased in both CGHD and PGHD children. There was also a tendency for increased bone mass.
You can read the abstract of the article at https://www.sciengine.com/JZJUMS/doi/10.3785/j.issn.1008-9292.2005.04.006;JSESSIONID=2117d91e-2bf5-4160-a148-869710f40bd9.
Binnerts A, Swart GR, Wilson JH, Hoogerbrugge N, Pols HA, Birkenhager JC, Lamberts SW. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition. Clin Endocrinol (Oxf) 1992 Jul;37(1):79–87.
The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition
The study aimed to investigate the impact of growth hormone administration to adult patients with growth hormone deficiency on various aspects of their health. Over a six-month period, growth hormone was given to eight adults at a dose of 25 micrograms/kg/day, with a maximum of 1.48 mg (4 IU) per day. The results showed improved subjective well-being in six patients and no change in two. Body composition assessments revealed a 4 kg increase in lean body mass (5% of body weight) and a 3 kg reduction in mean fat mass. Protein turnover studies indicated a temporary increase in fed state nitrogen balance, primarily due to increased protein synthesis. Growth hormone treatment affected bone markers, indicating bone remodeling activation, but not bone density. Glucose levels increased during treatment, while total cholesterol levels decreased. Thyroid hormone levels showed some alterations. Overall, growth hormone therapy led to increased lean body mass, improved well-being, and influenced various metabolic parameters.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2265.1992.tb02287.x?sid=nlm%3Apubmed.
Boot AM, van der Sluis IM, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density and body composition in adolescents with childhood-onset growth hormone deficiency. Horm Res. 2009;71(6):364–71. Epub 2009 Jun 9.
Bone mineral density and body composition in adolescents with childhood-onset growth hormone deficiency
This study aimed to assess the bone mineral density (BMD) and body composition of individuals with childhood-onset growth hormone (GH) deficiency (GHD) who received GH treatment during the transition period. The results showed that mean BMD and bone mineral apparent density (BMAD) at the lumbar spine, as well as total body BMD and lean body mass (LBM) standard deviation scores (SDS), were consistently lower than normal at final height and for the subsequent 2 years in all patients. The final height SDS was linked to changes in height SDS and LBM SDS during the first year of GH treatment. Additionally, individuals without GH treatment experienced a significant decrease in LBM SDS, while fat mass SDS increased in all patients.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/71/6/364/373401/Bone-Mineral-Density-and-Body-Composition-in?redirectedFrom=fulltext.
Shea HC, Levy RA. Transition Care of Growth Hormone Deficient Patients from Pediatric Endocrinologists to Adult Endocrinologists. Endocr Pract. 2011 Nov;8:1–34.
Transition Care of Growth Hormone Deficient Patients from Pediatric Endocrinologists to Adult Endocrinologists
This review assesses the continuation of growth hormone (GH) therapy into young adulthood when GH deficiency persists, examining recent evidence regarding its benefits during the transition period and addressing various debated issues in diagnosis, treatment, and care transition. Studies suggest that GH therapy should extend beyond final height attainment, promoting peak bone mass accrual and improving metabolic profiles in patients with persistent GH deficiency. Determining appropriate diagnostic criteria and testing methods for GH deficiency during the transition remains a challenge, while reevaluating the optimal GH dosage is essential. Effective communication between pediatric and adult endocrinologists is crucial to ensure a smooth transition and minimize therapy interruptions.
You can read the abstract of the article at https://www.endocrinepractice.org/article/S1530-891X(20)41494-6/fulltext.
Wüster C., Abs R., Bengtsson B.-A., et al. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. Journal of Bone and Mineral Research. 2001;16(2):398–405. doi: 10.1359/jbmr.2001.16.2.398.
The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density
An analysis was conducted to assess the impact of various factors on fracture risk and bone density in adult hypopituitary patients with growth hormone deficiency (GHD), utilizing data from the Pharmacia & Upjohn International Metabolic Database (KIMS) and comparing it with the European Vertebral Osteoporosis Study (EVOS) control population. The study involved 2084 hypopituitary patients from KIMS and 1176 individuals from EVOS. Hypopituitary patients with GHD had a 2.66 times higher prevalence of fractures compared to the non-GH-deficient EVOS population. Among hypopituitary patients, adult-onset GHD carried a higher fracture risk than childhood-onset disease, and isolated GHD patients exhibited similar fracture prevalence to those with multiple pituitary hormone deficiencies. Hormonal replacement therapies (L-thyroxine, glucocorticoids, sex steroids) did not impact fracture risk, while smoking was associated with a higher fracture rate in the KIMS group. In conclusion, this large-scale analysis supports increased fracture risk in adult hypopituitary patients with GHD, primarily attributed to GHD itself rather than other hormone deficiencies or replacement therapies.
You can read the full article at https://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.2001.16.2.398.
Mazziotti G., Bianchi A., Bonadonna S., et al. Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy. Journal of Bone and Mineral Research. 2006;21(4):520–528. doi: 10.1359/jbmr.060112.
Increased prevalence of radiological spinal deformities in adult patients with GH deficiency: influence of GH replacement therapy
In this cross-sectional study, a substantial number of untreated adult patients with growth hormone deficiency (GHD) developed radiological vertebral deformities. However, patients receiving recombinant human GH (rhGH) replacement treatment exhibited a notably lower prevalence of vertebral deformities compared to untreated patients, despite having similar bone mineral density (BMD) as assessed by DXA. Vertebral fractures were more common in GHD patients than in control subjects, with untreated GHD patients experiencing a higher fracture prevalence and number than those undergoing rhGH treatment. Furthermore, the study found that in untreated GHD patients, the prevalence of vertebral deformities correlated with T score and disease duration, whereas in treated GHD patients, it correlated with the timing of the initiation of rhGH replacement therapy. Overall, this study underscores the benefits of rhGH replacement therapy in reducing fracture rates in adult GHD patients.
You can read the full article at https://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.060112.
Akaltun İ, Çayır A, Kara T, Ayaydın H. Is growth hormone deficiency associated with anxiety disorder and depressive symptoms in children and adolescents?: A case-control study. Growth Horm IGF Res. 2018;41:23-27.
Is growth hormone deficiency associated with anxiety disorder and depressive symptoms in children and adolescents?: A case-control study
The aim of this study was to explore the relationship between growth hormone deficiency (GHD) and anxiety disorders and depression in children and adolescents. The study included 122 participants, consisting of 87 receiving GHD therapy, 35 before treatment, and 122 healthy volunteers. The results revealed that Generalized Anxiety Disorder (GAD) and Social Anxiety Disorder (SAD) were significantly more prevalent in children with GHD compared to the control group. Treatment for GHD significantly reduced the rates of GAD and SAD in the GHD-diagnosed group. Moreover, participants undergoing growth hormone therapy exhibited lower anxiety and depression scale scores. Overall, this study highlights the impact of GHD and its treatment on psychological well-being in children and adolescents, emphasizing the need for a comprehensive understanding of the underlying factors affecting these individuals.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637418300480?via%3Dihub.
Nicholas LM, Tancer ME, Silva SG, Underwood LE, Stabler B. Short stature, growth hormone deficiency, and social anxiety. Psychosom Med. 1997;59(4):372-5.
Short stature, growth hormone deficiency, and social anxiety
The objective of this follow-up study was to investigate whether the elevated rates of social phobia observed in adults with growth hormone deficiency (GHD) who had received growth hormone treatment during childhood were primarily a result of short stature. The study compared 21 GHD individuals with 21 age- and sex-matched non-GHD short adults. The findings revealed that 38% of GHD subjects met the criteria for social phobia, while only 10% of short subjects did. Additionally, GHD subjects scored significantly higher on various self-report questionnaires related to social anxiety and psychological distress. These results suggest that the increased prevalence of social phobia in GHD adults cannot be attributed solely to short stature, indicating a more complex relationship between GHD and social anxiety.
You can read the abstract of the article at https://journals.lww.com/psychosomaticmedicine/abstract/1997/07000/short_stature,_growth_hormone_deficiency,_and.6.aspx.
Stabler B. Impact of growth hormone (GH) therapy on quality of life along the lifespan of GH-treated patients. Horm Res. 2001;56 Suppl 1:55-8.
Impact of growth hormone (GH) therapy on quality of life along the lifespan of GH-treated patients
The link between growth hormone deficiency (GHD) and quality of life (QOL) is gaining clarity. Studies on short children with GHD referred for growth hormone treatment reveal poor QOL, often stemming from anxiety, depression, social isolation, and attention-related difficulties, which can impact academic performance and interpersonal skills. Recent observations indicate that these problems tend to diminish with GH therapy. However, young adults treated with GH in childhood may still experience QOL issues, potentially linked to previously unnoticed psychiatric disorders like anxiety, depression, panic disorder, and social phobia. Management should encompass lifelong consideration of QOL, possibly including GH therapy, psychotropic medication, or psychosocial support.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/56/Suppl.%201/55/371964/Impact-of-Growth-Hormone-GH-Therapy-on-Quality-of?redirectedFrom=fulltext.
Stabler B, Clopper RR, Siegel PT, et al. Links between growth hormone deficiency, adaptation and social phobia. Horm Res. 1996;45(1-2):30-3.
Links between growth hormone deficiency, adaptation and social phobia
Children referred for growth hormone (GH) treatment often face school performance issues, social skill deficits, and various behavior problems, including mood disorders, attention deficits, anxiety, and depression. Interestingly, these symptoms tend to decrease after GH replacement therapy initiation. Paradoxically, many GH-deficient individuals, even with successful growth responses to treatment, exhibit below-average quality of life in young adulthood. GH-deficient adults receiving GH therapy report enhanced psychological well-being and health, suggesting a potential central neuroendocrine role of GH. Notably, a substantial number of GH-deficient adults who were GH-deficient as children experience social phobia, a condition associated with GH secretion, indicating that the origins of psychiatric comorbidities in this group involve neuroendocrine and psychosocial factors.
You can read the abstract of the article at https://karger.com/hrp/article-abstract/45/1-2/30/370717/Links-between-Growth-Hormone-Deficiency-Adaptation?redirectedFrom=fulltext.
Brod M, Pohlman B, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Impact of adult growth hormone deficiency on daily functioning and well-being. BMC Research Notes. 2014;7:813. doi:10.1186/1756-0500-7-813.
Impact of adult growth hormone deficiency on daily functioning and well-being
Adult Growth Hormone Deficiency (AGHD) is a debilitating condition arising from various causes, including tumors, pituitary surgery, radiation, head injury, or hypothalamic-pituitary disease. A qualitative study involving seven focus groups and four telephone interviews across three countries aimed to comprehensively explore the effects and treatment outcomes of AGHD on patients’ daily lives. Thirty-nine adult patients, primarily with pituitary disease or tumors, shared their experiences. The study identified five key impact domains: 1) Psychological Health, encompassing body image changes and emotional distress; 2) Physical Health, including issues like sleep disturbances, fatigue, weight gain, and skin/hair problems; 3) Cognition, with difficulties in concentration and memory; 4) Energy Loss and its adverse consequences on productivity, social life, and motivation; and 5) Treatment Effect, highlighting the positive influence of growth hormone replacement therapy on various aspects of patients’ lives. Overall, untreated AGHD significantly affects patients’ well-being, but treatment can alleviate many of these challenges. Recognizing these diverse impacts is crucial for personalized treatment planning and assessing treatment outcomes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4255661/.
Wexler T, Gunnell L, Omer Z, et al. Growth Hormone Deficiency Is Associated with Decreased Quality of Life in Patients with Prior Acromegaly. The Journal of Clinical Endocrinology and Metabolism. 2009;94(7):2471-2477. doi:10.1210/jc.2008-2671.
Growth Hormone Deficiency Is Associated with Decreased Quality of Life in Patients with Prior Acromegaly
In a cross-sectional study conducted at a General Clinical Research Center, the quality of life was assessed in 45 patients with a history of acromegaly: 26 with growth hormone deficiency (GHD) and 19 with normal growth hormone levels post-cure. Various quality-of-life measures, including the Quality of Life Adult Growth Hormone Deficiency Assessment (QoL-AGHDA), Short-Form Health Survey (SF-36), and Symptom Questionnaire, were utilized. The results revealed that, except for the anger/hostility and anxiety subscales of the Symptom Questionnaire, patients with GHD exhibited significantly impaired quality of life compared to those with sufficient growth hormone levels post-cure. Furthermore, peak growth hormone levels after stimulation were associated with specific aspects of quality of life, suggesting the potential benefits of growth hormone replacement therapy for individuals with GHD following acromegaly cure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2708960/.
Mahajan T, Crown A, Checkley S, Farmer A, Lightman S. Atypical depression in growth hormone deficient adults, and the beneficial effects of growth hormone treatment on depression and quality of life. Eur J Endocrinol. 2004;151:325–332. doi: 10.1530/eje.0.1510325.
Atypical depression in growth hormone deficient adults, and the beneficial effects of growth hormone treatment on depression and quality of life
In a 4-month, double-blind, cross-over, placebo-controlled trial of growth hormone (GH) therapy, a comprehensive psychiatric assessment was conducted on 25 patients with adult-onset (AO) and childhood-onset (CO) growth hormone deficiency (GHD). At baseline, 61% of AO-GHD patients exhibited atypical depression, while none of the CO-GHD patients did. GH therapy led to significant improvements in depression rating scale scores, with notable enhancements in emotional reaction and social isolation observed after 1 month, and improvements in energy levels and sleep disturbance noted after 2 and 3 months, respectively. These findings suggest that a substantial proportion of GHD adults experience psychiatric issues, and GH therapy may rapidly alleviate symptoms of atypical depression, possibly through a direct central effect.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/151/3/325/6694454?redirectedFrom=fulltext&login=false.
Malik IA, Foy P, Wallymahmed M, Wilding JP, MacFarlane IA. Assessment of quality of life in adults receiving long-term growth hormone replacement compared to control subjects. Clin Endocrinol (Oxf) 2003;59:75–81. doi: 10.1046/j.1365-2265.2003.01799.x.
Assessment of quality of life in adults receiving long-term growth hormone replacement compared to control subjects
In a study of 120 adults with growth hormone deficiency (GHD) who had received growth hormone (GH) replacement for at least 1 year, their quality of life (QOL) was assessed using eight QOL questionnaires and an Energy Visual Analogue Scale (VAS). The results were compared with 83 control subjects without GHD. The GHD patients, despite GH replacement for an average of 3 years, exhibited significant impairments in various aspects of QOL compared to the control population. These findings suggest that factors beyond GH deficiency may influence the QOL of GHD patients, highlighting the need for additional strategies, including psychological and physical treatments, to improve their well-being.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2265.2003.01799.x?sid=nlm%3Apubmed.
Stouthart PJ, Deijen JB, Roffel M, HA D-v d W. Quality of life of growth hormone (GH) deficient young adults during discontinuation and restart of GH therapy. Psychoneuroendocrinology. 2003;28:612–626. doi: 10.1016/S0306-4530(02)00045-8.
Quality of life of growth hormone (GH) deficient young adults during discontinuation and restart of GH therapy
This study examined the impact of one year of growth hormone (GH) discontinuation followed by one year of GH treatment on the quality of life (QoL) in young adults with childhood-onset growth hormone deficiency (CO-GHD). Initially, during GH discontinuation, insulin-like growth factor I (IGF-I) levels decreased in the first 6 months, accompanied by increased psychological complaints and depression. However, after restarting GH treatment, IGF-I levels increased within the first 6 months, and QoL improved along with a decrease in anxiety and depression scores. Over the 2-year discontinuation and treatment period, IGF-I levels were correlated with various psychological parameters, and by the end of the treatment period, QoL had either improved or remained stable compared to the start of the discontinuation period.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0306453002000458?via%3Dihub.
McMillan CV, Bradley C, Gibney J, Healy ML, Russell-Jones DL, Sonksen PH. Psychological effects of withdrawal of growth hormone therapy from adults with growth hormone deficiency. Clin Endocrinol (Oxf) 2003;59:467–475. doi: 10.1046/j.1365-2265.2003.01870.x.
Psychological effects of withdrawal of growth hormone therapy from adults with growth hormone deficiency
This double-blind, placebo-controlled study investigated the psychological consequences of discontinuing growth hormone (GH) replacement therapy in adults with severe GH deficiency (GHD). After three months of GH withdrawal, patients on placebo reported psychological symptoms of GH withdrawal, including decreased energy, increased tiredness, pain, irritability, and depression. These symptoms were not observed in patients who continued GH treatment. The study highlighted the detrimental psychological effects of GH treatment discontinuation in adults with severe GHD.
You can read the full article at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2265.2003.01870.x?sid=nlm%3Apubmed.
Koltowska-Haggstrom M, Mattsson AF, Monson JP, Kind P, Badia X, Casanueva FF, Busschbach J, Koppeschaar HP, Johannsson G. Does long-term GH replacement therapy in hypopituitary adults with GH deficiency normalise quality of life. Eur J Endocrinol. 2006;155:109–119. doi: 10.1530/eje.1.02176.
Does long-term GH replacement therapy in hypopituitary adults with GH deficiency normalise quality of life
The study aimed to assess whether impaired quality of life (QoL) in adults with growth hormone deficiency (GHD) can be reversed with long-term growth hormone (GH) therapy and if there are variations in QoL responses among different dimensions and patient subgroups. The research involved comparing QoL data from general population samples in several countries with corresponding patient data from the Pfizer International Metabolic Database (KIMS) over 4-6 years of follow-up. Regardless of the initial degree of impairment, overall QoL improved significantly within the first 12 months of GH treatment, steadily progressing toward the population mean in each respective country. Memory and tiredness were the most significant burdens for untreated patients, followed by tenseness, self-confidence, and socializing issues. With GH treatment, these issues improved in reverse order, normalizing for the latter three dimensions. The study concluded that long-term GH replacement leads to sustained improvements in overall QoL and most impaired dimensions, with consistent response patterns across patient subgroups, supporting the hypothesis that GHD may underlie these patients’ psychological problems.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/155/1/109/6695718?redirectedFrom=fulltext&login=false.
Dahl RE, Birmaher B, Williamson DE, et al. Low growth hormone response to growth hormone-releasing hormone in child depression. Biol Psychiatry. 2000;48(10):981-8.
Low growth hormone response to growth hormone-releasing hormone in child depression
This study investigated the growth hormone (GH) response to growth hormone-releasing hormone (GHRH) in a large sample of depressed children in comparison to normal control children. Additionally, it examined test-retest reliability in control subjects and compared GH response in depressed children during episodes of depression and clinical recovery. The study found that depressed children exhibited a significantly lower GH response to GHRH compared to normal controls. Test-retest reliability was stable, and the low GH response persisted even during clinical remission from depression, suggesting a potential link between depression and reduced GH response to GHRH. Further research is needed to understand the mechanism and specificity of this observation.
You can read the abstract of the article at https://www.biologicalpsychiatryjournal.com/article/S0006-3223(00)00932-X/fulltext.
Deijen JB, De boer H, Blok GJ, Van der veen EA. Cognitive impairments and mood disturbances in growth hormone deficient men. Psychoneuroendocrinology. 1996;21(3):313-22.
Cognitive impairments and mood disturbances in growth hormone deficient men
To determine whether psychological complaints in adults with pituitary hormone deficiencies are linked to growth hormone (GH) deficiency or other hormone deficits, researchers evaluated emotional well-being and cognitive performance in 31 men with multiple pituitary hormone deficiencies (MPHD) and 17 men with isolated growth hormone deficiency (IGHD). Compared to a control group of 41 healthy men, MPHD patients displayed lower vigor scores, higher state anxiety, impaired perceptual-motor skills, and memory problems. In contrast, IGHD patients exhibited subnormal memory performance. These findings suggest that cognitive impairment in both MPHD and IGHD is associated with GH deficiency, while reduced vigor in MPHD patients may be attributed to low testosterone levels. The study highlights the distinct psychological profiles of MPHD and IGHD adults.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/030645309500050X?via%3Dihub.
Peabody CA, Warner MD, Markoff E, Hoffman AR, Wilson DM, Csernansky JG. Growth hormone response to growth hormone releasing hormone in depression and schizophrenia. Psychiatry Res. 1990;33(3):269-76.
Growth hormone response to growth hormone releasing hormone in depression and schizophrenia
In a study involving 6 normal controls, 10 schizophrenic subjects, and 7 depressed subjects, the administration of growth hormone releasing hormone (GHRH-44) at a dose of 1 microgram/kg intravenously resulted in a notably reduced growth hormone (GH) response in both the schizophrenic and depressed groups. The study also assessed two molecular forms of GH, 22K GH and 20K GH, which did not reveal further distinctions between the three subject groups.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0165178190900435?via%3Dihub.
Baker LD, Barsness SM, Borson S, et al. Effects of Growth Hormone–Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults: Results of a Controlled Trial. Archives of neurology. 2012;69(11):1420-1429. doi:10.1001/archneurol.2012.1970.
Effects of Growth Hormone–Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults: Results of a Controlled Trial
In a randomized, double-blind, placebo-controlled trial involving 152 adults (66 with mild cognitive impairment), aged 55 to 87 years, participants self-administered daily subcutaneous injections of tesamorelin, a stabilized analog of human growth hormone-releasing hormone (GHRH), or a placebo for 20 weeks. The study found that GHRH had favorable effects on cognition, with similar benefits observed in both adults with mild cognitive impairment and healthy older adults. The completer analysis provided stronger evidence for these cognitive benefits. GHRH treatment also increased insulin-like growth factor 1 levels and reduced body fat, with mild adverse events reported in a portion of the participants. Longer-duration trials are recommended to explore the potential therapeutic benefits of GHRH in brain health during normal and pathological aging.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764914/.
Krishnan KR, Manepalli AN, Ritchie JC, et al. Growth hormone-releasing factor stimulation test in depression. Am J Psychiatry. 1988;145(1):90-2.
Growth hormone-releasing factor stimulation test in depression
The authors conducted a growth hormone-releasing factor (GRF) stimulation test on 19 patients with major depression and 19 age- and sex-matched control subjects to investigate if a reduced growth hormone (GH) response to clonidine indicates central alpha 2-adrenergic receptor subsensitivity in depression. Surprisingly, the GH response to GRF was notably higher in the depressed patients compared to the control group, primarily due to three depressed patients displaying exceptionally elevated GH responses to GRF. These findings suggest that the diminished GH response to clonidine in individuals with depression is not linked to a pituitary dysfunction in GH secretion.
You can read the abstract of the article at https://ajp.psychiatryonline.org/doi/10.1176/ajp.145.1.90?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Steiger A, Guldner J, Colla-müller M, Friess E, Sonntag A, Schier T. Growth hormone-releasing hormone (GHRH)-induced effects on sleep EEG and nocturnal secretion of growth hormone, cortisol and ACTH in patients with major depression. J Psychiatr Res. 1994;28(3):225-38.
Growth hormone-releasing hormone (GHRH)-induced effects on sleep EEG and nocturnal secretion of growth hormone, cortisol and ACTH in patients with major depression
Studies in both normal human subjects and animals have indicated that the neuropeptide growth hormone-releasing hormone (GHRH) plays a role in regulating sleep EEG and hormone secretion during the night. To investigate the effects of GHRH on individuals with acute major depression, 4 x 50 micrograms of GHRH were administered in a pulsatile manner to 10 inpatients (four females, six males) during their depressive episode. Surprisingly, GHRH distinctly increased growth hormone (GH) secretion and reduced rapid-eye-movement (REM) density in the second half of the night, but no significant changes were observed in other sleep-endocrine activities like slow wave sleep, cortisol, and ACTH secretion. This suggests that the impact of GHRH on the hypothalamic-pituitary-adrenocortical system and slow wave sleep may be limited during acute depression, despite its ability to stimulate GH and reduce REM density.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/7932284/.
Burman P, Hetta J, Wide L, Månsson JE, Ekman R, Karlsson FA. Growth hormone treatment affects brain neurotransmitters and thyroxine [see comment]. Clin Endocrinol (Oxf). 1996;44(3):319-24.
Growth hormone treatment affects brain neurotransmitters and thyroxine
In a 21-month double-blind, placebo-controlled trial with a cross-over design involving 24 patients with acquired GH deficiency in adulthood, the long-term effects of GH on cerebrospinal fluid (CSF) neurotransmitters and thyroid hormones relevant to mood and cognition were examined. CSF analysis at the end of the two treatment periods revealed a correlation between GH concentration and administered rhGH dose. Following rhGH treatment, the dopamine metabolite homovanillic acid (HVA) decreased significantly, while the excitatory acid aspartate increased notably. No significant effects were observed on serotonin and noradrenaline metabolites or on glutamate, glycine, and beta-endorphin. However, both CSF and serum levels of free T4 decreased, while total T3 levels increased in serum. These findings suggest that GH has an impact on mood and behavior akin to the effects of antidepressant drugs, potentially signifying a beneficial influence of GH on these aspects.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2265.1996.617439.x?sid=nlm%3Apubmed.
Dinan TG. Psychoneuroendocrinology of depression. Growth hormone. Psychiatr Clin North Am. 1998;21(2):325-39.
Psychoneuroendocrinology of depression. Growth hormone
The regulation of growth hormone (GH) release in the anterior pituitary involves hypothalamic peptides, notably GH-releasing hormone (GHRH), and somatostatin, which are under the influence of classic neurotransmitters like noradrenaline, dopamine, and acetylcholine, as well as feedback from GH and insulin-like growth factor-1. Studies in individuals with depression have primarily focused on this axis. The most consistently reported anomaly is related to noradrenaline-mediated GH release, mediated through GHRH-containing neurons. Some researchers have also reported abnormalities in ACh-induced GH release through the somatostatin system, GABA, and GHRH-stimulated release.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0193953X05700083?via%3Dihub
Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience. 2015;9:37. doi:10.3389/fnins.2015.00037.
Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods
Sex hormones play crucial roles in processes like neurite outgrowth, synaptogenesis, dendritic branching, and myelination, all of which are essential for neural plasticity. This review explores evidence from both animal experiments and human studies regarding the interactions between sex hormones and key neurotransmitters, including serotonin, dopamine, GABA, and glutamate. It summarizes findings under various physiological and pathological conditions and discusses existing theories on how sex hormones may induce neuroplasticity changes through these neurochemical systems. Several brain regions, such as the amygdala, hypothalamus, and hippocampus, express high concentrations of estrogen and progesterone receptors. Given the hippocampus’s significance in mediating structural plasticity in the adult brain, the review places particular emphasis on evidence linking behavioral differences, neurochemical patterns, and hippocampal structure to hormonal fluctuations. Furthermore, it explores how naturally occurring hormonal transitions in humans can serve as models for understanding the impact of changing sex hormones on functional connectivity, neurotransmission, and brain structure in vivo.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4335177/.
Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues in Clinical Neuroscience. 2002;4(1):7-20.
Pathophysiology of depression and mechanisms of treatment
Major depression, a highly significant sociological and clinical disorder, has seen significant advancements since the discovery of antidepressant drugs in the 1950s. Initially, the biochemical hypothesis centered on central monoaminergic dysfunction as the primary cause. However, ongoing research across various neuroscience fields, including genetics, as well as the development of new antidepressants, has revolutionized our comprehension of depression’s mechanisms and drug efficacy. While the monoaminergic system remains a cornerstone in these mechanisms, it’s crucial to consider its intricate interactions with other brain systems and their role in regulating central nervous system function. Despite substantial progress, numerous unanswered questions still await resolution in the future.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181668/.
Terry LC, Crowley WR, Johnson MD. Regulation of Episodic Growth Hormone Secretion by the Central Epinephrine System: STUDIES IN THE CHRONICALLY CANNULATED RAT. Journal of Clinical Investigation. 1982;69(1):104-112.
Regulation of Episodic Growth Hormone Secretion by the Central Epinephrine System: STUDIES IN THE CHRONICALLY CANNULATED RAT
Catecholamines are believed to play a role in regulating growth hormone (GH) secretion by influencing the release of somatostatin and GH-releasing factors in the hypothalamus. Research has shown that central norepinephrine and dopamine have a stimulatory effect on GH, while the role of epinephrine (EPI) is less clear. In this study, the specific inhibition of CNS EPI synthesis using N-methyltransferase inhibitors, SK & F 64139 and LY 78335, resulted in the complete suppression of episodic GH secretion in rats. This depletion of central EPI did not affect dopamine or norepinephrine levels. The findings suggest that the central EPI system plays a significant role in stimulating episodic GH release and that drugs affecting CNS adrenergic systems may have potential applications in diagnosing and treating GH secretion disorders.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC371173/.
Nyberg F. Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol. 2000;21(4):330-48.
Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance
Over the past decade, research has revealed significant effects of growth hormone (GH) on the central nervous system (CNS). GH replacement therapy has shown to enhance psychological capabilities in adults with GH deficiency (GHD) and improve memory, mental alertness, motivation, and working capacity. Children with GHD also benefit behaviorally from GH treatment. Studies indicate that GH therapy influences cerebrospinal fluid levels of hormones and neurotransmitters and can cross the blood-brain barrier. GH receptors are found in various brain regions, including the choroid plexus, hippocampus, hypothalamus, and spinal cord, with their density decreasing with age. Recent discoveries have identified GH receptors in the rat and human brain, suggesting their involvement in regulating hormone secretion and possibly memory and cognitive functions.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0091302200902000?via%3Dihub.
Available at https://link.springer.com/chapter/10.1007/978-1-4684-5505-2_8.
Ghigo E, Arvat E, Bellone J, Ramunni J, Camanni F. Neurotransmitter control of growth hormone secretion in humans. J Pediatr Endocrinol. 1993;6(3-4):263-6.
Neurotransmitter control of growth hormone secretion in humans
The secretion of growth hormone is primarily controlled by the interaction between growth hormone-releasing hormone (GHRH) and somatostatin, two specific neurohormones that act on the pituitary gland. Besides GHRH and somatostatin, various neurotransmitters and neuropeptides impact GH secretion, primarily at the hypothalamic level. This discussion focuses on the stimulating effects of acetylcholine, arginine, and galanin, as well as the inhibitory role of catecholamines, mediated through beta-adrenergic receptors. We’ll also explore how the neural regulation of GH secretion changes with age, from childhood to old age.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/7522825/#:~:text=Growth%20hormone%20secretion%20is%20mainly,acting%20at%20the%20hypothalamic%20level..
Martin JB. Functions of central nervous system neurotransmitters in regulation of growth hormone secretion. Fed Proc. 1980;39(11):2902-6.
Functions of central nervous system neurotransmitters in regulation of growth hormone secretion
Pituitary growth hormone (GH) secretion is governed by two hypothalamic factors: somatostatin, an identified tetradecapeptide that inhibits secretion, and GH-releasing factor, which remains unidentified but stimulates secretion. Various biogenic amines, such as norepinephrine, dopamine, serotonin, acetylcholine, and gamma-aminobutyric acid, exert either excitatory or inhibitory effects at specific brain sites to modulate hypothalamic control over GH secretion. Among these, alpha-adrenergic mechanisms play a crucial role in regulating physiological GH secretion, characterized by episodic surges.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/6105974/#:~:text=Pituitary%20growth%20hormone%20(GH)%20secretion,%2C%20unidentified%2C%20which%20stimulates%20secretion..
Brod M, Pohlman B, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Impact of adult growth hormone deficiency on daily functioning and well-being. BMC Research Notes. 2014;7:813. doi:10.1186/1756-0500-7-813.
Impact of adult growth hormone deficiency on daily functioning and well-being
Adult Growth Hormone Deficiency (AGHD), caused by various factors like tumors or head injury, was investigated in a qualitative study involving 39 adult patients. The majority had pituitary disease or tumors as the underlying cause. The study, conducted across three countries, involved interviews and analysis, revealing five domains of impact: 1) Psychological Health, involving body image changes and negative emotions; 2) Physical Health, encompassing sleep problems, fatigue, weight gain, and more; 3) Cognition, with memory and concentration issues; 4) Energy Loss, affecting productivity and motivation; and 5) Treatment Effect, where treatment improved energy and sleep, enhancing patients’ lives. A conceptual model of AGHD impacts was developed, emphasizing the importance of addressing these aspects in treatment and assessment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4255661/.
Maggi M, Buvat J, Corona G, Guay A, Torres LO. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA). J Sex Med. 2013;10(3):661-77.
Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA)
This review explores the impact of various endocrine disorders on male sexual dysfunction (MSD), specifically focusing on prolactin (PRL), growth hormone (GH), thyroid hormones, and adrenal androgens. Severe hyperprolactinemia, typically related to pituitary tumors, negatively affects sexual function by reducing sexual desire, testosterone production, and erectile function due to both mass effect and PRL-induced suppression of gonadotropin secretion. Hyperthyroidism is associated with premature ejaculation and possibly erectile dysfunction (ED), while hypothyroidism mainly affects sexual desire and the ejaculatory reflex. The impact of acromegaly on sexual function remains debated, and dehydroepiandrosterone (DHEA) administration does not appear to improve male sexual function significantly. More research is needed to fully understand the role of these hormones in regulating male sexual function.
You can read the abstract of the article at https://academic.oup.com/jsm/article-abstract/10/3/661/6940230?redirectedFrom=fulltext&login=false.
Ginzburg E, Lin A, Sigler M, Olsen D, Klimas N, Mintz A. Testosterone and growth hormone normalization: a retrospective study of health outcomes. Journal of multidisciplinary healthcare. 2008;1:79-86.
Testosterone and growth hormone normalization: a retrospective study of health outcomes
In a long-term retrospective study, the impact of testosterone and/or growth hormone (GH) supplementation on body composition and quality of life (QoL) was examined in 91 men and 97 women aged 25-82. Different treatment groups were based on hormonal status: control (dehydroepiandrosterone only), testosterone only (Tes), GH only (GH), and testosterone plus GH (Tes+GH). After approximately 3 years of treatment, women in the control and Tes+GH groups experienced weight loss, while men’s weight remained stable in all groups. Both Tes and Tes+GH led to significant increases in lean mass, reductions in fat mass, and improved bone mineral density (BMD) in both genders, while GH produced similar changes in women. All groups showed improvements in QoL and mood, and treatments were generally well-tolerated and safe. This retrospective study suggests favorable effects of testosterone and/or GH treatment in men and women spanning a broad age range.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3004552/.
Brod M, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Assessing the impact of growth hormone deficiency and treatment in adults: development of a new disease-specific measure. J Clin Endocrinol Metab. 2014;99(4):1204-12.
Assessing the impact of growth hormone deficiency and treatment in adults: development of a new disease-specific measure
In the context of approximately 50,000 adults in the United States diagnosed with growth hormone (GH) deficiency, a patient-reported outcome measure (PRO) called the Treatment-Related Impact Measure-Adult Growth Hormone Deficiency (TRIM-AGHD) was developed and validated to assess the impact of GH deficiency and its treatment. The development followed Food and Drug Administration guidelines and involved concept elicitation, qualitative analysis, and cognitive debriefing. The measure was psychometrically validated with a US clinic-based population, resulting in four domains: energy level, physical health, emotional health, and cognitive ability. The TRIM-AGHD, consisting of 26 items, demonstrated reliability and validity, making it a valuable tool for assessing the impact of GH deficiency and its treatment in adults.
You can read the full article at https://academic.oup.com/jcem/article/99/4/1204/2537260?login=false.
Available at https://www.researchgate.net/publication/12600670_Effects_of_growth_hormone_on_male_reproductive_functions.
Effects of growth hormone on male reproductive functions
This concise review summarizes the evidence supporting the physiological role of growth hormone (GH) in male reproductive development and function, as well as how excessive GH release can negatively affect male sexual behavior and fertility. It also discusses the broader context of interactions between the somatotropic and hypothalamic-pituitary-testicular (H-P-T) axis, drawing on historical studies and recent research involving GH-deficient mutants, recombinant GH, and transgenic mouse models to shed light on the role of GH in reproduction.
You can read the abstract of the article at
Galdiero M, Pivonello R, Grasso LF, Cozzolino A, Colao A. Growth hormone, prolactin, and sexuality. J Endocrinol Invest. 2012;35(8):782-94.
Growth hormone, prolactin, and sexuality
GH and PRL, although not traditionally categorized as sexual hormones, may play roles in regulating sexual function in both men and women. PRL appears to influence sexual behavior by modulating dopaminergic and serotoninergic systems, potentially affecting sexual arousal and function. Chronic hyperprolactinemia is linked to hypogonadotropic hypogonadism and sexual dysfunction in both genders, with successful treatment typically restoring normal sexual function. The physiological role of GH in sexual function is less understood but is thought to influence the hypothalamus-pituitary-gonadal axis and genital sexual response, potentially impacting desire, arousability, and erectile function. However, the exact mechanisms and effects of GH on sexual function in men and women with GH deficiency or excess, such as acromegaly, remain complex and require further investigation.
You can read the abstract of the article at https://link.springer.com/article/10.1007/BF03345805.
Becker AJ, Uckert S, Stief CG, et al. Possible role of human growth hormone in penile erection. J Urol. 2000;164(6):2138-42.
Possible role of human growth hormone in penile erection
Recombinant human growth hormone (rhGH) was found to have dose-dependent relaxant effects on human corpus cavernosum tissue in vitro, correlating with increased intracellular cyclic guanosine monophosphate (cGMP) levels. In vivo, peripheral and cavernous blood levels of growth hormone did not significantly differ during various penile functional conditions. The highest increase in growth hormone occurred during penile tumescence, followed by a temporary decrease afterward. These findings suggest that rhGH may contribute to penile erection by stimulating cGMP activity in human corpus cavernosum smooth muscle.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/11061943/.
Becker AJ, Uckert S, Stief CG, et al. Serum levels of human growth hormone during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology. 2002;59(4):609-14
Serum levels of human growth hormone during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction
The study aimed to observe growth hormone (GH) serum level changes during various penile conditions in patients with erectile dysfunction compared to healthy men. It’s known that GH plays a role in male reproductive function, and its deficiency can lead to issues like fatigue, loss of sexual desire, and erectile problems. The study found that GH levels in healthy men increased during penile tumescence, followed by a temporary decline from tumescence to rigidity and detumescence. In patients with erectile dysfunction, GH levels during penile flaccidity were significantly lower than in healthy men. However, during penile tumescence, GH levels increased similarly in psychogenic patients as in healthy men, while organogenic patients showed negligible increases. These findings suggest that GH may play a crucial role in maintaining male erectile capability, possibly by stimulating cyclic guanosine monophosphate generation in cavernous smooth muscle, and decreased GH release may contribute to erectile dysfunction.
You can read the abstract of the article at https://www.goldjournal.net/article/S0090-4295(01)01594-1/fulltext.
Otunctemur A, Ozbek E, Sahin S, et al. Low serum insulin-like growth factor-1 in patients with erectile dysfunction. Basic and Clinical Andrology. 2016;26:1. doi:10.1186/s12610-015-0028-x.
Low serum insulin-like growth factor-1 in patients with erectile dysfunction
This study aimed to investigate the relationship between insulin-like growth factor-1 (IGF-1) levels and erectile dysfunction (ED), considering the role of endothelial dysfunction and microvascular damage in ED pathogenesis. Two groups were formed: one with 80 patients suffering from ED for over a year and another with 80 individuals without ED (control group). ED diagnosis was based on the International Index of Erectile Function Score-5. The results revealed significantly lower plasma IGF-1 levels in the ED group compared to the control group (96.5 ± 38.3 vs. 132.5 ± 53.3 ng/mL, respectively, P < 0.001). Furthermore, IGF-1 levels showed a positive correlation with ED scores, suggesting that serum IGF-1 levels may serve as a specific predictor of ED, potentially aiding in its early detection in the male population.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4730635/.
Rajfer J. Growth Factors and Gene Therapy for Erectile Dysfunction. Reviews in Urology. 2000;2(1):34.
Growth Factors and Gene Therapy for Erectile Dysfunction
Erectile dysfunction resulting from radical pelvic surgery is often caused by damage to the cavernous nerves near the prostate. The nerve-sparing radical prostatectomy technique was developed to mitigate this issue, but its success varies. Even with tools like the Caver-Map to identify nerves, erectile dysfunction can still occur. One experimental approach involves nerve transplantation to bridge the surgical gap, showing promise in animal studies. However, the chemical factors promoting the acceptance of such neural grafts in vivo remain unclear. In a study by Jung et al., growth factors like insulin-like growth factor (IGF-1) and transforming growth factor (TGF-β2) were found to potentially enhance cavernous nerve regeneration. Whether patients at risk for nerve injury during pelvic surgery will benefit from neural transfer or treatment with these growth factors remains to be determined.
You can read the abstract of the article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476094/.
Pastuszak AW, Liu JS, Vij A. IGF-1 levels are significantly correlated with patient-reported measures of sexual function. International journal of impotence research. 2011; 23(5):220-6.
IGF-1 levels are significantly correlated with patient-reported measures of sexual function
Supplementing with growth hormone (GH) may help maintain erectile function. A study involving 65 men who completed sexual health questionnaires and had their insulin-like growth factor 1 (IGF-1) levels measured found a significant correlation between IGF-1 levels and sexual function scores. This suggests that the GH axis may play a role in erectile function, as evidenced by the positive association between IGF-1 levels and sexual health measures. No significant correlations were observed between IGF-1 levels and other factors like Gleason score or testosterone levels, highlighting the specific impact on sexual function.
You can read the abstract of the article at https://www.nature.com/articles/ijir201131.
Pastuszak AW, Liu JS, Vij A, et al. IGF-1 levels are significantly correlated with patient-reported measures of sexual function. Int J Impot Res. 2011;23(5):220-6.
IGF-1 levels are significantly correlated with patient-reported measures of sexual function
A study on 65 men found a significant correlation between serum insulin-like growth factor 1 (IGF-1) levels, which are indicative of growth hormone levels, and sexual function scores from the Sexual Health Inventory for Men (SHIM) and Expanded Prostate Cancer Index Composite (EPIC) questionnaires. There was no link found between IGF-1 levels and testosterone or between SHIM scores and testosterone. This suggests the growth hormone axis may play a role in preserving erectile function.
El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999;11:123–32. doi: 10.1038/sj.ijir.3900392.
Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model
Erectile dysfunction is a common occurrence in individuals with diabetes mellitus, yet the underlying molecular mechanisms remain unclear. To investigate this phenomenon, an animal model was employed, involving 40 male rats divided into two groups. The experimental group was induced with diabetes using Streptozotocin (STZ), while the control group received a vehicle injection. Eight weeks later, erectile function was assessed, revealing a significant decrease in NOS-containing nerve fibers and reduced maximal intracavernosal pressure in the diabetic group. Molecular analyses indicated down-regulation of various factors, including nNOS, iNOS, and ER-beta, at both mRNA and protein levels, suggesting potential molecular changes underlying the link between diabetes and erectile dysfunction.
You can read the abstract of the article at https://www.nature.com/articles/3900392.
Soh J, Katsuyama M, Ushijima S, Mizutani Y, Kawauchi A, Yabe-Nishimura C, et al. Localization of increased insulin-like growth factor binding protein-3 in diabetic rat penis: Implications for erectile dysfunction. Urology. 2007;70:1019–23. doi: 10.1016/j.urology.2007.07.057.
Localization of increased insulin-like growth factor binding protein-3 in diabetic rat penis: Implications for erectile dysfunction
The study aimed to uncover the molecular mechanisms contributing to diabetes-induced erectile dysfunction (ED) by conducting gene expression profiling in the penises of streptozotocin-induced diabetic rats. The research revealed a significant increase in the expression of insulin-like growth factor binding protein 3 (IGFBP-3) and the reduction of various other genes associated with growth and cellular processes. Elevated IGFBP-3 mRNA levels were observed as early as two weeks after hyperglycemia induction, with increased protein localized in the penile endothelium, urethral epithelium, and corpus cavernosum smooth muscle. Smooth muscle depletion was noted in diabetic rats at eight weeks, and a decrease in intracavernous pressure was observed at 12 weeks, suggesting that IGFBP-3 overexpression during hyperglycemia may contribute to ED development.
You can read the abstract of thel article at https://www.goldjournal.net/article/S0090-4295(07)01913-9/fulltext.
Pu XY, Zheng XG, Zhang Y, Xiao HJ, Xu ZP, Liu JM, et al. Higher expression of mRNA and protein of insulin-like growth factor binding protein-3 in old rat penile tissues: implications for erectile dysfunction. J Sex Med. 2011;8:2181–90. doi: 10.1111/j.1743-6109.2011.02318.x.
Higher expression of mRNA and protein of insulin-like growth factor binding protein-3 in old rat penile tissues: implications for erectile dysfunction
In this study, the researchers aimed to investigate the mRNA and protein expression of insulin-like growth factor binding protein-3 (IGFBP-3) in young and aging rat penile tissues and its association with erectile dysfunction (ED) related to aging. They found a significant increase in IGFBP-3 expression in aging rats compared to young rats, both at the mRNA and protein levels. IGFBP-3 protein was notably present in the urethral epithelium, penile endothelium, and corpus cavernosum smooth muscle. Additionally, aging rats exhibited a significant reduction in smooth muscle density, decreased nitric oxide synthase (NOS) activity, lower cyclic guanosine 3′,5′-cyclic monophosphate (cGMP) levels, and impaired intracavernous pressure, all of which are indicative of ED. These findings suggest that elevated IGFBP-3 expression in aging rats may contribute to the development of ED.
You can read the abstract of the article at https://academic.oup.com/jsm/article-abstract/8/8/2181/6843913?redirectedFrom=fulltext&login=false.
Khorram O, Laughlin GA, Yen SS. Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women. J Clin Endocrinol Metab. 1997;82(5):1472-9.
Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women
In this study involving age-advanced men and women, the researchers investigated the effects of a GHRH analog on the GH and IGF-I axis, metabolic parameters, and overall well-being. Over a 5-month single-blind, randomized, placebo-controlled trial, participants self-administered either saline placebo or [Nle27]GHRH-(1-29)-NH2, a GHRH analog, for 16 weeks. The GHRH analog promptly increased GH levels, which were sustained throughout the study. It also led to elevated IGF-I and IGFBP-3 levels, along with increased GHBP concentrations in women. Men experienced additional benefits, including increased lean body mass, improved insulin sensitivity, enhanced general well-being, and heightened libido. Both genders showed an increase in skin thickness, and there was a trend towards a positive nitrogen balance. The only noted side effect was temporary hyperlipidemia, which resolved by the end of the study. These findings suggest that GHRH analog administration activates the somatotropic axis and elicits gender-specific anabolic effects, particularly favoring men. Further research is needed to explore the observed gender differences in response to GHRH analog treatment.
You can read the full article at https://academic.oup.com/jcem/article/82/5/1472/2823341?login=false.
Rubinek T, Rubinfeld H, Hadani M, Barkai G, Shimon I. Nitric oxide stimulates growth hormone secretion from human fetal pituitaries and cultured pituitary adenomas. Endocrine. 2005;28(2):209-16.
Nitric oxide stimulates growth hormone secretion from human fetal pituitaries and cultured pituitary adenomas
This study aimed to investigate the impact of nitric oxide (NO) on human growth hormone (GH) and prolactin (PRL) secretion using primary cell cultures of human fetal pituitaries and hormone-secreting adenomas. When exposed to sodium nitroprusside (SNP), an NO donor, fetal pituitaries showed a significant increase in GH secretion, similar to the effect of growth hormone-releasing hormone (GHRH). However, PRL secretion remained unaffected. SNP also stimulated GH release in a majority of cultured GH-secreting adenomas. The addition of cyclic GMP (cGMP), a second messenger for NO, further enhanced GH secretion in both fetal pituitaries and adenomas. Neuronal nitric oxide synthase (nNOS) was detected in normal pituitary tissues and adenomas, and its functional expression led to increased GH release, which was blocked by specific inhibitors. These findings suggest that NO plays a role in stimulating human GH secretion through the involvement of cGMP.
You can read the abstract of the article at https://link.springer.com/article/10.1385/ENDO:28:2:209.
Valverde I, Peñalva A, Ghigo E, Casanueva FF, Dieguez C. Involvement of nitric oxide in the regulation of growth hormone secretion in dogs. Neuroendocrinology. 2001;74(4):213-9.
Involvement of nitric oxide in the regulation of growth hormone secretion in dogs
This study examined the role of nitric oxide (NO) pathways in growth hormone (GH) secretion by administering L-arginine, an NO precursor, and L-NAME, an NO synthase (NOS) inhibitor, to adult beagle dogs. L-arginine infusion resulted in a modest increase in plasma GH compared to controls, and when combined with growth hormone-releasing hormone (GHRH), it enhanced GH release. L-NAME, on its own, had no impact on baseline GH levels but completely suppressed GH release induced by GHRH or GHRP-6. Additionally, it reduced the effect of both peptides when administered together. Administration of the 5-HT1D agonist sumatriptan (SUM) led to significant GH secretion, a response unaffected by the concurrent administration of L-NAME. These findings suggest that NO inhibition diminishes GHRH or GHRP-6-induced GH release, possibly by reducing hypothalamic somatostatin release.
You can read the abstract of the article at https://karger.com/nen/article-abstract/74/4/213/225390/Involvement-of-Nitric-Oxide-in-the-Regulation-of?redirectedFrom=fulltext.
Rigamonti AE, Cella SG, Marazzi N, Müller EE. Nitric oxide modulation of the growth hormone-releasing activity of Hexarelin in young and old dogs. Metab Clin Exp. 1999;48(2):176-82.
Nitric oxide modulation of the growth hormone-releasing activity of Hexarelin in young and old dogs
The growth hormone (GH)-releasing effects of Hexarelin, a potent GH-releasing peptide (GHRP) analog, were investigated in young and old beagle dogs after pretreatment with erythrityl tetranitrate, a liposoluble nitric oxide (NO) donor, and/or indomethacin, an inhibitor of cyclooxygenase enzymes, as well as N-nitro-L- or N-nitro-D-arginine methylester (L-NAME and D-NAME), active and inactive NO synthase (NOS) inhibitors, respectively. Erythrityl tetranitrate significantly enhanced Hexarelin-induced GH secretion in both young and aged dogs. L-NAME suppressed GH release in young dogs but potentiated it in old dogs, while D-NAME had no effect. Indomethacin eliminated the NO-donor-induced enhancement of GH response to Hexarelin in both young and old dogs without affecting the GH peak triggered by the peptide alone. These findings suggest that NO donors can further amplify Hexarelin’s GH-releasing activity, and prostaglandins play a role in mediating this process, with age-related differences in NOS inhibition affecting somatotrope function in older dogs.
You can read the abstract article at https://www.metabolismjournal.com/article/S0026-0495(99)90030-6/pdf.
Doi SQ, Jacot TA, Sellitti DF, et al. Growth hormone increases inducible nitric oxide synthase expression in mesangial cells. J Am Soc Nephrol. 2000;11(8):1419-25.
Growth hormone increases inducible nitric oxide synthase expression in mesangial cells
Transgenic mice expressing bovine growth hormone (GH) develop progressive glomerulosclerosis, but the underlying signaling events leading to increased matrix deposition remain unclear. The L-arginine metabolic pathway components, particularly inducible nitric oxide (NO) synthase (iNOS), ornithine aminotransferase (OAT), and ornithine decarboxylase (ODC), have been linked to glomerular scarring. This study exposed mesangial cells to GH and assessed iNOS, ODC, and OAT expression using reverse transcription-PCR. GH dose-dependently increased iNOS transcript levels, peaking at 20 to 50 ng/ml GH. This upregulation of iNOS transcripts correlated with a significant rise in nitrite levels in conditioned media, blocked by L-N(G)-monomethylarginine. GH (50 ng/ml) induced nitrite production as effectively as bacterial lipopolysaccharide (10 microg/ml). However, GH did not alter OAT and ODC expression at any tested concentration. Mesangial cells also expressed GH receptor mRNA independently of GH concentration, suggesting GH’s direct modulation of the L-arginine/NO pathway by regulating iNOS expression and NO production without affecting the arginase/OAT/ODC pathway.
You can read the full article at https://journals.lww.com/jasn/fulltext/2000/08000/growth_hormone_increases_inducible_nitric_oxide.6.aspx.
Available at http://erj.ersjournals.com/content/31/4/815.
Deniz Tuncel, Fatma Inanc Tolun, and Ismail Toru, “Serum Insulin-Like Growth Factor-1 and Nitric Oxide Levels in Parkinson’s Disease,” Mediators of Inflammation, vol. 2009, Article ID 132464, 4 pages, 2009.
Serum Insulin-Like Growth Factor-1 and Nitric Oxide Levels in Parkinson’s Disease
This study aimed to explore the roles of circulating growth hormone (GH), insulin-like growth factor-1 (IGF-1), IGF binding protein-3 (IGFBP-3), and nitric oxide (NO) concentrations in Parkinson’s disease (PD) patients. The study included 25 PD patients and 25 matched healthy individuals as controls. PD patients exhibited significantly lower NO levels (2.3 +/- 0.4 micromol/L) compared to the control group (2.8 +/- 0.6 micromol/L) (P:.011). While GH, IGF-1, and IGF BP-3 levels showed no significant differences between the two groups, this preliminary study observed reduced NO and mildly elevated IGF-1 levels in PD patients, possibly indicating adaptive or protective mechanisms in neurodegenerative disease processes like PD. Further research is needed to validate these findings.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2655363/.
Available at https://clinicaltrials.gov/ct2/show/NCT00470002.
Effects of Growth Hormone on the Nitric Oxide Pathway
Nitric oxide (NO) is a potent endogenous vasodilator that inhibits key processes of atherosclerosis, such as monocyte adhesion, platelet aggregation, and vascular smooth muscle cell proliferation. Endothelial NO production impairment is a significant feature of endothelial dysfunction, an early step in atherosclerotic vascular disease. Recent studies have reinforced the connection between NO pathway parameters and cardiovascular disease, shedding light on pathophysiological mechanisms. Insulin resistance is closely related to the endogenous NO synthase inhibitor, asymmetric dimethylarginine (ADMA). Furthermore, evidence suggests that elevated ADMA plasma levels independently predict mortality and cardiovascular outcomes in hemodialysis patients. Patients with growth hormone deficiency have a 1.9-fold higher risk of cardiovascular disease-related death, and alterations in the NO pathway are implicated in this increased risk. Growth hormone deficiency patients exhibit reduced systemic NO production, which is normalized with recombinant growth hormone treatment. Growth hormone’s effects on NO may be mediated by insulin-like growth factor-I (IGF-I), known to stimulate NO synthesis in vitro. This study aims to further investigate GH’s in vivo impact on the NO pathway and NO-mediated cardiovascular functions.
You can read the abstract of the article at https://classic.clinicaltrials.gov/ct2/show/NCT00470002.
Böger RH , Skamira C , Bode-Böger SM , Brabant G , von zur Muhlen A , Frolich JC. 1996. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 98:2706–2713.
Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study
In a double-blind, placebo-controlled trial, we investigated the impact of recombinant growth hormone (r-hGH) on systemic nitric oxide (NO) production and hemodynamics in adult patients with acquired growth hormone deficiency. Initially, 30 patients received either r-hGH (2.0 IU/d) or placebo for 12 months. In the subsequent year, both groups received r-hGH. Urine and plasma samples were collected regularly to measure urinary nitrate and cyclic GMP as indicators of systemic NO production, along with plasma IGF-1 levels. Cardiac output was assessed using echocardiography. r-hGH led to a fourfold increase in plasma IGF-1 levels within the first month. Patients with growth hormone deficiency had lower baseline urinary nitrate and cyclic GMP excretion compared to healthy controls. r-hGH significantly increased NO production during the first year in the GH group and during the second year in the placebo group. While blood pressure remained unchanged, cardiac output increased by 30-40%, and total peripheral resistance decreased by about 30% in both groups during r-hGH treatment. In the second year, when both groups received r-hGH, there were no significant differences in the studied parameters between them. In summary, untreated growth hormone-deficient patients exhibit reduced systemic NO production. Treatment with r-hGH normalizes NO production, through IGF-1 stimulation of endothelial NO formation, while also reducing peripheral arterial resistance. This increased NO production may contribute to the improved cardiovascular performance observed during growth hormone therapy for acquired hypopituitarism.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507734/.
Thum T , Fleissner F , Klink I , Tsikas D , Jakob M , Bauersachs J , Stichtenoth DO. 2007. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab 92:4172–4179.
Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I
In a study involving healthy middle-aged volunteers (n = 16), we examined the relationship between improved systemic nitric oxide (NO) bioavailability and increased levels of circulating endothelial progenitor cells (EPC) following recombinant human GH treatment. GH treatment led to significant increases in plasma IGF-I levels, elevated urinary cGMP levels, and reduced diastolic blood pressure (P < 0.05). Plasma nitrate and nitrite levels increased, while the NO synthase inhibitor asymmetric dimethylarginine decreased. IGF-I treatment in cultured human endothelial cells increased the expression of enzymes involved in asymmetric dimethylarginine metabolism. IGF-I levels correlated with cGMP concentrations (r = 0.51; P < 0.05). GH treatment also increased EPC numbers, which correlated with markers of NO bioavailability. These findings were replicated in mice treated with GH for 7 days, where GH treatment increased aortic endothelial NO synthase expression. Notably, blocking the IGF-I receptor in vivo eliminated GH-mediated effects on markers of increased NO bioavailability. In conclusion, GH treatment enhances systemic NO bioavailability through IGF-I, potentially offering cardiovascular benefits in specific conditions.
You can read the full article at https://academic.oup.com/jcem/article/92/11/4172/2598165?login=false.
Mani maran RR, Sivakumar R, Ravisankar B, et al. Growth hormone directly stimulates testosterone and oestradiol secretion by rat Leydig cells in vitro and modulates the effects of LH and T3. Endocr J. 2000;47(2):111-8.
Growth hormone directly stimulates testosterone and oestradiol secretion by rat Leydig cells in vitro and modulates the effects of LH and T3
In vitro, we examined the impact of GH on testosterone and oestradiol secretion by purified rat Leydig cells, focusing on basal, LH, and T3-mediated secretion. Purified Leydig cells (1 x 10(3)) were cultured with varying concentrations of rat GH (5-400 ng/mL) for 48 hours at 34 degrees C, following an initial 24-hour culture at 37 degrees C. GH dose-dependently increased both testosterone and oestradiol secretions. Testosterone secretion reached saturation at 50 ng GH, while oestradiol secretion plateaued at 150 ng GH before decreasing. Co-administering a minimal effective dose of GH (10 ng) with minimal (25 ng) or maximal (100 ng) effective doses of LH significantly reduced testosterone secretion. Conversely, combining maximal effective doses of rGH (50 ng) and oLH (100 ng) increased testosterone secretion. T3, at both minimal (25 ng) and maximal (50 ng) doses, inhibited GH-mediated testosterone secretion in vitro. For oestradiol, its concentration in the culture medium increased when any dose of rGH was co-administered with minimal or maximal effective doses of oLH. T3 (50 ng) boosted oestradiol secretion by Leydig cells in the presence of GH. These findings demonstrate that GH stimulates testosterone and oestradiol secretion by Leydig cells, acting as a gonadotrophin, and modulates LH or T3-induced steroid secretion depending on their respective stimulatory intensity.
You can read the abstract of the article at https://www.jstage.jst.go.jp/article/endocrj1993/47/2/47_2_111/_article.
Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51-8.
Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations
In our study, we investigated the separate and combined influences of age and sex on GH secretion patterns. We conducted 24-hour GH secretion profiles in 10 young women, 10 young men, 8 postmenopausal women, and 8 older men. Our analysis showed that total GH secretion (IGHC) was higher in women than in men and greater in the young compared to the older individuals. Pulsatile GH secretion, characterized by pulse frequency, duration, amplitude, and the fraction of GH secreted in pulses (FGHP) over 24 hours, was also more pronounced in the young participants. While the serum level of free estradiol correlated with GH parameters, including IGHC, pulse amplitude, and FGHP, the influence of age on FGHP was reduced after accounting for estradiol levels. These findings suggest that age and sex independently impact GH secretion, largely influenced by variations in estradiol levels, indicating an amplifying role of estradiol in regulating pulsatile GH release.
You can read the abstract of the article at https://academic.oup.com/jcem/article-abstract/64/1/51/2653487?redirectedFrom=fulltext&login=false.
Bancroft J. The endocrinology of sexual arousal. J Endocrinol. 2005;186(3):411-27.
The endocrinology of sexual arousal
This review explores the significance of testosterone, estradiol, and certain peptides (oxytocin, beta-endorphin, and prolactin) in human sexual arousal. It considers behavioral studies, the distribution of gonadal steroid receptors in the brain, and brain imaging evidence. Testosterone plays a crucial role in adult males, supported by consistent findings in hypogonadal and eugonadal men. Its roles in sexual arousability development and aging males are less clear, with aromatization and non-sexual effects requiring further understanding. In females, testosterone’s role is more complex, possibly due to greater variability in responsiveness. A ‘desensitization hypothesis’ is proposed to explain gender differences. Estradiol’s direct impact on female sexual arousability is limited, and the extent of testosterone’s action via conversion to estradiol or increasing free estradiol remains uncertain. Peptides’ roles in sexual arousal remain uncertain due to their multiple roles and sites of action, with oxytocin and beta-endorphin having both excitatory and inhibitory effects. Prolactin has been suggested as an inhibitory factor through dopaminergic activity inhibition, but evidence is inconclusive, presenting a more intricate role for peptides in sexual arousal compared to traditional hormones.
You can read the full article at https://joe.bioscientifica.com/view/journals/joe/186/3/1860411.xml.
Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertility and sterility. 2015;104(5):1051-1060. doi:10.1016/j.fertnstert.2015.08.033.
Normal male sexual function: emphasis on orgasm and ejaculation
Orgasm and ejaculation, although often challenging to distinguish, are distinct physiological processes. Orgasm involves intense, transient pleasure and altered consciousness with associated physical changes, while ejaculation consists of emission and expulsion phases influenced by complex neurological and hormonal pathways. Despite extensive research, much remains unknown, and ejaculatory dysfunction lacks a definitive cure. Understanding these processes aids in developing treatments for such dysfunctions. This article reviews current literature on orgasm and ejaculation physiology, including organ anatomy, erection physiology, and the intricate neural, neurochemical, and hormonal regulation of ejaculation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4896089/.
Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63:811–59.
Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction
Erection is primarily a spinal reflex influenced by various penile afferents and supraspinal stimuli, involving neurotransmitters like dopamine, acetylcholine, nitric oxide (NO), oxytocin, and adrenocorticotropin/α-melanocyte-stimulating hormone. The balance between contractant and relaxant factors regulates penile smooth muscle contraction, affecting the functional state of the penis. Erectile dysfunction (ED), defined as the inability to achieve or maintain a satisfactory erection, has diverse causes, including psychogenic, vasculogenic, neurologic, and endocrinologic factors. Many ED patients respond well to available pharmacological treatments, which often replace malfunctioning endogenous mechanisms. However, there’s a need for alternative therapies, necessitating the identification of new therapeutic targets and innovative approaches, with promising avenues emerging from ongoing research.
You can read the abstract of the article at https://pharmrev.aspetjournals.org/content/63/4/811.long.
Dail WG, Moll MA. Localization of vasoactive intestinal polypeptide in penile erectile tissue and in the major pelvic ganglion of the rat. Neuroscience. 1983;10:1379–86.
Localization of vasoactive intestinal polypeptide in penile erectile tissue and in the major pelvic ganglion of the rat
Vasoactive intestinal polypeptide (VIP) was identified through immunocytochemical techniques in the major pelvic ganglion and penile erectile tissue of rats. VIP fibers were notably concentrated in the penile crura with decreasing density towards the distal regions. The helicine arteries displayed dense innervation, while fewer fibers were observed around the deep artery of the penis. Moderate VIP innervation was found in the intrinsic smooth muscle of the cavernous bodies. Vascular structures, including the deep dorsal vein, in the dorsal region were also innervated by VIP fibers. VIP immunoreactive cell bodies were localized in the major pelvic ganglion, concentrated at one end. Retrograde studies confirmed that neurons in the major pelvic ganglion projected to the penis, and combined studies revealed that all the labeled neurons were VIP immunoreactive. This suggests that VIP fibers innervate all vascular beds in the rat penis, with innervation extent correlating with smooth muscle occurrence, originating from neurons in the major pelvic ganglion.
You can read the abstract article at https://www.ibroneuroscience.org/article/0306-4522(83)90119-7/pdf.
Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol. 1991;308:293–310.
Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat
The organization of calcitonin gene-related peptide-like immunoreactive (CGRPir) innervation in the rat’s amygdala and caudate-putamen was investigated using immunohistochemistry for CGRP, combined with retrograde transport of fluoro-gold and anterograde transport of Phaseoleus vulgaris leucoagglutinin (PHA-L). The central nucleus of the amygdala’s lateral part and the amygdalostriatal transition zone displayed dense CGRPir terminals at all anterior-posterior levels. More posteriorly, the lateral part of the caudate-putamen also exhibited numerous CGRPir terminals. Fluoro-gold injections into the amygdala and amygdalostriatal transition area, followed by CGRP immunohistochemistry, revealed double-labeled neurons in specific thalamic nuclei and the peripeduncular nucleus. Caudate-putamen injections showed double-labeled neurons in the lateral parts of the same nuclear complex. PHA-L injections into the posterior thalamic nuclei, the source of CGRPir projections, confirmed the medial-to-lateral organization of projections to the amygdala and striatum. The subparafascicular and lateral subparafascicular nuclei mainly projected to the medial amygdala and amygdalostriatal transition area, while the more lateral cell groups, including the caudal part of the lateral parafascicular, posterior intralaminar, and peripeduncular nuclei, projected to the lateral amygdala and caudate-putamen. These CGRPir projections may play a role in mediating conditioned autonomic and behavioral responses to acoustic or somatosensory stimuli.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1002/cne.903080212?sid=nlm%3Apubmed.
Hedlund P, Ekström P, Larsson B, Alm P, Andersson KE. Heme oxygen-ase and NO-synthase in the human prostate—relation to adrenergic, cholinergic and peptide-containing nerves. J Auton Nerv Syst. 1997;63:115–26.
Heme oxygen-ase and NO-synthase in the human prostate—relation to adrenergic, cholinergic and peptide-containing nerves
In the human prostate, the distribution of heme oxygenase (HO-1 and HO-2), nitric oxide synthase (NOS), tyrosine hydroxylase (TH), acetylcholine-esterase (AChE), and some peptidergic nerve structures were examined. Nerve trunks contained cell bodies and nerve fibers expressing HO-1, HO-2, NOS, TH, and vasoactive intestinal polypeptide (VIP) immunoreactivities and were AChE-positive, but HO and NOS immunoreactivities were found in separate nerves. HO-1, NOS, and VIP immunoreactive nerves, along with AChE-positive fibers, were observed along smooth muscle strands, intraglandular septa, and around acini. Double immunostaining showed co-localization of NOS and VIP immunoreactivities in varicose nerve terminals. Some TH-IR terminals resembled NOS, HO-1, or VIP-IR terminals but weren’t identical. Neuropeptide Y (NPY) nerves were distributed abundantly, while calcitonin gene-related peptide (CGRP) nerves were less numerous. NOS and CGRP-IR terminals had similar profiles but didn’t co-localize. Nitric oxide and electrical nerve stimulation relaxed noradrenaline-contracted prostatic stroma preparations, and inhibiting nitric oxide synthesis abolished electrically induced relaxations. VIP had slight relaxant effects, and carbon monoxide had no effect on noradrenaline-contracted strips. This innervation pattern and functional evidence suggest that the L-arginine/nitric oxide pathway may play a role in regulating human prostatic smooth muscle activity and secretory neurotransmission, while the physiological role of carbon monoxide in the prostate remains to be clarified.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165183896001397?via%3Dihub.
Jen PY, Dixon JS, Gosling JA. Co-localization of nitric oxide synthase, neuropeptides and tyrosine hydroxylase in nerves supplying the human postnatal vas deferens and seminal vesicle. Br J Urol. 1997;80:291–9.
Co-localization of nitric oxide synthase, neuropeptides and tyrosine hydroxylase in nerves supplying the human postnatal vas deferens and seminal vesicle
The objective of this study was to investigate the distribution and co-localization patterns of nitric oxide synthase (NOS), neuropeptides, and tyrosine hydroxylase (TH) in intrinsic nerves of the human post-natal vas deferens and seminal vesicle. Tissue samples were obtained from 10 male infants and children within 12 hours of death. The findings revealed that most nerves supplying the muscle coat of both organs were TH-immunoreactive (IR), with the majority also containing neuropeptide Y (NPY), and a smaller proportion containing both NPY and NOS. Some intramuscular nerves additionally contained calcitonin gene-related peptide (CGRP), galanin (GAL), met-enkephalin (m-ENK), or vasoactive intestinal polypeptide (VIP). Non-TH-IR intramuscular nerves were less common and mostly contained NPY along with either VIP or NOS. Presumptive secretomotor nerves formed subepithelial plexuses in both organs, mainly containing NPY co-localized with either VIP or NOS, and minor populations containing CGRP and/or GAL. TH- and substance P (SP)-IR nerves were absent subepithelially. Perivascular nerve plexuses were primarily composed of TH-IR varicose nerves, with most co-localizing NPY and CGRP, a smaller portion containing NPY and NOS, and minor populations containing VIP, m-ENK, SP, or GAL. These findings suggest that the autonomic control of the human vas deferens and seminal vesicle involves various immunohistochemically distinct nerve populations, and NOS is present in a portion of both noradrenergic and non-noradrenergic nerves. Further pharmacological studies are needed to clarify the specific roles of nitric ox
You can read the abstract article at https://pubmed.ncbi.nlm.nih.gov/9284205/.
King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin Receptors, Melanotropic Peptides and Penile Erection. Current topics in medicinal chemistry. 2007;7(11):1098-1106.
Melanocortin Receptors, Melanotropic Peptides and Penile Erection
Penile erection is a complex physiological process influenced by the central nervous system’s melanocortinergic (MC) receptors. Activation of these receptors, particularly MC3 and MC4 subtypes, can initiate and enhance spontaneous erections. While MC4R appears to be the primary mediator of MC-induced erections, the role of MC3R is less understood. Recent research suggests that blocking MC3R in the forebrain may boost melanocortin-induced erections. Additionally, melanocortin agents may interact with oxytocinergic pathways at various neural levels. Current erectile dysfunction treatments target vascular tissue, but manipulating MC receptors offers a potentially effective, centrally mediated approach for sexual dysfunctions. PT-141, a potent MC agonist, has advanced to phase II human trials. Melanocortin agonists may hold promise for treating erectile dysfunction and addressing issues like decreased sexual motivation and libido.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2694735/.
Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005;26(10):1972–7.
Melanocortinergic control of penile erection
Activation of melanocortin receptors in the forebrain, spinal cord, and other regions can induce penile erection in both rats and humans, offering insights into the neural circuits involved in sexual behavior. This review explores the roles of melanocortin receptors and neurons in various parts of the central nervous system, including the hypothalamus, hindbrain, spinal cord, and peripheral nerves, in regulating erectile function. Recent findings on neuropeptides involved in penile erection further enhance our understanding of the central control of sexual behavior. Melanocortin agonists hold promise as potential alternatives for addressing common issues like erectile dysfunction, presenting new therapeutic avenues.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4768007/.
Wessells H, et al. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J. Urol. 1998;160(2):389–93.
Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study
We conducted a double-blind, placebo-controlled crossover study to assess the erectogenic effects of Melanotan-II, a novel cyclic alpha-melanocyte-stimulating hormone analogue, in the treatment of psychogenic erectile dysfunction in ten men. Real-time RigiScan monitoring was employed to record the presence, duration, and rigidity of erections during a 6-hour period. Eight out of ten men treated with Melanotan-II experienced clinically significant erections, with a mean duration of tip rigidity greater than 80% lasting 38.0 minutes, compared to 3.0 minutes with the placebo (p=0.0045). Some transient side effects such as nausea, stretching, yawning, and decreased appetite were reported more frequently with Melanotan-II but were manageable and did not require treatment. In conclusion, Melanotan-II demonstrates potent erectogenic properties in men with psychogenic erectile dysfunction, with manageable side effects at a dose of 0.025 mg/kg.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/9679884/.
Zipf WB, Payne AH, Kelch RP. Prolactin, growth hormone, and luteinizing hormone in the maintenance of testicular luteinizing hormone receptors. Endocrinology. 1978;103(2):595-600.
Prolactin, growth hormone, and luteinizing hormone in the maintenance of testicular luteinizing hormone receptors
To investigate the pituitary’s impact on testicular function, we examined how PRL, GH, and LH, either individually or in combinations, influenced testicular LH receptor concentration and testosterone synthesis responsiveness to LH in hypophysectomized adult rats. Hypophysectomy led to an 80% reduction in LH receptor concentration and a 70% decrease in testicular responsiveness to LH. Treatment with PRL (75 or 150 μg/day) or GH (75 or 150 μg/day), initiated shortly after surgery and continued for 6 days, partially mitigated the loss of LH receptors. Combining PRL (150 μg/day) with GH (150 μg/day) had an additive effect on LH receptor concentration. When LH (5 μg/day), PRL, and GH were administered together, it prevented any loss of LH receptors. Notably, LH’s positive effect on its receptor was enhanced in the presence of PRL. These findings demonstrate that maintaining LH receptors depends partially on PRL and GH, and PRL prevents LH from negatively affecting LH receptor concentration. However, PRL treatments alone did not enhance testicular responsiveness to LH, which only occurred when LH was administered along with PRL and/or GH, maintaining responsiveness at intact control levels. These results suggest distinct hormonal regulations for LH receptor concentration and testicular responsiveness to LH.
You can read the abstract of the article at https://academic.oup.com/endo/article-abstract/103/2/595/2618576?redirectedFrom=fulltext&login=false.
Doessing S, Heinemeier KM, Holm L, et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. The Journal of Physiology. 2010;588(Pt 2):341-351. doi:10.1113/jphysiol.2009.179325.
Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis
In skeletal muscle and tendons, the extracellular matrix plays a crucial role in providing tensile strength and aiding tissue regeneration after injury. Mechanical loading influences musculoskeletal tissue adaptation by regulating growth factors such as growth hormone (GH) and insulin-like growth factor-I (IGF-I). To test whether GH promotes collagen synthesis in musculotendinous tissue, a 14-day administration of 33-50 microg kg(-1) day(-1) recombinant human GH (rhGH) was conducted in healthy young individuals. This led to increased levels of serum GH, serum IGF-I, and IGF-I mRNA expression in both tendon and muscle. Collagen I mRNA expression and collagen protein synthesis significantly increased in tendons (3.9-fold and 1.3-fold, respectively) and muscles (2.3-fold and 5.8-fold, respectively). Notably, GH elevation had no effect on myofibrillar protein synthesis, indicating that GH primarily enhances matrix collagen synthesis in adult human musculotendinous tissue, strengthening it without significantly impacting muscle cell hypertrophy.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2821728/.
Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am J Kidney Dis. 2015;65:327–336.
Insulin-like growth factors and kidney disease
Insulin-like growth factors (IGF-1 and IGF-2) are essential for normal growth and development, sharing structural similarities with proinsulin. They play a crucial role in promoting cell proliferation, differentiation, survival, and insulin-like metabolic effects across various cell types and tissues. Specifically, IGFs are vital for kidney development before and after birth. IGF-1 is a key mediator of growth hormone actions, and imbalances in growth hormone levels can affect kidney function. IGFs influence renal hemodynamics directly and indirectly through interactions with the renin-angiotensin system. The IGF system comprises IGF-1, IGF-2/mannose-6-phosphate, insulin receptors, and a family of 6 high-affinity IGF-binding proteins that modulate IGF functions. Dysregulation of the IGF system is implicated in various kidney diseases. Early diabetic nephropathy and polycystic kidneys exhibit enhanced IGF activity, while chronic kidney failure often involves IGF resistance. IGFs may have a role in promoting stem cell repair in kidney injuries. Most IGF effects are mediated by the tyrosine kinase IGF-1 receptor, and there have been recent developments in the creation of inhibitors. Further research is needed to determine the optimal use of IGF-based therapies in kidney disease.
You can read the abstract of the article at https://www.ajkd.org/article/S0272-6386(14)01000-2/fulltext.
Devesa J, Almengló C, Devesa P. Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clinical Medicine Insights Endocrinology and Diabetes. 2016;9:47-71. doi:10.4137/CMED.S38201.
Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth?
In this review, we examine the impact of growth hormone on various tissues and organs, along with its potential role in an organism’s longitudinal growth. We conclude that this hormone plays a crucial role in maintaining tissue and organ integrity during normal human development and post-injury recovery. Its growth-promoting effects do not appear significant during fetal development or early infancy but become prominent during childhood and puberty, primarily mediated by insulin-like growth factor I (IGF-I). Proper GH secretion is essential for IGF-I transcription, although in many tissues, IGF-I production occurs independently of GH. We propose that GH might function as a prohormone rather than a hormone, as it undergoes tissue-specific proteolytic cleavage, yielding shorter GH forms with unclear activity in many tissues and organs.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5063841/.
Scheepens A, Williams CE, Breier BH, Guan J, Gluckman PD. A role for the somatotropic axis in neural development, injury and disease. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1483–1491.
A role for the somatotropic axis in neural development, injury and disease
This review article explores the roles of the somatotropic axis in the growth and development of the normal central nervous system (CNS) and its involvement in brain injury recovery. Traditionally, pituitary-derived growth hormone (GH) was believed to primarily act through the induction of hepatic insulin-like growth factor-I (IGF-I). However, GH receptors (GHRs) have been identified in various body tissues, and they exert both endocrine and local effects, some of which are independent of IGF-I. In the CNS, GHRs are widely distributed among different cell types, yet their functions and endogenous ligands remain poorly understood. It is increasingly recognized that GH, similar to IGF-I, plays a crucial role in CNS growth and development. After brain injury, IGF-I mRNA is induced, particularly in reactive microglia, and the resulting IGF-I protein serves as both a neurotrophic and anti-apoptotic agent for stressed cells, as well as a precursor for neuroprotective peptides. Research suggests that a GH-like substance is upregulated after brain injury, specifically associated with stressed neurons and glia. Central administration of GH shortly after brain injury in juvenile rats has been shown to offer significant neuroprotection, distinct from the neuroprotection provided by IGF-I. These findings have important implications for CNS growth, development, and injury response.
You can read the abstract of the article at https://www.degruyter.com/document/doi/10.1515/jpem-2000-s623/html.
Pathipati P, Gorba T, Scheepens A, Goffin V, Sun Y, Fraser M. Growth hormone and prolactin regulate human neural stem cell regenerative activity. Neuroscience. 2011;190:409–427.
Growth hormone and prolactin regulate human neural stem cell regenerative activity
In previous research, it was demonstrated that the growth hormone (GH) and prolactin (PRL) axis plays a significant role in regulating neuroprotective and neurorestorative mechanisms in the brain, particularly through actions on neural stem cells (NSCs). In this study using NSCs with properties similar to neurogenic radial glia from fetal human forebrains, it was found that both GH and PRL promote NSC proliferation, even in the absence of epidermal growth factor or basic fibroblast growth factor. When applied to differentiating NSCs, they both stimulate neuronal progenitor proliferation, while only PRL affects glial progenitors’ proliferation. Additionally, GH and PRL enhance NSC migration, especially at higher concentrations. While GH activates both GH and PRL receptors in humans, research suggests that some of these effects may be mediated via the PRL receptor, particularly in promoting migration. The specific mechanisms of receptor signaling in NSC proliferation, however, require further investigation. In summary, GH and PRL have intricate effects on NSC activity, potentially playing a role in brain injury-related recovery processes.
You can read the abstract of the article at https://www.ibroneuroscience.org/article/S0306-4522(11)00578-1/fulltext.
Devesa P, Reimunde P, Gallego R, Devesa J, Arce V. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 2011;25:503–510.
Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury
The main objective of this study was to investigate the impact of growth hormone (GH) treatment on the proliferation of endogenous neural progenitor cells in the dentate gyrus (DG) of the brain, which had been stimulated by kainic acid (KA)-induced neurotoxicity. Neurotoxicity was induced by administering KA through intraperitoneal injection, and GH treatment was carried out for 4 days, either immediately following neurotoxic insult or after a 10-day delay. Proliferating cells were identified through immunodetection following labeling with 5-bromodeoxyuridine (BrdU), and GH expression was monitored using in situ hybridization and immunofluorescence. The results showed that KA administration stimulated the proliferation of hippocampal precursors, and this effect was significantly amplified by GH treatment. Furthermore, hippocampal GH expression increased in response to KA administration. These findings suggest that the proliferative response observed in the rat hippocampus following KA and GH treatment may result from a collaborative action between exogenous and locally-produced hormones, as well as their synergy with other mitogenic factors generated in response to neurotoxic damage. Consequently, GH treatment could potentially be employed in conjunction with other physiological or pathological stimuli to promote cell proliferation.
You can read theabstract of the article at https://www.tandfonline.com/doi/abs/10.3109/02699052.2011.559611.
David Aberg N, Lind J, Isgaard J, Georg KH. Peripheral growth hormone induces cell proliferation in the intact adult rat brain. Growth Horm IGF Res. 2010;20:264–269.
Peripheral growth hormone induces cell proliferation in the intact adult rat brain
Growth hormone (GH) and insulin-like growth factor I (IGF-I) have been shown to enhance cell genesis in various brain regions of GH-IGF-I-deficient hypophysectomized rats. However, the extent to which GH treatment promotes adult cell genesis in pituitary-intact rodents remains unclear. To investigate this, we examined the impact of peripheral administration of bovine growth hormone (bGH) on cell proliferation in different brain regions of normal adult female rats. Cellular division was monitored using bromodeoxyuridine (BrdU) administration over five days. Our study focused on areas with ongoing neurogenesis: the subventricular zone (SVZ), the dentate gyrus (DG) of the hippocampus, as well as the corpus callosum, striatum, and the parietal and piriform cortices. Following bGH treatment, the numbers of BrdU-positive cells increased by 2.0- to 2.5-fold in all brain regions, except for the SVZ, where no increase was observed. This study demonstrates that peripheral bGH administration can boost the generation of new brain cells in normal adult female rats. Consequently, bGH may stimulate cellular proliferation not only in cases of GH deficiency but also under physiological conditions, presenting important implications for GH treatment strategies in patients with normal or near-normal GH or IGF-I levels.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637409001609?via%3Dihub.
Aberg ND, Johansson UE, Aberg MA, et al. Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology. 2007;148:3765–3772.
Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats
In previous studies, we demonstrated that recombinant human (rh) IGF-I promotes cell proliferation and neurogenesis in the hippocampus of hypophysectomized rats. In this investigation, we explored the effects of rhIGF-I on proliferation and differentiation in the cerebral cortex. Adult hypophysectomized rats were administered bromodeoxyuridine (BrdU) to label newborn cells (once daily for the first 5 days), while rhIGF-I was peripherally administered for either 6 or 20 days. In the cerebral cortex, the number of BrdU-labeled cells increased after 20 days but not after 6 days of rhIGF-I infusion, suggesting that rhIGF-I enhances the survival of newborn cells in the cerebral cortex. We also observed an increase in oligodendrogenesis in the cerebral cortex, confirmed by changes in myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Additionally, an increase in capillary-associated BrdU-positive cells was noted. While rhIGF-I did not affect cortical astrogliogenesis or neurogenesis, its capacity to induce cortical oligodendrogenesis may hold significance for cortical regenerative potential.
You can read the full article at https://academic.oup.com/endo/article/148/8/3765/2502105?login=false.
Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNF alpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107.
IGF-1 protects oligodendrocyte progenitors against TNF alpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway
In the context of periventricular leukomalacia (PVL), a common brain injury in preterm infants attributed to proinflammatory cytokine-induced damage to oligodendrocyte progenitor cells (OPCs), strategies to prevent OPC death are critical. This study employed an in vitro cell culture system to investigate insulin-like growth factor-1 (IGF-1) as a potential protector against tumor necrosis factor-alpha (TNFalpha)-induced OPC injury. Isolated OPCs from neonatal rat optic nerves were exposed to TNFalpha, resulting in OPC death. However, IGF-1 demonstrated a dose-dependent protective effect against TNFalpha cytotoxicity. This protection was mediated through the PI3K/Akt pathway, as evidenced by partial abrogation with Akt inhibitors. IGF-1’s downstream effects included the phosphorylation of BAD, prevention of Bax translocation to the mitochondrial membrane, and inhibition of caspase-9 and caspase-3 activation, ultimately interrupting the mitochondrial apoptotic pathway. These findings suggest that IGF-1 shields OPCs by interfering with the mitochondrial apoptotic pathway via PI3K/Akt activation.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1002/glia.20530.
Gonzalez-Perez O, Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev. 2011;67:147–156.
Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor
Demyelinating diseases, which involve the loss of oligodendrocytes and myelin sheaths from nerve fibers, are a significant cause of disability, especially in young adults, with no effective treatment currently available. Neural stem cells (NSCs) located in the adult subventricular zone (SVZ) have shown promise in brain repair therapies. SVZ Type-B cells, a subtype of NSCs, have the ability to self-renew and generate both neurons and glia. Recent research has revealed that these cells, when stimulated with epidermal growth factor (EGF), become highly mobile and proliferative. Notably, a subset of EGF-activated cells expresses markers characteristic of oligodendrocyte precursor cells (OPCs). Upon EGF withdrawal, OPCs originating from SVZ-derived progenitors differentiate into myelinating and pre-myelinating oligodendrocytes in various white matter regions, such as the corpus callosum, fimbria fornix, and striatum. In the presence of demyelinating injuries, OPCs derived from EGF-stimulated SVZ progenitors actively contribute to myelin repair. Due to their migratory potential and capacity to become myelin-forming cells, SVZ NSCs offer a valuable intrinsic source of OPCs for maintaining the oligodendrocyte population in white matter and promoting recovery in cases of demyelinating damage.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3109119/.
Pan SN, Ma HM, Su Z, Zhang CX, Zhu SY, Du ML. Epidermal growth factor receptor signaling mediates growth hormone-induced growth of chondrocytes from sex hormone-inhibited adolescent rats. Clin Exp Pharmacol Physiol. 2011;38:534–542.
Epidermal growth factor receptor signaling mediates growth hormone-induced growth of chondrocytes from sex hormone-inhibited adolescent rats
In this study, the mechanisms underlying the growth-promoting effects of growth hormone (GH) in children with central precocious puberty treated with gonadotropin-releasing hormone analogue (GnRHa) were investigated. Using cultured growth plate chondrocytes from adolescent rats treated with GnRHa, the researchers explored the role of the epidermal growth factor receptor (EGFR) pathway in GH-induced growth. Chondrocytes treated with GH showed increased proliferation, accompanied by enhanced phosphorylation of Erk1/2 and EGFR, as well as elevated expression of epidermal growth factor (EGF). Pre-treatment with inhibitors targeting different components of this pathway, including Janus tyrosine kinase (JAK) 2, EGFR kinase, extracellular signal-regulated kinase (Erk) activation, or a neutralizing antibody against EGF, partially or completely inhibited chondrocyte proliferation and the activation of Erk1/2 and EGFR. These findings suggest that GH promotes chondrocyte proliferation by activating EGFR signaling.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1440-1681.2011.05547.x.
Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459.
The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation
The decline in central nervous system (CNS) remyelination efficiency with age has significant implications for conditions like multiple sclerosis (MS). To understand the factors contributing to this age-related slowdown, researchers investigated whether it was due to a decrease in the recruitment of oligodendrocyte progenitors (OPs) or a hindrance in the differentiation of OPs into remyelinating oligodendrocytes. They compared the OP response during remyelination in young and old rats with toxin-induced CNS demyelination. By examining two OP markers, platelet-derived growth factor-alpha receptor and the OP transcription factor myelin transcription factor 1 (MyT1), they found delayed OP colonization in the old rats compared to the young ones. Additionally, there was a progressively delayed OP differentiation in older animals. These findings suggest that the decline in remyelination efficiency with age is linked to both impaired OP recruitment and differentiation, necessitating strategies that address both aspects of the regenerative process.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6758320/.
Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, Dall R, et al. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:1505–1512.
Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study
The impact of GH on bone remodeling in healthy adults was systematically investigated in a multicenter study. Ninety-nine healthy volunteers received either placebo or recombinant human GH for 28 days, followed by a 56-day wash-out period. Biochemical markers related to bone and collagen turnover were assessed. GH administration led to significant increases in these markers, with the 0.2 IU/kg-day group showing a more pronounced effect. Procollagen type III and osteocalcin levels remained elevated even after GH withdrawal, while levels of C-terminal propeptide of type I procollagen and type I collagen telopeptide declined after day 42. Gender differences were observed, with females exhibiting a less responsive reaction to GH treatment. These findings suggest that GH-induced modifications in specific markers, especially procollagen type III and osteocalcin, may serve as indicators of GH abuse detection.
You can read the full article at https://academic.oup.com/jcem/article/85/4/1505/2852750?login=false.
Powrie JK, Bassett EE, Rosen T, Jorgensen JO, Napoli R, Sacca L, et al. Detection of growth hormone abuse in sport. Growth Horm IGF Res. 2007;17:220–226.
Detection of growth hormone abuse in sport
The use of growth hormone (GH) by athletes for its anabolic and lipolytic effects is well-documented, although GH is a banned substance on the World Anti-Doping Agency (WADA) list. Detecting GH abuse has been challenging, but two approaches have emerged. One method involves measuring pituitary GH isoforms, and the other relies on markers of GH action. Pituitary GH contains various isoforms, while recombinant human GH consists solely of the 22-kDa isoform. Immunoassays that differentiate these isoforms form the basis of the test adopted by WADA during the Athens Olympic Games. As of now, no athlete has tested positive using this method. The GH-2000 project introduced an alternative test based on measuring insulin-like growth factor-I (IGF-I) and type III pro-collagen (P-III-P) levels, which increase in response to GH in a dose-dependent manner. When combined with discriminant function analysis, these markers successfully distinguished between individuals taking GH and those on a placebo in double-blind placebo-controlled trials. Subsequent studies demonstrated the test’s applicability across various ethnicities and its resistance to interference from injuries.
You can read the abstract of the article at https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/dta.59.
Ueland T, Fougner SL, Godang K, Schreiner T, Bollerslev J. Serum GH and IGF-I are significant determinants of bone turnover but not bone mineral density in active acromegaly: a prospective study of more than 70 consecutive patients. Eur J Endocrinol. 2006;155:709–715.
Serum GH and IGF-I are significant determinants of bone turnover but not bone mineral density in active acromegaly: a prospective study of more than 70 consecutive patients
The study aimed to investigate the impact of gonadal status and disease activity on bone metabolism in individuals with active acromegaly, a condition characterized by excessive GH secretion. In a cohort of 73 consecutive patients (40 women and 33 men), bone turnover was significantly increased in relation to disease activity. Acromegalic women exhibited an increased bone area and slightly reduced bone mineral content, leading to a notable decrease in bone mineral density (BMD) in various regions. However, no differences in bone turnover or BMD were observed between those with normal and low gonadal function. Multivariate analysis identified age, body mass index (BMI), and gender as independent predictors of total BMD in acromegaly. These findings suggest that women with active acromegaly, regardless of gonadal status or disease activity, experience reduced total body BMD, with bone turnover significantly increased, possibly offsetting the anabolic effects of excess GH/IGF-I. The study emphasizes the importance of biomechanical analyses in investigating endocrine disorders that affect bone size and distribution between compartments.
You can read the abstract of the article at https://academic.oup.com/ejendo/article-abstract/155/5/709/6695752?redirectedFrom=fulltext&login=false.
Dioufa N, Schally AV, Chatzistamou I, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18611-18615. doi:10.1073/pnas.1013942107.
Acceleration of wound healing by growth hormone-releasing hormone and its agonists
This study unveils a novel role of growth hormone-releasing hormone (GHRH) in wound healing and tissue repair, primarily through its action on wound-associated fibroblasts. Both cultured mouse embryonic fibroblasts (MEFs) and in vivo wound-associated fibroblasts expressed GHRH receptor splice variant SV1. GHRH and its agonist, JI-38, induced the expression of α-smooth muscle actin (αSMA) in fibroblasts, promoting their migration and accelerating wound healing in mice. The treated wounds exhibited increased fibroblast abundance during early stages and faster reformation of the epithelium during later stages. These findings suggest a potential clinical use of GHRH for enhancing the healing of skin wounds caused by various factors.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2972940/.
Kiaris H, et al. Ligand-dependent and -independent effects of splice variant 1 of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA. 2003;100:9512–9517.
Ligand-dependent and -independent effects of splice variant 1 of growth hormone-releasing hormone receptor
Current evidence suggests that growth hormone-releasing hormone (GHRH), aside from its neuroendocrine functions, directly influences various nonpituitary tissues, especially tumors, by promoting cell proliferation. This study focused on a receptor splice variant, SV1, expressed in normal and tumor tissues, including HEC-1A human endometrial carcinoma cells. SV1 exhibited intrinsic, ligand-independent activity and played a role in promoting cell proliferation and colony formation. SV1’s impact was evident in reducing cell sensitivity to GHRH when exogenously added. Notably, SV1 expression was observed in around 43% of human primary endometrial carcinoma specimens. These findings highlight SV1’s capacity to operate independently of ligands and suggest its potential association with carcinogenesis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC170949/.
Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609.
Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction
The study aimed to investigate whether growth hormone-releasing hormone (GHRH) directly activates cardiac repair mechanisms independently of the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis. After myocardial infarction (MI) in rats, they received either placebo, rat recombinant GH, or JI-38, a potent GHRH agonist, for four weeks. JI-38 did not elevate GH or IGF-1 levels but significantly mitigated cardiac functional decline and remodeling post-injury. In contrast, GH administration increased GH, IGF-1, and body/heart weight but failed to counteract cardiac structural and functional decline. JI-38 and GH both enhanced cardiac precursor cell proliferation, but only JI-38 increased antiapoptotic gene expression. The presence of GHRH receptors on myocytes supported the notion of direct cardiac signal activation by GHRH. These results suggest a potential cardiac repair signaling pathway involving GHRH in the heart, with therapeutic implications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2823907/.
Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312.
Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart
The study aimed to explore the effects of the hypothalamic neuropeptide growth hormone-releasing hormone (GHRH) on cardiac myocyte cell survival and the underlying signaling mechanisms. GHRH receptor (GHRH-R) mRNA was detected in adult rat ventricular myocytes (ARVMs) and rat heart H9c2 cells. GHRH prevented cell death and caspase-3 activation induced by serum starvation and isoproterenol in ARVMs. The GHRH-R antagonist JV-1-36 negated GHRH’s protective effects. GHRH-induced cardiac cell protection involved extracellular signal-regulated kinase (ERK)1/2, phosphoinositide-3 kinase (PI3K)/Akt activation, and adenylyl cyclase/cAMP/protein kinase A signaling. GHRH also blocked pro-apoptotic effects induced by isoproterenol. Similar results were observed in H9c2 cardiac cells. Additionally, GHRH improved left ventricular recovery and reduced infarct size in rat hearts subjected to ischaemia-reperfusion (I/R) injury, involving PI3K/Akt signaling. These findings highlight a novel cardioprotective role of GHRH in promoting cardiac myocyte survival through multiple signaling pathways and protecting against I/R injury.
You can read the full article at https://academic.oup.com/cardiovascres/article/83/2/303/324318?login=false.
Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623–12628.
Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets
Therapeutic approaches for pancreatic islet cell transplantation urgently seek to enhance beta-cell mass through increased proliferation and prolonged cell survival. This study highlights the expression of the biologically active splice variant-1 (SV-1) of the growth hormone-releasing hormone (GHRH) receptor in rat insulinoma (INS-1) cells and human pancreatic islets. In vitro experiments with INS-1 cells revealed that the GHRH agonist JI-36 significantly boosted cell proliferation and reduced apoptosis, leading to increased islet size and enhanced glucose-stimulated insulin secretion in rat islets. Transplantation of islets treated with the GHRH agonist JI-36 into diabetic mice achieved normoglycemia faster and more consistently than untreated islets, indicating the potential of GHRH agonists as a pharmacological therapy for promoting islet graft growth and proliferation in diabetic patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2906543/.
Kiaris H, Schally AV, Armatis P. Direct action of growth hormone-releasing hormone agonist JI-38 on normal human fibroblasts: Evidence from studies on cell proliferation and c-myc proto-oncogene expression. Regul Pept. 2001;96:119–124.
Direct action of growth hormone-releasing hormone agonist JI-38 on normal human fibroblasts: Evidence from studies on cell proliferation and c-myc proto-oncogene expression
Growth hormone-releasing hormone (GHRH), known for its role in stimulating growth hormone release from the pituitary, has also been implicated in promoting the proliferation of cancer cells. In this study, the effects of JI-38, an agonistic analog of GHRH, on cultured normal human diploid dermal fibroblasts (NHF) were explored. Exposure to 10(-7) M JI-38 significantly increased the proliferation rate of early passage NHF by approximately 100%, along with up to a 3.5-fold increase in c-myc proto-oncogene mRNA levels when exposed to JI-38 concentrations ranging from 10(-8) to 5×10(-6) M for 24 hours. However, this proliferative effect was lost in late-passage NHF. Interestingly, prolonged exposure to 10(-7) M JI-38 over multiple passages reduced NHF proliferation rates, indicating a dual role for GHRH agonist in both stimulating and inhibiting cell growth at different developmental stages.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S016701150000166X?via%3Dihub.
Kiaris H, Schally AV. Decrease in telomerase activity in U-87MG human glioblastomas after treatment with an antagonist of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1999;96:226–231.
Decrease in telomerase activity in U-87MG human glioblastomas after treatment with an antagonist of growth hormone-releasing hormone
In this study, we explored the impact of growth hormone-releasing hormone (GH-RH) antagonist MZ-5-156 on telomerase activity and its potential role in tumor growth inhibition. Using nude mice with U-87MG human glioblastomas, we found that treatment with MZ-5-156 reduced telomerase activity compared to controls. Additionally, when various cancer cells were cultured in vitro, MZ-5-156 at 3 microM also inhibited telomerase activity. Further analysis in U-87MG glioblastomas revealed that MZ-5-156 down-regulated the hTRT gene, responsible for the telomerase catalytic subunit, without significantly affecting mRNA levels of other telomerase-related genes or the c-myc protooncogene. These findings suggest that GH-RH antagonists, like MZ-5-156, may inhibit tumor growth by reducing telomerase activity, specifically by down-regulating the hTRT gene. Further research is necessary to explore the broader implications of GH-RH antagonists on telomerase inhibition in different tumor types.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC15121/.
Bellyei S, et al. GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro. Cancer Lett. 2010;293:31–40
GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro
We examined the impact of the GHRH antagonist, MIA-602, on the metastatic processes of three human cancers (DBTRG-05 glioblastoma, MDA-MB-468 estrogen-independent breast cancer, and ES-2 clear cell ovarian cancer) in vitro. GHRH receptors and their main splice variant, SV1, were present on all three cell lines. Upon treatment with MIA-602, a significant decrease in cell viability was observed, accompanied by substantial inhibition of cell invasion and reduced release of MMPs. Cancer cell attachment to fibronectin and matrigel was notably impaired. Wound-healing experiments demonstrated decreased cellular motility in all three cell lines. Furthermore, MIA-602 led to the upregulation of caveolin-1 and E-cadherin while significantly downregulating NF-kappaB and beta-catenin. These findings highlight the potential clinical utility of potent GHRH antagonists in cancer therapy, as they exhibit inhibitory effects on proliferation and the development of metastasis.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0304383509007307?via%3Dihub.
Schally AV, Comaru-Schally AM. Growth Hormone Secretagogues. In: Bercu BB, Walker RF, editors. Clinical Practice. Dekker, New York: 1998. pp. 131–142.
McAnulty RJ. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671.
Fibroblasts and myofibroblasts: Their source, function and role in disease
Fibroblasts are versatile cells found throughout the body, with various phenotypes, including non-contractile fibroblasts, contractile myofibroblasts, and intermediate forms like protomyofibroblasts. They play crucial roles in regulating extracellular matrices, interstitial fluid balance, wound healing, and tissue repair. Fibroblast populations can be maintained or expanded by resident cell proliferation, but emerging evidence suggests their derivation from processes like epithelial-mesenchymal transition and mesenchymal stem cells. Dysregulation of fibroblast function is linked to numerous diseases, impacting tissue structure and function, often causing significant health issues. Currently, there are no specific therapies targeting fibroblast-related pathologies, but ongoing research offers hope for improved treatments in the future.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1357272506003256?via%3Dihub.
Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283.
Available at http://www.medicineatmichigan.org/web-exclusives/2016/february/medsport-studies-hgh-acl-repair.
Khorram O, Yeung M, Vu L, Yen SS. Effects of [norleucine27]growth hormone-releasing hormone (GHRH) (1-29)-NH2 administration on the immune system of aging men and women. J Clin Endocrinol Metab. 1997;82(11):3590-6.
Effects of [norleucine27]growth hormone-releasing hormone (GHRH) (1-29)-NH2 administration on the immune system of aging men and women
Aging in humans is linked to the decline of both the GH-insulin-like growth factor I (IGF-I) axis and the immune system. Lymphocytes express GH-IGF-I, GHRH, and their respective receptors, suggesting that restoring this axis in elderly individuals with GHRH administration may boost immune cell function. In a single-blind, randomized, placebo-controlled trial lasting 5 months, healthy elderly subjects self-administered nightly subcutaneous placebo for 4 weeks, followed by 16 weeks of [norleucine27]GHRH (1-29)-NH2 at a dose of 10 micrograms/kg. The treatment led to significant increases in GH secretion and serum IGF-I levels in both men and women, which lasted up to 12 and 16 weeks, respectively. Immune system activation occurred within 4 weeks, with various lymphocyte subsets showing significant increases, along with enhanced responsiveness to mitogens and elevated cytokine-related markers. These findings suggest that GHRH analog administration has substantial immune-enhancing effects, potentially benefiting individuals with compromised immune function.
You can read the full article at https://academic.oup.com/jcem/article/82/11/3590/2865942?login=false.
Brown PA, Davis WC, Draghia-akli R. Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation. Mol Ther. 2004;10(4):644-51.
Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation
In this study, the impact of plasmid-mediated GHRH treatment on immune function and the overall health of Holstein heifers was assessed. Thirty-two heifers received GHRH-expressing plasmid injections in the third trimester of pregnancy, while 20 heifers served as controls. No adverse effects were observed from the plasmid delivery or GHRH expression. The GHRH-treated animals exhibited increased numbers of various immune cells, such as T-cells and natural killer lymphocytes, which were sustained long-term. These improvements in immune function correlated with plasmid expression. Additionally, the treated heifers showed enhanced body condition scores and reduced hoof pathology, ultimately resulting in lower mortality rates compared to controls. These findings demonstrate the effectiveness of myogenic GHRH plasmid administration in improving immune health and overall well-being in large mammals, suggesting its potential for therapeutic protein production and research in relevant animal models.
You can read the full article at https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(04)01310-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1525001604013103%3Fshowall%3Dtrue.
Botteri FM, Van der Putten H, Wong DF, Sauvage CA, Evans RM. 1987 Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen. Mol Cell Biol . 7:3178–3184.
Unexpected thymic hyperplasia in transgenic mice harboring a neuronal promoter fused with simian virus 40 large T antigen
Transgenic mice were created with the human GRF promoter fused to simian virus 40 early region sequences to investigate GRF gene regulation. Surprisingly, these mice exhibited severe thymic hyperplasia, despite normal hypothalamic function. Immunohistochemical analysis revealed the expression of large T antigen in thymic epithelial cells with endocrine properties. These cells normally produce thymic hormones, and the observed thymic hyperplasia appeared to result from the inappropriate production of T-cell maturation factors by these epithelial cells, possibly involving increased self-renewal of normal T stem cells in the thymus.
You can read the abstract of the article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC367952/.
Blazar BR, Brennan CA, Broxmeyer HE, Schultz LD, Vallera DA. 1995 Transgenic mice expressing either bovine growth hormone (bGH) or human GH releasing hormone (hGRH) have increased splenic progenitor cell colony formation and DNA synthesis in vitro and in vivo. Exp Hematol . 22:1397–1406.
Transgenic mice expressing either bovine growth hormone (bGH) or human GH releasing hormone (hGRH) have increased splenic progenitor cell colony formation and DNA synthesis in vitro and in vivo
To investigate the potential impact of growth hormone (GH) on the hematopoietic system, transgenic mice expressing bovine GH (bGH) or human growth hormone-releasing hormone (hGRH) genes, leading to constant GH overproduction, were examined for hematopoietic progenitor cells in the spleen and bone marrow (BM). These transgenic mice exhibited splenic hyperplasia, characterized by increased numbers of erythroid and megakaryocytic progenitor cells and enhanced megakaryocyte development. In hGRH mice, the number of day-10 CFU-S colonies, indicating multilineage progenitor cell effects, was significantly higher than in nontransgenic controls. A substantial proportion of splenic progenitors were actively cycling in transgenic mice, in contrast to controls. While these effects were prominent in the spleen, similar changes were not observed in BM or peripheral blood parameters, except for megakaryocyte colony formation. Overall, these findings highlight the primarily splenic hematopoietic effects resulting from constitutive bGH or hGRH expression.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/7498369/.
Siriani MC, Annibale B, Tagliaferi F, et al. 1992 Modulation of human natural killer activity by vasoactive intestinal polypeptide (VIP) family. VIP, glucagon, and GHRF specifically inhibit NK activity. Regul Pept . 38:79–87.
Modulation of human natural killer activity by vasoactive intestinal polypeptide (VIP) family. VIP, glucagon, and GHRF specifically inhibit NK activity
Vasoactive intestinal polypeptide (VIP), a neuropeptide known to modulate immune functions, has been found to diminish the natural killer (NK) cell activity in human large granular lymphocytes (LGL), exhibiting maximal inhibition at doses between 10^(-8) to 10^(-6) M. Other neuropeptides within the VIP family, including secretin, peptide histidine isoleucine (PHI), glucagon, and human growth hormone-releasing factor (GHRF), were also tested. Among these, secretin and PHI had no significant impact on NK cell activity, while glucagon and GHRF showed inhibitory effects. GHRF exhibited a similar potency to VIP with a D50 of 10^(-9) M, while glucagon had a D50 of 10^(-8) M. An antagonist to VIP (4Cl-D-Phe6-Leu17) was able to completely reverse the inhibitory effect of VIP on NK activity and partially reverse the effects of glucagon and GHRF, suggesting the presence of a VIP receptor on human LGL with NK activity.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0167011592900745?via%3Dihub.
Clark R. 1997 The somatogenic hormones and insulin-like growth factor-I: stimulators of lymphopoiesis and immune function. Endocr Rev . 18:157–179.
Auernhammer CJ, Strasburger CJ. 1995 Effect of growth hormone and insulin-like growth factor I on the immune system. Eur J Endocrinol . 133:635–645.
Effect of growth hormone and insulin-like growth factor I on the immune system
The concept of a neuro-endocrine-immune axis was introduced over 50 years ago, with growth hormone (GH) being a crucial component of this axis, influencing various immune functions at molecular and cellular levels. GH plays a role in thymocyte proliferation, natural killer cell cytotoxicity, lymphocyte proliferation, and other immune processes. While GH binding to its receptors on lymphocytes triggers insulin-like growth factor I (IGF-I) production, mediating GH’s effects on cell proliferation, some of GH’s impacts on the immune system appear to be direct, like enhancing monocyte hydrogen peroxide production and stimulating neutrophils to release superoxide anions, enhancing phagocytic activity. Despite these functions and GH being produced in immunological tissues like the thymus and spleen, GH deficiency in humans isn’t typically associated with immune deficiency. The potential use of GH and IGF-I for immunotherapy has been explored, particularly in adults with acquired immunodeficiency syndrome to combat wasting, where GH has shown promise in increasing CD8+ cell counts, albeit with limited impact on CD4+ cell counts due to resistance. Further research is needed to explore their potential as immunotherapies.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.1997.tb18377.x.
Siriani MC, Annibale B, Tagliaferi F, et al. 1992 Modulation of human natural killer activity by vasoactive intestinal polypeptide (VIP) family. VIP, glucagon, and GHRF specifically inhibit NK activity. Regul Pept . 38:79–87.
Modulation of human natural killer activity by vasoactive intestinal polypeptide (VIP) family. VIP, glucagon, and GHRF specifically inhibit NK activity
Vasoactive intestinal polypeptide (VIP), a neuropeptide with immune-modulating properties, has been shown to reduce natural killer (NK) cell activity in human large granular lymphocytes (LGL). This study reveals that VIP’s inhibition of NK cell activity is most pronounced at doses between 10(-8) and 10(-6) M. Neuropeptides related to VIP, such as secretin, glucagon, peptide histidine isoleucine (PHI), and human growth hormone-releasing factor (GHRF), were also investigated. Among these, secretin and PHI had no effect on NK cell activity, while glucagon and GHRF exhibited inhibitory effects. GHRF’s inhibitory effect was similar to that of VIP, with a D50 of 10(-9) M, while glucagon had a D50 of 10(-8) M. The study also introduced a VIP antagonist (4Cl-D-Phe6-Leu17), which effectively reversed VIP-induced NK cell inhibition, as well as the inhibitory effects of glucagon and GHRF, albeit to a lesser extent than VIP. These findings shed light on the physiological role of LGL, suggesting the presence of a VIP receptor on human LGL associated with NK activity.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0167011592900745?via%3Dihub.
Mowles TF, Stricker P, Felix AM, Soike KF, Campbell RM. 1991 Effect of human growth hormone-releasing factor and a potent analog on antibody formation in African Green Monkey. Horm Metab Res . 23:530–534.
Effect of human growth hormone-releasing factor and a potent analog on antibody formation in African Green Monkey
African Green monkeys were subcutaneously injected twice daily for six months with human GRF(1-44)-NH2 (10 micrograms/kg BW) or a more potent analog, [desNH2Tyr1,Ala15]-hGRF(1-29)-NH2 (2 micrograms/kg BW) to assess their potential to induce antibody production. Blood samples, diluted at 1:100, were tested every two weeks for their ability to bind radioiodinated hGRF. One monkey in the hGRF(1-44)-NH2 group (N = 6) developed low-titer GRF antibodies at 6 weeks (19% binding), persisting throughout the 24-week treatment (average = 50-60% binding). Similarly, one monkey in the hGRF analog group (N = 6) displayed low-titer GRF antibodies at 18 weeks (14% binding), with the highest binding observed at 24 weeks (51% binding). Further dilutions (1:1,000 and 1:3,000) confirmed that higher GRF antibody titers were not concealed by antibody excess. Dialyzed sera from these two monkeys did not impact the ability of hGRF(1-44)-NH2 or [desNH2Tyr1,Ala15]-hGRF(1-29)-NH2 to stimulate GH secretion by rat pituitary cells in vitro. After 20 weeks of treatment, significant GH responses were observed following hGRF or hGRF analog injection, indicating that the low-titer GRF antibodies detected during six months of treatment with hGRF or a potent analog were biologically non-neutralizing.
You can read the abstract of the article at https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2007-1003747.
Rapaport R, Oleske J, Ahdieh H, Solomon S, Delfaus C, Denny T. Suppression of immune function in growth hormone-deficient children during treatment with human growth hormone. J Pediatr. 1986;109(3):434-9.
Suppression of immune function in growth hormone-deficient children during treatment with human growth hormone
We examined immune functions, including immunoglobulins, cell surface markers, mitogen responses, and polymorphonuclear cell function, in eight children aged 1 to 17 years with growth hormone deficiency before and during 12 to 16 months of treatment with human growth hormone. Immune functions were normal in all children before treatment. During treatment, serum immunoglobulins and polymorphonuclear cell function were not significantly affected, and the percentage of T cells remained unchanged. However, the percentage of B cells decreased to subnormal levels in seven out of seven patients. T helper/suppressor ratios decreased in all patients, falling below normal levels in seven of eight, and mitogen responses dropped below normal in all. The declines in percentage of B cells, T helper/suppressor ratios, and mitogen responses were mostly transient. Although these immune function depressions did not lead to an increased infection rate during the observation period, caution is advised regarding the indiscriminate use of human growth hormone, as the long-term effects on immune function are still unknown.
You can read the abstract of the article at https://www.jpeds.com/article/S0022-3476(86)80113-5/pdf.
Meazza C, Pagani S, Travaglino P, Bozzola M. Effect of growth hormone (GH) on the immune system. Pediatr Endocrinol Rev. 2004;1 Suppl 3:490-5.
Effect of growth hormone (GH) on the immune system
A growing body of evidence points to a bidirectional relationship between the neuroendocrine system and immune functions. Lymphoid organs like the thymus, spleen, and peripheral blood produce growth hormone (GH), and GH receptors are expressed on various lymphocyte subpopulations. Numerous in vitro and animal studies have demonstrated GH’s significant role in immunoregulation, including the stimulation of T and B cell proliferation, immunoglobulin synthesis, myeloid progenitor cell maturation, and modulation of cytokine responses. However, in humans, GH deficiency (GHD) is
typically not associated with immunodeficiency, and only minor immune function abnormalities have been reported compared to GHD animals. It’s plausible that locally produced GH within the human immune system compensates for the lack of systemic GH. This review summarizes GH’s primary actions on the immune system in vitro, in animal models, and in humans.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/16444180/#:~:text=GH%20stimulates%20T%20and%20B,able%20to%20modulate%20cytokine%20response..
Weigent DA. Expression of Lymphocyte-derived Growth Hormone (GH) and GH-releasing Hormone Receptors in Aging Rats. Cellular immunology. 2013;282(2):71-78. doi:10.1016/j.cellimm.2013.04.009.
Expression of Lymphocyte-derived Growth Hormone (GH) and GH-releasing Hormone Receptors in Aging Rats
In this study, we have observed increased levels of lymphocyte GH in spleen cells from aging animals when compared to their younger counterparts. Additionally, we found that leukocytes in primary and secondary immune tissues, as well as splenic T and B cells in aging rats, exhibit heightened expression of GHRH receptors compared to their younger counterparts, with bone marrow and splenic T cells showing the highest receptor levels in aging animals. Notably, the proliferation and GH induction in spleen cells from aging animals remained largely unchanged after treatment with GHRH. These findings collectively provide new insights into the alterations in GH synthesis and GHRH receptor expression in immune system cells, which may have implications for understanding immune responses in the context of aging.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3736351/.
Arlt W, Hewison M. Hormones and immune function: implications of aging. Aging Cell. 2004;3(4):209-16.
Hormones and immune function: implications of aging
Aging is characterized by immunosenescence, a decline in immune function. This is often associated with reduced production of various hormones, such as the decline in ovarian estrogen during menopause. However, other hormonal changes during aging, including melatonin release from the pineal gland, pituitary growth hormone, adrenal dehydroepiandrosterone, and tissue-specific active vitamin D levels, may also impact immune function. The exact role of these hormonal changes in immunosenescence is still unclear and warrants further research. This review summarizes established knowledge about the secretion and function of these hormones, which generally decline with age and may influence the immune system.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1111/j.1474-9728.2004.00109.x.
Morrhaye G, Kermani H, Legros J-J, et al. Impact of Growth Hormone (GH) Deficiency and GH Replacement upon Thymus Function in Adult Patients. Unutmaz D, ed. PLoS ONE. 2009;4(5):e5668. doi:10.1371/journal.pone.0005668.
Impact of Growth Hormone (GH) Deficiency and GH Replacement upon Thymus Function in Adult Patients
In this study, researchers investigated thymic function in adults with growth hormone deficiency (AGHD) and the impact of growth hormone (GH) treatment on thymopoiesis. They examined 22 AGHD patients, measuring plasma IGF-1 concentrations, signal-joint T-cell receptor excision circle (sjTREC) frequency, and sj/beta TREC ratio. After a one-month GH treatment interruption, both IGF-1 levels and sjTREC frequency decreased significantly. The decline in IGF-1 and sjTREC levels were correlated. Intrathymic T cell proliferation, indicated by the sj/beta TREC ratio, also decreased. However, one month after GH treatment was resumed, IGF-1 and sjTREC levels returned to pre-withdrawal levels, and sj/beta TREC ratio increased, though not fully to the pre-withdrawal level. These findings highlight the importance of the GH/IGF-1 axis in maintaining thymus function in adults and its potential restoration with GH treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2682582/.
Smith TJ. Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacological Reviews. 2010;62(2):199-236. doi:10.1124/pr.109.002469.
Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases?
This focused review delves into the connection between the immune system and insulin-like growth factors (IGF-I and IGF-II), as well as the proteins involved in their actions, such as IGF-I receptor (IGF-IR) and IGF-I binding proteins. The IGF/IGF-IR pathway holds diverse roles in tissue development and function, influencing processes like cell cycle progression, apoptosis, and protein translation. Recent discoveries have revealed the impact of IGF-I and IGF-IR on immune function, potentially serving as a critical switch governing the nature and strength of immune responses. Furthermore, this pathway may play a role in autoimmune diseases, although the relationship remains complex and underexplored. While IGF-I appears to protect against diabetes, antibodies targeting IGF-IR have been found in Graves’ disease patients. The increased presence of IGF-IR+ B and T cells in these patients suggests the pathway’s involvement in autoimmune diseases, presenting a potential therapeutic target. Strategies for targeting IGF-IR have been explored in cancer drug development, showing promise and good tolerability. Recognizing the broader role of IGF-IR in regulating both normal and pathological immune responses may offer valuable therapeutic opportunities for challenging diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879913/.
Ge R-T, Mo L-H, Wu R, et al. Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine. Scientific Reports. 2015;5:7735. doi:10.1038/srep07735.
Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine
This study investigates how insulin-like growth factor-1 (IGF1) influences monocyte (Mo) behavior to mitigate intestinal immune inflammation, focusing on chronic conditions like inflammatory bowel disease. The research reveals that intestinal epithelial cells produce IGF1, whose levels can be increased by exposure to CpG-oligodeoxynucleotides (CpG-ODN). When CpG-ODN-primed intestinal epithelial cells and monocytes are cultured together or monocytes are exposed to IGF1, the monocytes start expressing IL-10. These IGF1-primed monocytes exhibit immune-suppressive properties that effectively dampen immune inflammation in the mouse colon, shedding light on a potential mechanism for mitigating chronic inflammation in the intestines.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295102/.
Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases?. Pharmacol Rev. 2010;62(2):199-236.
Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases
This concise review explores the intricate connection between the immune system and insulin-like growth factors (IGF-I and IGF-II) and their associated proteins, including the IGF-I receptor (IGF-IR) and IGF-binding proteins. The IGF/IGF-IR pathway serves multifaceted roles in tissue development and function, governing processes such as cell cycle progression, apoptosis, and protein translation. Recent discoveries have illuminated its role in regulating immune function, potentially acting as a pivotal switch dictating the quality and magnitude of immune responses. Furthermore, there is emerging evidence suggesting the involvement of IGF-I and IGF-IR in autoimmune diseases, although the intricacies of this relationship remain relatively uncharted. While IGF-I appears to protect against insulin-deficient diabetes in experimental animals, patients with Graves’ disease, characterized by overexpressed IGF-IR in multiple cell types, exhibit activating antibodies against IGF-IR. This pathway’s potential role in autoimmune disease pathogenesis presents an appealing avenue for therapeutic exploration. Notably, IGF-IR has been targeted in cancer drug development, utilizing small molecules and monoclonal antibodies, with generally favorable tolerability. Recognizing the broader impact of IGF-IR in regulating both normal and pathological immune responses opens the door to critical therapeutic possibilities for challenging diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879913/.
Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases?. Pharmacol Rev. 2010;62(2):199-236.
Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases?
This review delves into the connection between the immune system and insulin-like growth factors (IGF-I and IGF-II) and their associated proteins. The IGF/IGF-IR pathway is pivotal in tissue development, regulating cell cycles, apoptosis, and protein translation. Recent findings suggest this pathway may be crucial in modulating immune responses and could be involved in autoimmune diseases. While IGF-I might protect against diabetes in experimental animals, increased IGF-IR activity has been observed in patients with Graves’ disease. The potential role of IGF-I and IGF-IR in autoimmune disease pathogenesis indicates they could be significant therapeutic targets, especially as treatments targeting IGF-IR for cancer.
Bilbao D, Luciani L, Johannesson B, Piszczek A, Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Molecular Medicine. 2014;6(11):1423-1435. doi:10.15252/emmm.201303376.
Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease
The recent surge in autoimmune diseases presents growing challenges to healthcare systems worldwide, often with limited and side-effect-prone treatment options. In this study, we demonstrate that recombinant human insulin-like growth factor-1 (rhIGF-1) promotes the proliferation of regulatory T (Treg) cells in both human and mouse models in vitro. When delivered systemically via continuous minipump, rhIGF-1 effectively halts the progression of autoimmune diseases in mouse models of type 1 diabetes (STZ and NOD) and multiple sclerosis (EAE) in vivo. This treatment boosts Treg cell populations in affected tissues while preserving their regulatory functions. Furthermore, genetically eliminating the IGF-1 receptor in Treg cells abolishes the beneficial effects of rhIGF-1 on multiple sclerosis symptoms in the EAE model, confirming the direct impact of IGF-1 on Treg cell proliferation. These findings establish systemically administered rhIGF-1 as a promising approach for stimulating Treg cell activity, highlighting its clinical potential for mitigating autoimmune diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4237469/.
Spadaro O, Camell C, Bosurgi L, et al. IGF1 shapes the macrophage activation in response to immunometabolic challenge. Cell reports. 2017;19(2):225-234. doi:10.1016/j.celrep.2017.03.046.
IGF1 shapes the macrophage activation in response to immunometabolic challenge
In addition to their phagocytic functions, macrophages play a crucial role in regulating host immunometabolic responses by producing specific cytokines and metabolites. This study reveals that M2-like macrophages differentiated by IL-4 secrete insulin-like growth factor-1 (IGF1), previously believed to be exclusively produced by the liver. Eliminating IGF1 receptors in myeloid cells reduces phagocytosis, increases adipose tissue macrophages, raises adiposity, decreases energy expenditure, and induces insulin resistance in mice on a high-fat diet. In obese myeloid IGF1 receptor knockout (MIKO) mice, adipose tissue macrophages exhibit reduced transcripts associated with M2-like macrophage activation. MIKO mice infected with the helminth Nippostrongylus brasiliensis experience delayed infection resolution but maintain normal insulin sensitivity. Surprisingly, cold exposure fails to induce a pronounced M2-like state or tyrosine hydroxylase expression in adipose tissue macrophages of both control and MIKO mice. These findings highlight the role of IGF1 signaling in shaping macrophage activation phenotypes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513500/.
Johannesson B, Sattler S, Semenova E, et al. Insulin-like growth factor-1 induces regulatory T cell-mediated suppression of allergic contact dermatitis in mice. Disease Models & Mechanisms. 2014;7(8):977-985. doi:10.1242/dmm.015362.
Insulin-like growth factor-1 induces regulatory T cell-mediated suppression of allergic contact dermatitis in mice
Allergic contact dermatitis (ACD) results from an excessive immune response to harmless chemicals and stands as the most common occupational skin condition globally. While various immune cells contribute to ACD, regulatory T (Treg) cells play a critical role in dampening the inflammation. Insulin-like growth factor-1 (IGF-1), known for regulating cell growth, differentiation, and tissue regeneration, has recently been associated with protecting against autoimmune inflammation through Treg cell expansion. This study demonstrates that introducing IGF-1 into mouse skin suppresses ACD, specifically enhancing Treg cell numbers in the affected area and promoting lymphocytes to produce the anti-inflammatory cytokine interleukin 10. The therapeutic benefits extend to systemic or topical IGF-1 administration, suggesting its potential as a novel treatment for ACD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4107326/.
Chapes SK, Simske SJ, Forsman AD, Bateman TA, Zimmerman RJ. Effects of space flight and IGF-1 on immune function. Adv Space Res. 1999;23(12):1955-64.
Effects of space flight and IGF-1 on immune function
We tested the hypothesis that insulin-like growth factor-1 (IGF-1) could mitigate the immune system effects induced by space flight. Twelve male Sprague-Dawley rats with surgically implanted mini osmotic pumps were exposed to a 10-day space flight on STS-77. Six rats received a daily dose of 10 mg/kg of IGF-1, while six rats received saline. Space flight led to lymphocytopenia and granulocytosis, both of which were reversed by IGF-1. Flight rats exhibited higher corticosterone levels than ground controls, but IGF-1 did not impact this stress hormone. The reversed granulocytosis did not correlate with serum corticosterone levels. Space flight and IGF-1 together induced monocytopenia, not observed in ground controls receiving IGF-1 or in space flight animals given saline. While vivarium animals treated with IGF-1 showed a significant increase in spleen weights, this change did not occur in flight animals. Flight animal lymph node cell proliferation in response to agonists was reduced compared to ground controls, and IGF-1 treatment did not enhance this effect. Peritoneal macrophages from flight animals exhibited increased secretion of TNF, IL-6, and NO compared to vivarium controls, while O2(-) secretion remained unaffected. These findings indicate that IGF-1 can alleviate some space flight effects on the immune system, but space flight can also impact the normal response to IGF-1. Grant Numbers: NAGW-1197, NAGW-2328.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/11710377/.
Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999;10(1):5-14.
Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation
In recent years, significant efforts have focused on unraveling the intricate interactions between the endocrine and immune systems. Growth hormone (GH) primarily exerts its effects through insulin-like growth factor-1 (IGF-1), a key endocrine regulator of growth and development in normal circumstances. Beyond its role in growth, IGF-1 also plays a central role in immune regulation and the modulation of inflammatory responses. This article explores the participation of IGF-1 in both innate and adaptive immunity as well as host-defense mechanisms. Additionally, it discusses the influence of IGF-1 on various inflammatory disorders, such as sepsis, sepsis-induced catabolism, and degenerative arthritis. Drawing from recent insights, the article examines the underlying pathophysiology, potential challenges, and future prospects of IGF-1 supplementation therapy for these conditions.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1359610198000227?via%3Dihub.
Granata R., Trovato L., Gallo M. P., et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovascular Research. 2009;83(2):303–312. doi: 10.1093/cvr/cvp090.
Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart
The study aimed to investigate the impact of growth hormone-releasing hormone (GHRH) on the survival of cardiac myocytes and the underlying signaling pathways. The research revealed that GHRH prevented cell death and caspase-3 activation in cardiac myocytes under conditions of serum starvation and beta-adrenergic receptor stimulation. This protective effect was mediated through the GHRH receptor and involved the activation of extracellular signal-regulated kinase (ERK)1/2, phosphoinositide-3 kinase (PI3K)/Akt, and adenylyl cyclase/cAMP/protein kinase A signaling pathways. GHRH also counteracted the pro-apoptotic effects of isoproterenol. In addition, GHRH improved left ventricular recovery and reduced infarct size in rat hearts subjected to ischemia-reperfusion injury, with these effects relying on PI3K/Akt signaling. Overall, the study highlights a novel cardioprotective role of GHRH in promoting cardiac myocyte survival through various signaling mechanisms.
You can read the full article at https://academic.oup.com/cardiovascres/article/83/2/303/324318?login=false.
Penna C., Settanni F., Tullio F., et al. GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology. 2013;154(4):1624–1635. doi: 10.1210/en.2012-2064.
GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways
Growth hormone-releasing hormone (GHRH) stimulates the synthesis and release of growth hormone from the pituitary and has direct effects on extrapituitary tissues. In a study involving isolated rat hearts subjected to ischemia/reperfusion (I/R), GHRH administered at reperfusion reduced myocardial reperfusion injury and improved heart function. The cardioprotective effects of GHRH were inhibited by various antagonists and inhibitors, including JV-1-36, 5-hydroxydecanoate, atractyloside, Wortmannin (WM), and tyrphostin-AG490. Western blot analysis revealed that GHRH activated multiple signaling pathways, including the reperfusion injury salvage kinases (RISK), phosphoinositide 3-kinase/Akt, ERK1/2, glycogen synthase kinase-3β, and signal transducer and activator of transcription-3 (STAT-3), as part of the survivor activating factor enhancement (SAFE) pathway. GHRH-mediated activation of these pathways was blocked by specific inhibitors. Additionally, GHRH increased the phosphorylation of endothelial nitric oxide synthase and AMP-activated protein kinase while preserving postischemic nicotinamide adenine dinucleotide (NAD(+)) levels. These findings suggest that GHRH protects the heart from I/R injury through receptor-mediated mechanisms, leading to the activation of RISK and SAFE pathways, which may converge on mitochondria and AMP-activated protein kinase.
You can read the full article at https://academic.oup.com/endo/article/154/4/1624/2423622?login=false.
Kanashiro-Takeuchi R. M., Szalontay L., Schally A. V., et al. New therapeutic approach to heart failure due to myocardial infarction based on targeting growth hormone-releasing hormone receptor. Oncotarget. 2015;6(12):9728–9739. doi: 10.18632/oncotarget.3303.
New therapeutic approach to heart failure due to myocardial infarction based on targeting growth hormone-releasing hormone receptor
In previous studies, growth hormone-releasing hormone (GHRH) agonists demonstrated cardioprotective effects after myocardial infarction (MI). New potent GHRH agonists were evaluated for their in vitro and in vivo activities in promoting cardiac repair. These agonists improved cell survival by reducing calcium influx in cultured H9c2 cells subjected to serum deprivation. In rats with cardiac infarction, selected GHRH agonists (JI-38, MR-356, MR-409) reduced MI size, increased cardiac c-kit+ cells, mitotic divisions, and vascular density. MR-409 also lowered plasma levels of inflammatory cytokines one week post-MI. Gene expression analysis revealed that MR-409 treatment inhibited pro-apoptotic and pro-fibrotic pathways while elevating bone morphogenetic proteins. These findings suggest that GHRH agonists may reduce inflammation post-MI and enhance mechanisms involved in cardiac healing and remodeling, making them a potential therapeutic option for patients with cardiac dysfunction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4496393/.
Available at http://www.jimmunol.org/content/166/6/4195.
Mora Rodríguez JA, Porchia LM, Camargo F, López-Bayghen E. The use of insulin-like growth factor 1 improved the parameters of the seminogram in a patient with severe oligoasthenoteratozoospermia. SAGE Open Med Case Rep. 2019;7:2050313X19834154. Published 2019 Mar 5. doi:10.1177/2050313X19834154.
The use of insulin-like growth factor 1 improved the parameters of the seminogram in a patient with severe oligoasthenoteratozoospermia
Male patients with oligoasthenoteratozoospermia often struggle to achieve pregnancy, even with assisted reproductive technologies. Growth hormone and insulin-like growth factor 1 (IGF-1) have been identified as regulators of sperm quality parameters, suggesting that supplementation with IGF-1 could enhance sperm parameters. In a case study, a 47-year-old male received daily intradermal injections of 1.5 IU of IGF-1 for 2 months. Seminogram analysis before and after treatment revealed a significant 15.5-fold improvement in sperm concentration (1.1 × 106 vs 18.3 × 106 per mL), a 71.4% increase in volume (0.7 vs 1.2 mL), enhanced progressive motility (2% vs 43%), and a substantial increase in the total volume of sperm with progressive motility (0% vs 23.6%). These findings demonstrate the potential of daily IGF-1 administration to improve sperm quality parameters in oligoasthenoteratozoospermia patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6404043/.
Magon N, Agrawal S, Malik S, Babu KM. Growth hormone in the management of female infertility. Indian J Endocrinol Metab. 2011;15 Suppl 3(Suppl3):S246–S247. doi:10.4103/2230-8210.84876.
Growth hormone in the management of female infertility
Growth hormone (GH) plays a significant role in regulating both male and female infertility and has been utilized in managing infertility in both genders. It is noteworthy that GH is produced not only by the pituitary gland but also by the ovary itself. GH contributes to the growth of single ovarian follicles, and it is often used as an adjunctive therapy in ovarian stimulation and Assisted Reproductive Technologies (ART). GH supplementation has demonstrated its effectiveness in improving pregnancy rates, particularly in cases of poor responders. While GH cotherapy is a valuable tool in ovarian stimulation, it should be judiciously considered and not employed indiscriminately for all patients undergoing ovarian stimulation or assisted reproductive technology.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3183519/.
Volpe A, Coukos G, Barreca A, Giordano G, Artini PG, Genazzani AR. Clinical use of growth hormone-releasing factor for induction of superovulation. Hum Reprod. 1991;6(9):1228-32.
Clinical use of growth hormone-releasing factor for induction of superovulation
A substantial body of both in vivo and in vitro evidence indicates that insulin-like growth factor-I (IGF-I) and potentially growth hormone (GH) play a stimulating role in regulating the ovarian follicular cycle. Promising results have been observed when GH is administered in superovulation induction protocols. In this study, ten patients with previously normal responses to gonadotrophins were given subcutaneous GH-releasing factor (GRF) in combination with gonadotrophins for superovulation induction as part of an in-vitro fertilization/embryo transfer-gamete intra-Fallopian transfer (IVF/ET-GIFT) program. The administration of GRF led to a shortened stimulatory cycle and reduced the total number of gonadotrophin ampoules used per patient compared to a previous cycle with gonadotrophins alone. Elevated levels of follicular fluid IGF-I and plasma GH throughout the GRF cycle suggest that GRF enhances the ovarian response to gonadotrophins by stimulating the GH-IGF-I axis. The study also discusses the potential direct effects of GRF, emphasizing the importance of evaluating individual pituitary GH responses before using GRF for superovulation in infertile patients.
You can read the abstract of the article at https://academic.oup.com/humrep/article-abstract/6/9/1228/616067?redirectedFrom=fulltext&login=false.
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

