GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Book a Free Consultation
Overall Health Benefits of ARA 290
ARA 290 benefits include promoting tissue repair, relieving neuropathic pain, fighting diabetes, and boosting immune function, making it a valuable therapeutic for enhancing overall health and addressing specific chronic conditions.
- Promotes tissue repair [1-18]
- Relieves neuropathic pain [9, 14, 19-35]
- Fights diabetes [11, 15, 36-43]
- Boosts immune function [44-54]
Key Takeaways
- Promotes Tissue Repair: ARA 290 helps in the regeneration of tissues, which is particularly beneficial for recovery from injuries and surgeries.
- Relieves Neuropathic Pain: It effectively alleviates pain associated with nerve damage, offering relief for patients suffering from various neuropathic conditions.
- Fights Diabetes: ARA 290 has a role in managing and potentially mitigating symptoms of diabetes by influencing glucose metabolism and insulin sensitivity.
- Boosts Immune Function: The peptide enhances the immune response, helping the body defend against infections and diseases more effectively.
- Multi-functional Therapeutic: With its diverse effects, ARA 290 holds potential in treating a variety of conditions, making it a versatile addition to therapeutic regimens targeting complex diseases.
What is ARA 290?
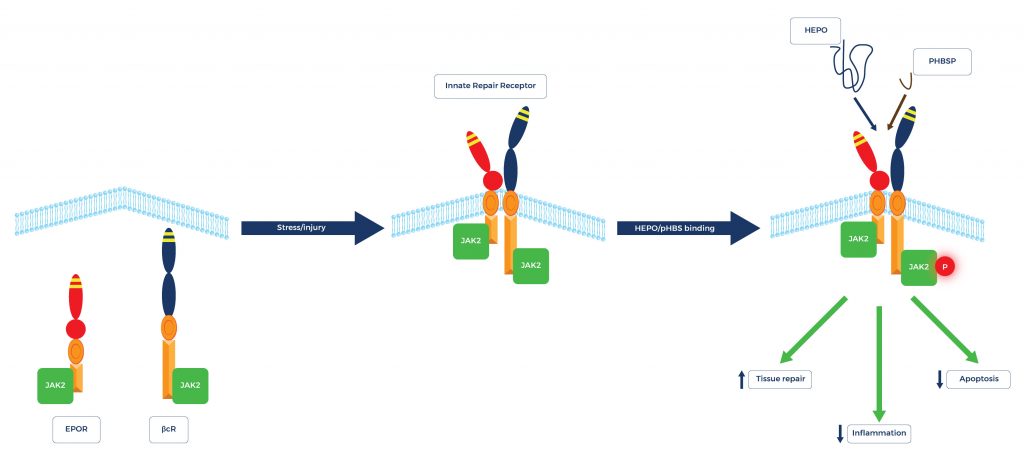
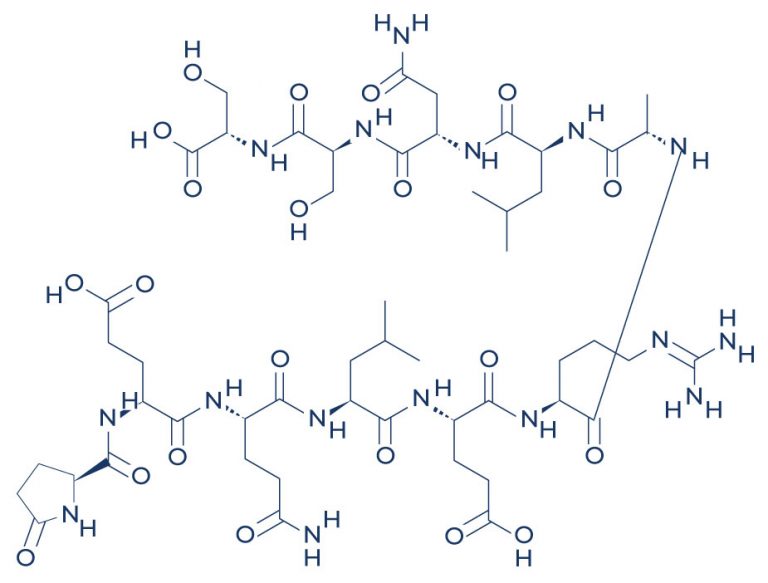
ARA 290, also known as cibinetide, is an 11–amino acid peptide that has potent tissue-protective and tissue-regenerative properties. It is called “nonhematopoietic peptide” because ARA 290 exerts its beneficial effects without stimulating erythropoiesis or red blood cell production. Preclinical and clinical studies have shown that by selectively interacting with the innate repair receptor, ARA 290 mediates tissue repair and regeneration, offering therapeutic potential for various conditions.
How ARA 290 Works
ARA 290 is designed from the structure of erythropoietin. It mediates tissue protection by selectively interacting with the innate repair receptor. This in turn stimulates tissue repair and decreases inflammation and apoptosis (programmed cell death).
Chemical Structure of ARA 290
Research on ARA 290
A. Promotes Tissue Repair
By selectively interacting with the innate repair receptor, ARA 290 exerts its regenerative effects on different body tissues. This targeted mechanism of action makes ARA 290 a promising candidate for therapeutic interventions aimed at promoting tissue repair and regeneration.
- Studies found that ARA 290 relieved neuropathic pain in patients with sarcoidosis by improving corneal nerve fiber abundance. This suggests that ARA 290 may have a beneficial effect on nerve regeneration and repair in the cornea, contributing to the reduction of neuropathic pain symptoms. The increase in corneal nerve fiber density indicates potential improvements in nerve function and sensory perception in the affected individuals.[1-2]
- In mice with deep partial-thickness cutaneous burn injury, ARA 290 mitigated the innate inflammatory response and decreased the burn depth area. These findings, as reported in the journal “Molecular Pain” (Mol Pain), highlight the potential of ARA 290 in treating burn injuries by reducing inflammation and promoting healing. Further research in “Mol Pain” suggests that ARA 290’s ability to modulate the immune response and enhance tissue repair mechanisms could be beneficial in various other inflammatory conditions. Understanding these mechanisms could lead to the development of new therapeutic approaches for managing acute and chronic inflammatory injuries.[3]
- In mice, ARA 290 administration protected against rhabdomyolysis-induced acute kidney injury. This suggests that ARA 290 may have a role in preserving kidney function under conditions of muscle breakdown. Further investigation into its tertiary structure and mechanism of action could elucidate its potential therapeutic applications in kidney injury. Understanding the tertiary structure of ARA 290 is crucial for delineating its interactions with cellular targets and optimizing its pharmacological properties. Characterizing the tertiary structure of ARA 290 may provide insights into its stability, bioavailability, and mode of action, facilitating its development as a therapeutic agent. Analyzing the tertiary structure of ARA 290 can also aid in the design of analogs with improved efficacy and specificity. Investigating the tertiary structure of ARA 290 is essential for assessing its stability under physiological conditions and predicting its pharmacokinetic properties. Deciphering the tertiary structure of ARA 290 may unveil novel binding sites and interaction partners, expanding our understanding of its biological functions.[4]
- A 2018 mice study published in the Journal of Cellular and Molecular Medicine also found that ARA 290 prevents programmed cell death (apoptosis) of kidney cells. This suggests that erythropoietin mediates tissue protection by preserving cellular integrity in renal tissues.. [5]
- In a rat model of inflammatory pain, ARA 290 induced regeneration of nerve fibers. This suggests its potential therapeutic efficacy in conditions characterized by nerve damage and inflammation, such as neuropathic pain in spinal nerve ligated rats.. [6]
- In mice, ARA 290 protected islets of the pancreas from cytokine-induced damage and apoptosis. This indicates the potential of ARA 290 in mitigating tissue injury associated with inflammatory responses. Understanding the mechanisms by which ARA 290 protects against tissue injury could inform its therapeutic applications in various conditions characterized by inflammation and tissue damage.. [7]
- In rats, ARA 290 protected against early renal allograft injury (tissue damage associated with a kidney transplant) by reducing macrophage infiltration. This protection highlights the therapeutic potential of ARA 290 in transplant medicine. Erythropoietin effectively ameliorates tissue damage and inflammatory responses in various models of organ injury, suggesting a broader application for peptides like ARA 290 derived from erythropoietin. Further studies are needed to determine if erythropoietin effectively ameliorates other forms of transplant-related injuries, thereby improving graft survival and function. Understanding these effects can lead to the development of new strategies for managing and preventing organ transplant complications.[8]
- In patients with sarcoidosis-associated small nerve fiber loss, ARA 290 stimulated eye tissue repair by increasing corneal nerve fiber density. In patients with sarcoidosis-associated small nerve fiber loss, ARA 290 stimulated eye tissue repair by increasing corneal nerve fiber density. This enhancement in corneal nerve fiber density suggests potential therapeutic benefits in alleviating symptoms associated with small fiber neuropathy affecting the eyes. By promoting the regeneration of corneal nerve fiber density, ARA 290 may contribute to improved sensory function and reduced discomfort in individuals with neuropathic conditions impacting ocular health.[9]
- A 2009 study published in the Journal of Molecular Medicine found that nonerythropoietic tissue protective compounds such as ARA 290 are highly effective facilitators of wound healing. In addition to promoting wound healing, ARA 290 has shown significant efficacy in reducing pain symptoms. For instance, in animal models, ARA 290 effectively alleviated hind paw mechanical allodynia, a condition characterized by increased sensitivity to pain in response to mechanical stimuli. These findings suggest that ARA 290 can address both tissue repair and pain management. Further research is needed to confirm these effects and explore the therapeutic potential of ARA 290 in clinical settings, particularly for conditions involving hind paw mechanical allodynia and other neuropathic pain symptoms.[10]
- In rats with spinal cord injury, ARA 290 treatment resulted in increased activity of the cells of the spinal cord. This suggests a potential role for ARA 290 in modulating spinal cord function and promoting recovery following injury. By targeting the spinal cord, ARA 290 may offer therapeutic benefits in mitigating the effects of spinal cord injury and improving neurological outcomes. Understanding the precise mechanisms by which ARA 290 affects spinal cord activity could lead to further advancements in the treatment of spinal cord injuries and related conditions. [11]
- In rats with nerve inflammation, ARA 290 treatment suppressed inflammation and exhibited tissue protection. This finding suggests that ARA 290 may hold promise as a therapeutic intervention for chronic pain conditions associated with nerve inflammation. Chronic pain condition often result from ongoing inflammation and nerve damage, making treatments that can reduce inflammation and protect tissues particularly valuable. Further research is needed to explore the potential of ARA 290 in managing chronic pain conditions and to understand its mechanisms of action. This could lead to new therapeutic options for patients suffering from long-term pain and improve their quality of life.[12]
- In mouse cells, ARA 290 restored tissue homeostasis by modulating pro-inflammatory signaling pathways. This modulation is particularly significant because chronic inflammation can lead to tissue damage and contribute to various diseases. The ability of ARA 290 to restore balance in pro-inflammatory signaling pathways suggests it could be beneficial for conditions involving the central nervous system, where inflammation often plays a critical role.[13]
- In a model of retinal ischemia (insufficient blood flow to the eye), ARA 290 prolonged cell survival and enhanced repair of blood vessels. These findings suggest that ARA 290 could be a promising therapeutic agent for retinal diseases involving ischemia. To further investigate its efficacy and safety, a double-blind pilot study would be essential. Such a study would help determine the optimal dosage and assess the therapeutic benefits of ARA 290 in patients with retinal ischemia. Additionally, a double-blind pilot study could provide valuable insights into the broader applications of ARA 290 in other ischemic conditions, ensuring rigorous evaluation of its potential. By conducting well-designed clinical trials, researchers can confirm the preliminary findings from animal models and move closer to potential clinical use in treating retinal ischemia and possibly other ischemic diseases.[14]
- A cell study found that ARA 290 stimulated the survival and repair of cells involved in blood vessel regeneration. This indicates the potential of ARA 290 in promoting vascular health and repairing damaged blood vessels. However, to fully understand its therapeutic potential, it is important to evaluate the effects of chronic administration of ARA 290. Chronic administration studies would help determine the long-term benefits and safety of ARA 290 in maintaining blood vessel integrity and function. Furthermore, exploring the impacts of chronic administration on various cell types involved in vascular repair could provide deeper insights into the mechanisms through which ARA 290 supports regeneration and tissue protection. These findings underscore the necessity for long-term studies to establish the efficacy and safety profile of ARA 290 for chronic conditions requiring sustained vascular repair and regeneration.[15]
- In mice, ARA 290 suppressed the production of inflammatory cytokines, thus, prolonging the survival of transplanted islet cells. [16]
- A study reported that ARA 290 can help speed up wound healing by reducing inflammation and cell death. [17]
- In mice with diabetic wounds, daily injections with ARA 290 improved angiogenesis (formation of new blood vessels), scar strength, and time to complete wound closure. [18]
B. Relieves Neuropathic Pain
Neuropathic pain is often debilitating because it presents as a shooting or burning pain. Sometimes, it can resolve on its own but is usually chronic in nature. In worst cases, it comes and goes and can significantly alter one’s quality of life. Interestingly, there is an overwhelming body of clinical evidence suggesting that ARA 290 can offer long-term relief for neuropathic pain associated with nerve damage or a malfunctioning nervous system:
- A 2016 study published in the Peptides journal found that ARA 290-mediated analgesic effect is achieved by blocking or influencing receptors in pain sensation. This suggests its potential in managing chronic pain conditions. This research sheds light on the mechanisms underlying the alleviation of chronic pain with ARA 290, paving the way for further investigations into its therapeutic applications. Understanding how ARA 290 interacts with pain receptors can lead to more targeted and effective treatments for chronic pain sufferers.[14]
- In mice, ARA 290 relieved capsaicin-induced mechanical allodynia by inhibiting capsaicin-evoked TRPV1 channel activity, a modulator of nerve inflammation and pain sensation. This suggests its potential in managing mirror image neuropathic pain conditions.. [19]
- In patients with type 2 diabetes who are suffering from neuropathic symptoms, ARA 290 showed significant potential for the treatment of diabetic small fiber neuropathy. Neuropathic symptoms, such as tingling, burning pain, and numbness, are common manifestations of diabetic neuropathy. By targeting these neuropathic symptoms, ARA 290 may offer relief and improve the quality of life for individuals with diabetic neuropathy. Further research is warranted to explore the efficacy and safety of ARA 290 in managing neuropathic symptoms associated with diabetic small fiber neuropathy.[20]
- In patients with neuropathic pain related to sarcoidosis, an inflammatory disease affecting multiple body organs, ARA 290 significantly reduced pain and improved quality of life. These results highlight the potential of ARA 290 as a therapeutic option for individuals suffering from neuropathic pain coupled with inflammatory conditions like sarcoidosis. [9, 21-25]
- A 2016 study published in the Pain Reports journal found that ARA 290 treats neuropathic pain by effectively reprogramming the pro-inflammatory and tissue-damaging process into healing and tissue repair. This mechanism suggests that ARA 290 may be a promising therapeutic option for neuropathic pain management. [26]
- In a rat neuritis (nerve inflammation) model, ARA 290 prevented the development of mechanical allodynia (increased pain sensitivity to non-painful stimuli). This suggests that ARA 290 may have potential as a treatment for neuropathic pain conditions. Further research is needed to explore whether ARA 290 produces long-term relief from neuropathic pain symptoms and its underlying mechanisms of action. Understanding the duration and sustainability of ARA 290’s analgesic effects is crucial for assessing its clinical utility in managing chronic neuropathic pain. Investigating the persistence of ARA 290’s efficacy over time will provide valuable insights into its potential as a long-term therapeutic option for individuals suffering from neuropathic pain..[27]
- In a mouse model of diabetic neuropathy, ARA 290 induced repair of small autonomic nerve fibers within the sympathetic ganglia (nerve cell bodies along the spinal cord). This suggests its potential in targeting nerve regeneration processes originating from the spinal cord.. [28]
- In mice with pain associated with sciatic nerve injury, ARA 290 prevented the development of mechanical allodynia. This finding suggests that ARA 290 may hold promise as a therapeutic intervention for neuropathic pain management. Sciatic nerve injury often leads to severe neuropathic pain, and targeting this condition could significantly improve patient outcomes. However, further investigation is necessary to determine whether ARA 290 produces long-term relief from neuropathic pain symptoms. Understanding the duration and sustainability of ARA 290’s analgesic effects is essential for evaluating its potential clinical efficacy in chronic neuropathic pain conditions. Additionally, elucidating the underlying mechanisms through which ARA 290 exerts its analgesic effects on the sciatic nerve will provide valuable insights into its long-term therapeutic utility. Further preclinical and clinical studies are warranted to assess the persistence of ARA 290’s efficacy over time and its potential as a long-term treatment option for individuals suffering from neuropathic pain associated with sciatic nerve injury. [29]
- In rats, weekly injections with ARA 290 produced long-term relief of neuropathic pain. This observation suggests that ARA 290 may hold promise as a therapeutic intervention for managing neuropathic pain conditions. Further research is needed to explore the duration and sustainability of ARA 290’s analgesic effects, particularly in the context of the neuropathic pain triad. Understanding how ARA 290 impacts the neuropathic pain triad, which typically includes sensory abnormalities, neuronal damage, and neuroinflammation, will provide valuable insights into its potential as a long-term treatment option for individuals suffering from neuropathic pain. Moreover, exploring the relief of neuropathic pain through sustained use of ARA 290 will help in assessing its effectiveness over extended periods. Additional preclinical and clinical studies are warranted to investigate the mechanisms underlying ARA 290’s efficacy and its ability to address the complexities of the neuropathic pain triad effectively. These studies will be crucial in determining whether ARA 290 can consistently provide relief of neuropathic pain and improve the quality of life for patients with chronic pain conditions.[30]
- In mice with spinal cord injury, ARA 290 reduced neuropathic pain by suppressing spinal inflammatory mediators such as CCL2. This indicates its potential in modulating spinal cord inflammation and alleviating associated pain symptoms. [31]
- In a murine model, ARA 290 reduced neuropathic pain by reducing the levels of the inflammatory substance TNF-α. This suggests that ARA 290 may have a significant anti-inflammatory effect, contributing to its pain-relieving properties. Furthermore, the ability of certain cells to attenuate neuropathic pain highlights the potential of cellular therapies in managing chronic pain conditions. Understanding how cells attenuate neuropathic pain through the modulation of inflammatory pathways like TNF-α could lead to the development of more effective treatments. Additionally, further research into how ARA 290 and specific cells attenuate neuropathic pain can provide deeper insights into the mechanisms behind these therapeutic effects. [32]
- Studies found that ARA 290 relieved neuropathic pain in patients with sarcoidosis by improving corneal nerve fiber abundance. This suggests that ARA 290 may have a beneficial effect on nerve regeneration and repair in the cornea, contributing to the reduction of neuropathic pain symptoms. The increase in corneal nerve fiber density indicates potential improvements in nerve function and sensory perception in the affected individuals.[33-34]
- In rats and mice with peripheral nerve injury, ARA 290 produced significant relief of allodynia. This suggests that ARA 290 may hold promise as a therapeutic intervention for peripheral nerve injury-induced neuropathic pain. The alleviation of allodynia in animal models of peripheral nerve injury indicates the potential of ARA 290 to target neuropathic pain mechanisms associated with peripheral nerve damage. Further investigation into the efficacy and mechanisms underlying ARA 290’s effects on peripheral nerve injury-induced neuropathic pain is warranted to elucidate its therapeutic potential.. [35]
C. Fights Diabetes

Evidence also suggests that ARA290 possesses potent anti-diabetic properties that can be beneficial in patients with diabetes mellitus and chronically elevated blood sugar levels:
- In animal models of diabetic neuropathy, ARA 290 improved blood sugar levels by reducing the underlying inflammation. This suggests a potential therapeutic benefit of ARA 290 in modulating both the immune and nervous systems to address diabetic neuropathy.11]
- In patients with type 2 diabetes, ARA 290 administration at a dose of 4 mg daily for 28 days resulted in a significant improvement in hemoglobin A1c. This suggests a potential therapeutic benefit of ARA 290 in managing diabetic peripheral neuropathy, a common complication of diabetes characterized by nerve damage in the extremities. [15]
- In diabetic patients, ARA 290 administration restored glucose homeostasis (blood sugar balance). However, the effects of ARA 290 on sarcoidosis patients have not been extensively studied. Further research is needed to determine the potential benefits and risks of ARA 290 in this population. It’s essential to conduct well-designed clinical trials specifically targeting sarcoidosis patients to evaluate the safety and efficacy of ARA 290 in managing their condition. Until then, the use of ARA 290 in sarcoidosis patients should be approached with caution, considering the lack of robust clinical data in this particular patient group. [36]
- A study found that ARA 290 promotes glucose homeostasis by targeting the innate repair receptor. This indicates that ARA 290 not only has potential benefits for nerve repair but also for metabolic regulation. Additionally, ARA 290’s ability to target the innate repair receptor may produce relief from neuropathic pain, further enhancing its therapeutic potential. Understanding how ARA 290 can produce relief in both metabolic and neuropathic conditions could pave the way for its use in multi-faceted treatment approaches. Further research is needed to explore the full extent of ARA 290’s capabilities in these areas.. [37]
- In individuals with prediabetes and/or drug-naive type 2 diabetes, ARA 290 administration resulted in improved glucose tolerance, insulin secretion, insulin sensitivity, and long-term glucose control. [38]In type 2 diabetic Goto-Kakizaki (GK) rats, oral administration of ARA 290 for 4 weeks improved β-cell glucose metabolism and [Ca(2+)]i handling, resulting in enhanced insulin release. Furthermore, ARA 290 administration was associated with an increase in small nerve fiber density, which is essential for maintaining nerve function and potentially alleviating neuropathy symptoms. This dual action of ARA 290 on both metabolic and neural parameters suggests it may be a promising therapeutic agent for addressing both glucose metabolism issues and nerve damage in diabetic patients. Further research is warranted to explore these benefits and their implications for clinical treatment. [39-40]
- In a murine model of diabetes, ARA 290 reversed diabetes-induced autonomic nerve degeneration. This effect highlights the potential of ARA 290 as an erythropoietin-derived peptide that can address diabetic neuropathy. By reversing nerve degeneration, ARA 290 may help restore autonomic nerve function, contributing to improved overall health and quality of life for diabetic patients. Further research is needed to confirm these findings and explore the therapeutic applications of this erythropoietin-derived peptide in clinical settings.[41]
- In patients with prediabetes and type 2 diabetes, ARA 290 treatment for 2-4 weeks improved oral glucose tolerance tests and insulin secretion. This improvement in metabolic function highlights the therapeutic potential of ARA 290 in managing diabetes-related complications. Additionally, ARA 290’s effects on nerve health have been noteworthy. Studies have shown that ARA 290 not only targets damaged nerves but also appears to positively influence neighboring nerve fibers, promoting overall nerve regeneration and repair. Understanding how ARA 290 interacts with both injured and neighboring nerve fibers could pave the way for novel therapeutic strategies for diabetic neuropathy and other related conditions. Further investigation into these mechanisms is essential to fully harness ARA 290’s potential benefits in clinical applications.[42]
- In bone cancer rats, ARA 290 protected pancreatic islets (secrete insulin) from cytokine-induced damage and programmed cell death (apoptosis). This protective effect on pancreatic islets highlights the potential of ARA 290 in managing complications associated with cancer and diabetes. Researchers at Leiden University Medical Center have been at the forefront of studying ARA 290, exploring its various therapeutic benefits. Studies conducted at Leiden University Medical Center have shown promising results in preclinical models, paving the way for potential clinical applications. Continued research at Leiden University Medical Center is essential to fully understand the mechanisms by which ARA 290 confers protection and to develop effective treatment protocols for patients suffering from chronic conditions. The ongoing efforts at Leiden University Medical Center are crucial in translating these findings into clinical practice, potentially improving outcomes for individuals with bone cancer and diabetes. [43]
D. Boosts Immune Function
A growing body of evidence suggests that ARA 290 also has immune-modulating properties necessary for warding off a wide array of diseases:
- In a cell culture model, ARA 290 modulated the innate immune response and reduced invasion of the bacterium Escherichia coli. This suggests that ARA 290 may have potential therapeutic applications in managing bacterial infections by enhancing the body’s innate repair receptor. Understanding how ARA 290 affects the innate immune response could lead to further insights into its mechanisms of action and potential therapeutic uses in combating various infectious diseases. The modulation of the innate immune response by ARA 290 highlights its multifaceted potential in regulating immune functions and promoting tissue repair and homeostasis. Further studies investigating the specific pathways and mediators involved in ARA 290’s modulation of the innate immune response could provide valuable information for the development of novel immunomodulatory therapies.[44]
- Several studies also found that ARA 290 and other nonhematopoietic peptides have the potential to combat several inflammatory and infectious diseases. These peptides may modulate the activity of immune cells, reducing inflammation and promoting tissue repair.[45-47]
- A 2017 study published in Scientific Reports found that ARA 290 enhances immune cell function through its potent anti-inflammatory effects. This discovery underscores the potential of ARA 290 as an erythropoietin-derived peptide with immunomodulatory properties. By bolstering immune cell activity while reducing inflammation, ARA 290 may offer therapeutic benefits in conditions characterized by immune dysregulation and excessive inflammation. Further research into the mechanisms underlying the immunomodulatory effects of this erythropoietin-derived peptide is warranted to explore its clinical applications in various inflammatory disorders. [48]
- A 2016 study found that ARA 290 improves stem cells’ ability to find their destination (homing), thus, enhancing new blood vessel formation, tissue regeneration, and immune function. [49]
- A 2014 study published in the Journal of Immunology found that ARA 290 alters T cell function to suppress inflammation. [50]
- A study found that ARA 290 can help lower the risk of myocardial infarction by protecting against damage to the heart muscle (myocardium). [51]
- A study reported that ARA 290 has the ability to modulate graft rejection after organ transplant. [52]
- Studies found that the anti-inflammatory effects of ARA 290 can help treat autoimmune diseases such as Crohn’s disease, ulcerative colitis, and systemic lupus erythematosus (SLE). [53-54]
Associated Side Effects of ARA 290
ARA 290 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on ARA 290. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of ARA 290. Despite this, it was listed as a side effect associated with ARA 290 even though these associated side effects are very uncommon. These side effects may include gastrointestinal disturbances, such as nausea and diarrhea, and occasional reports of headaches or dizziness. However, further research is needed to fully understand the potential side effects of ARA 290 and their underlying mechanisms.
Side effects associated with ARA 290 may include the following:
- Elevated blood pressure
- Fast heart rate
- Liver enzyme elevation
- Nausea/vomiting
ARA 290 Dosage
ARA 290 is a synthetic peptide derived from erythropoietin (EPO) that shows promise in treating various inflammatory and neuropathic conditions. However, determining the appropriate dosage of ARA 290 is essential to ensure its therapeutic efficacy while minimizing potential side effects. The dosage of ARA 290 typically depends on factors such as the patient’s age, weight, overall health condition, and the severity of the condition being treated.
Clinical trials and research studies have provided valuable insights into the optimal dosage of ARA 290 for different conditions. For example, in trials focusing on neuropathic pain associated with diabetes, doses ranging from 10 to 30 micrograms per kilogram of body weight have been investigated. These studies have demonstrated that ARA 290 at appropriate dosages can effectively alleviate neuropathic pain symptoms and improve quality of life for patients suffering from diabetic neuropathy.
It’s important for healthcare providers to carefully assess each patient’s individual needs and response to treatment when determining the optimal dosage of ARA 290. Close monitoring of patients during treatment is crucial to evaluate the efficacy and safety of the chosen dosage regimen. Additionally, ongoing research may further refine our understanding of ARA 290 dosage requirements and its therapeutic potential in various inflammatory and neuropathic conditions.
ARA 290 Nerve Regeneration
ARA 290, a novel peptide derived from erythropoietin (EPO), has garnered attention for its potential in promoting nerve regeneration and repair. Nerve regeneration is a complex process involving the growth and reconnection of damaged corneal nerve fibers, and ARA 290 has shown promising results in preclinical studies and early clinical trials. This peptide acts by binding to the innate repair receptor (IRR), a receptor associated with tissue protection and repair, thereby exerting its neuroprotective and regenerative effects. Notably, ARA 290 has also demonstrated potential benefits in repairing small nerve fibers, which are critical for sensory functions and pain perception.
Early clinical trials investigating ARA 290 for nerve regeneration have shown promising results, with some studies reporting improvements in sensory function, pain relief, and overall quality of life in patients with neuropathic pain. Notably, improvements in corneal nerve fibers have also been observed, suggesting potential benefits for ocular neuropathic conditions. However, further research is needed to elucidate the optimal dosage, treatment duration, and long-term effects of ARA 290 therapy on nerve regeneration in humans. Continued investigation into the mechanisms underlying ARA 290’s neuroprotective and regenerative properties may pave the way for novel therapeutic approaches to treat nerve injuries and neuropathic disorders. The spared nerve injury (SNI) model has been particularly useful in studying neuropathic pain mechanisms and evaluating potential therapeutic interventions. Studies using the SNI model have provided valuable insights into the efficacy of ARA 290 in alleviating neuropathic pain and promoting nerve repair. These findings highlight the potential of ARA 290 as a promising therapeutic agent for nerve injury-induced neuropathic pain and its positive impact on corneal nerve fibers.
Early clinical trials investigating ARA 290 for nerve regeneration have shown promising results, with some studies reporting improvements in sensory function, pain relief, and overall quality of life in patients with neuropathic pain. However, further research is needed to elucidate the optimal dosage, treatment duration, and long-term effects of ARA 290 therapy on nerve regeneration in humans. Continued investigation into the mechanisms underlying ARA 290’s neuroprotective and regenerative properties may pave the way for novel therapeutic approaches to treat nerve injuries and neuropathic disorders. The spared nerve injury (SNI) model has been particularly useful in studying neuropathic pain mechanisms and evaluating potential therapeutic interventions. Studies using the SNI model have provided valuable insights into the efficacy of ARA 290 in alleviating neuropathic pain and promoting nerve repair. These findings highlight the potential of ARA 290 as a promising therapeutic agent for nerve injury-induced neuropathic pain.
FAQ
What is ARA 290 used for?
ARA 290 is primarily used for its potential to promote nerve regeneration and repair, particularly in conditions characterized by nerve damage such as diabetic neuropathy and peripheral neuropathy. Its mechanism of action involves modulating the immune system and targeting specific receptors associated with tissue repair and regeneration. By influencing the immune system, ARA 290 can help mitigate inflammation and promote healing processes, ultimately improving nerve function and reducing neuropathic symptoms. Understanding the interplay between ARA 290 and the immune system is crucial for optimizing its therapeutic effects in treating various neuropathic conditions.
Is neuropathic pain the same as nerve pain?
Yes, neuropathic pain is a type of nerve pain caused by damage or dysfunction of the nerves themselves, often resulting in sensations such as tingling, burning, or shooting pain. The spared nerve injury (SNI) model, commonly used in research, mimics aspects of neuropathic pain observed in clinical conditions. This model involves surgically sparing one nerve while injuring others, leading to the development of neuropathic pain-like symptoms in the spared nerve. Researchers use the SNI model to study the mechanisms underlying neuropathic pain and to evaluate potential therapeutic interventions. Studies utilizing this model have provided valuable insights into the pathophysiology of neuropathic pain and have contributed to the development of new treatments for this challenging condition.
What peptides are good for neuropathy?
Peptides such as ARA 290 and CNTX-4975 are being investigated for their potential in treating neuropathy by promoting nerve regeneration and reducing neuropathic pain. These peptides offer a novel approach to addressing neuropathic conditions by targeting alternative erythropoietin-mediated signaling pathways involved in tissue repair and regeneration. By modulating these pathways, ARA 290 and CNTX-4975 may enhance the body’s natural healing mechanisms, leading to improved nerve function and reduced pain perception. Research into the therapeutic effects of these peptides is ongoing, with preclinical and clinical studies exploring their safety and efficacy in neuropathic pain management. The identification of alternative erythropoietin-mediated signaling pathways opens up new possibilities for the development of innovative treatments for neuropathy, offering hope for patients who suffer from debilitating symptoms..
What is the half life of ARA290?
The half-life of ARA 290 is approximately 3 to 4 hours, allowing for multiple daily dosing if necessary to maintain therapeutic levels in the body. This characteristic is vital because it ensures that the medication remains effective throughout the day. In conditions where the spinal cord contributes to ongoing symptoms, such as neuropathic pain or inflammation, frequent dosing can help manage these symptoms effectively. Adjusting the dosing schedule based on individual patient responses and the specific condition being treated is essential to achieve optimal therapeutic outcomes. By targeting the spinal cord, ARA 290 may offer a direct means of addressing symptoms and promoting recovery in conditions where spinal cord dysfunction plays a significant role. Therefore, the ability to administer ARA 290 multiple times a day provides clinicians with the flexibility to tailor treatment regimens and optimize patient care.
Is Ara 290 safe?
ARA 290 has shown promising safety profiles in clinical trials, with minimal reported adverse effects, making it an attractive candidate for addressing mechanical and cold allodynia. Clinical trials evaluating ARA 290 have employed various outcome measures, including the neuropathic pain symptom inventory, to assess its efficacy in alleviating neuropathic pain symptoms. These assessments provide valuable insights into the impact of ARA 290 on different aspects of neuropathic pain, helping researchers and clinicians better understand its therapeutic potential and optimize treatment strategies.
Can you live a long life with small fiber neuropathy?
Small fiber neuropathy doesn’t typically affect life expectancy, but it can significantly impact quality of life without proper management. Effective treatment strategies often focus on symptom relief and improving daily functioning. Emerging therapies that target specific inflammatory pathways, such as those involving tumor necrosis factor, have shown promise in alleviating symptoms and improving patient outcomes. For example, certain medications that inhibit tumor necrosis factor can reduce inflammation and neuropathic pain, offering potential relief for those with small fiber neuropathy. Integrating such targeted therapies into treatment plans may enhance quality of life by addressing the underlying inflammatory processes contributing to nerve damage and pain.
What is the most successful treatment for neuropathy?
Treatment success varies depending on the underlying cause, but medications targeting nerve pain, physical therapy, and lifestyle modifications are commonly used. Emerging evidence suggests that certain interventions not only alleviate nerve pain but also improve metabolic control. For instance, maintaining optimal blood glucose levels is critical in diabetic neuropathy management, as improves metabolic control can significantly reduce the progression of nerve damage.
Additionally, treatments that improve metabolic control, such as ARA 290, have shown promise in preclinical studies. These interventions not only target neuropathic pain but also contribute to better overall metabolic health, which is essential for preventing further nerve damage. Thus, integrating therapies that improve metabolic control into the treatment regimen may enhance outcomes for patients suffering from neuropathic pain.
What stops nerve pain immediately?
Immediate relief from nerve pain can be achieved with medications like gabapentin or pregabalin, as well as topical treatments or nerve blocks. In addition to these treatments, understanding the tertiary structure of erythropoietin (EPO) can provide valuable insights into its function and therapeutic applications. The tertiary structure of erythropoietin is crucial for its ability to interact with specific receptors on nerve cells, thereby modulating pain and promoting nerve repair. Advanced studies on the tertiary structure of erythropoietin have shown that modifications in its structure can enhance its tissue-protective effects without stimulating erythropoiesis, which is important for its use in treating neuropathic pain. Furthermore, the detailed knowledge of the tertiary structure of erythropoietin allows for the development of novel EPO derivatives, such as ARA 290, which retain the protective properties while minimizing side effects. As researchers continue to explore the tertiary structure of erythropoietin, new therapeutic avenues may emerge, offering improved pain relief and neuroprotection for patients suffering from nerve injuries and neuropathic conditions.
Are there any peptides that help with neuropathy?
Peptides like ARA 290 have shown potential in preclinical studies for neuropathic pain management, but further research is needed for clinical applications. In particular, studies have demonstrated that ARA 290 can improve the corneal nerve fiber area, suggesting its role in nerve regeneration and repair. By increasing the corneal nerve fiber area, ARA 290 may help alleviate neuropathic pain and enhance sensory function. This highlights the importance of understanding the peptide’s mechanisms of action and efficacy in clinical settings. Further research focusing on the effects of ARA 290 on corneal nerve fiber area and its potential therapeutic benefits could pave the way for novel treatments for neuropathic pain.
What is the protocol for ARA290?
The protocol for ARA 290 typically involves subcutaneous injections at specific dosages, as determined by a healthcare provider based on individual patient needs and condition severity. Injured nerves impairs the body’s ability to repair and regenerate, making precise dosing crucial to maximize therapeutic effects. Understanding that injured nerves impairs overall function and sensation underlines the importance of personalized treatment plans to address the specific needs of each patient effectively.
What is ARA 290?
ARA 290 is a novel peptide-based drug developed for treating conditions associated with nerve damage and inflammation, such as neuropathic pain. ARA 290 is a novel peptide-based drug developed for treating conditions associated with nerve damage and inflammation, such as neuropathic pain. Its mechanism of action involves modulating the innate repair receptor, leading to tissue protection and regeneration. Clinical studies have shown promising results in relieving neuropathic pain symptoms, suggesting ARA 290 as a potential therapeutic option for individuals suffering from this debilitating condition.
What are the benefits of ARA290?
ARA 290 has shown promise in preclinical and early clinical studies for its ability to promote nerve repair, reduce inflammation, and alleviate neuropathic pain. Its unique mechanism of action involves targeting the innate repair receptor, which plays a crucial role in tissue protection and regeneration. By modulating this receptor, ARA 290 facilitates the repair processes within the nervous system, leading to improvements in neuropathic pain symptoms. These findings suggest that ARA 290 holds potential as a therapeutic intervention for individuals suffering from neuropathic pain..
How long does it take for ARA 290 to work?
The onset of action for ARA 290 can vary, but some patients may experience symptom improvement within weeks to months of starting treatment. The efficacy of ARA 290 in improving symptoms and promoting nerve repair has been demonstrated in various preclinical studies, including animal models such as diabetic mouse sympathetic ganglia. These findings suggest the potential therapeutic benefits of ARA 290 in managing neuropathic conditions associated with diabetic neuropathy.
Which peptide is best for nerve repair?
The peptide ARA 290 has shown promising potential for nerve repair in preclinical studies, demonstrating its ability to reduce inflammation and promote regeneration of damaged nerves. Animal models, including the spared nerve injury (SNI) model, have been instrumental in evaluating the efficacy of ARA 290 in promoting nerve regeneration and alleviating neuropathic pain. Studies utilizing the spared nerve injury (SNI) model have provided valuable insights into the mechanisms underlying neuropathic pain and the effects of potential therapeutic interventions, such as ARA 290. These findings support further investigation into the clinical application of ARA 290 for nerve repair and neuropathic pain management. Given its success in the SNI model, ARA 290 holds significant promise for translating these preclinical results into effective treatments for patients suffering from nerve injuries and related pain..
What is the newest treatment for neuropathy?
Emerging treatments for neuropathy include peptide-based therapies like ARA 290, stem cell therapy, and novel drug formulations targeting nerve regeneration pathways. Recent research has also focused on understanding the role of spinal microglia response in neuropathic pain and exploring potential interventions that modulate microglial activity to alleviate pain and promote nerve repair. These innovative approaches hold promise for improving the management of neuropathic conditions and enhancing the quality of life for affected individuals. Understanding the complex interactions between spinal microglia response and the nervous system may provide valuable insights into developing more effective therapies for neuropathic pain.
What is the life expectancy of a person with sarcoidosis?
Life expectancy can vary widely depending on the severity of sarcoidosis and its impact on vital organs, but many individuals with sarcoidosis live a normal lifespan. The primary injury response to sarcoidosis typically involves the activation of immune cells and the formation of granulomas in affected tissues.
Does sarcoidosis ever go away?
Sarcoidosis can go into remission spontaneously or with treatment, but it can also relapse, requiring ongoing management to control symptoms and prevent complications. In some cases, patients may experience peripheral inflammation-induced hyperalgesia, contributing to their discomfort and pain. This highlights the importance of comprehensive management strategies tailored to individual needs and the dynamic nature of sarcoidosis.
What triggers sarcoidosis flare-ups?
Triggers for sarcoidosis flare-ups are not fully understood, but factors like infections, environmental exposures, and stress may play a role in exacerbating symptoms. Experimental autoimmune neuritis, a model of Guillain-Barré syndrome, has been used to study the pathophysiology of autoimmune peripheral neuropathies.
What is the best treatment for sarcoidosis?
Treatment for sarcoidosis aims to manage symptoms and prevent organ damage, typically involving corticosteroids, immunosuppressants, and other medications depending on disease severity. However, emerging research suggests potential therapeutic avenues beyond conventional treatments, such as exploring the role of erythropoietin-mediated tissue protection in mitigating the inflammatory response associated with sarcoidosis. This highlights the need for continued research into novel treatment modalities to improve outcomes for individuals living with this complex condition. Understanding how erythropoietin-mediated mechanisms can modulate the inflammatory response could lead to breakthroughs in managing chronic inflammation. Additionally, developing therapies that specifically target the inflammatory response in sarcoidosis may help reduce disease progression and organ damage. Continued exploration into these innovative approaches is essential for advancing treatment strategies and enhancing the quality of life for those affected by sarcoidosis.
What are the symptoms of small fiber neuropathy?
Symptoms of small fiber neuropathy often include tingling, burning pain, numbness, and hypersensitivity in the affected areas, typically in the hands and feet. Additionally, small fiber neuropathy can lead to spinal cord histopathological alterations, indicating structural changes in the spinal cord tissue associated with nerve damage and dysfunction.
How to get rid of small fiber neuropathy?
Treatment for small fiber neuropathy typically revolves around symptom management and addressing the underlying causes, which may entail a combination of medications, physical therapy, lifestyle modifications, and pain management techniques. This multifaceted approach aims to alleviate pain and discomfort, improve nerve function, and enhance overall quality of life for individuals affected by small fiber neuropathy. It is important for healthcare providers to tailor treatment plans to each patient’s specific needs and symptoms, considering factors such as the severity of neuropathic symptoms, underlying medical conditions, and individual preferences.
What should I eat if I have SFN?
A diet rich in antioxidants, vitamins, and minerals, along with healthy fats and low-glycemic index carbohydrates, may support nerve health and overall well-being in small fiber neuropathy. Additionally, tissue protective peptides derived from natural sources could offer therapeutic benefits by promoting nerve regeneration and reducing inflammation. These peptides may help protect nerve cells from damage and support their repair processes, potentially improving symptoms and quality of life for individuals with neuropathic conditions. Incorporating foods and supplements rich in these tissue protective peptides into one’s diet may complement existing treatment strategies for small fiber neuropathy. The identification and characterization of novel tissue protective peptides derived from natural sources hold promise for the development of new therapeutic interventions aimed at preserving nerve function and mitigating the progression of neuropathic diseases.
Reference
Van rijt WG, Nieuwenhuijs-moeke GJ, Van goor H, et al. ARA290, a non-erythropoietic EPO derivative, attenuates renal ischemia/reperfusion injury. J Transl Med. 2013;11:9.
ARA290, a non-erythropoietic EPO derivative, attenuates renal ischemia/reperfusion injury
The study titled “ARA290, a non-erythropoietic EPO derivative, attenuates renal ischemia/reperfusion injury” investigated the potential protective effects of ARA290, a derivative of erythropoietin (EPO), in reducing renal ischemia/reperfusion injury. Ischemia/reperfusion injury is a condition that occurs when blood flow is temporarily reduced or interrupted to an organ (in this case, the kidneys) and then restored, leading to tissue damage.
ARA290 is a non-erythropoietic EPO derivative, which means it does not stimulate red blood cell production like traditional EPO. The study aimed to assess whether ARA290 could protect against kidney damage caused by ischemia and subsequent reperfusion.
For more details https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-11-9
Van rijt WG, Nieuwenhuijs-moeke GJ, Van goor H, Ottens PJ, Ploeg RJ, Leuvenink HG. Renoprotective capacities of non-erythropoietic EPO derivative, ARA290, following renal ischemia/reperfusion injury. J Transl Med. 2013;11:286.
Renoprotective capacities of non-erythropoietic EPO derivative, ARA290, following renal ischemia/reperfusion injury
The study titled “Renoprotective capacities of non-erythropoietic EPO derivative, ARA290, following renal ischemia/reperfusion injury” investigated the potential renoprotective effects of ARA290, a non-erythropoietic derivative of erythropoietin (EPO), in the context of renal ischemia/reperfusion injury. Ischemia/reperfusion injury occurs when blood flow to an organ, in this case, the kidneys, is temporarily reduced and then restored, leading to tissue damage.
ARA290 is unique because it does not stimulate the production of red blood cells, unlike traditional EPO. The study aimed to assess whether ARA290 could protect the kidneys from damage caused by ischemia and subsequent reperfusion.
For more details https://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-11-9
Bohr S, Patel SJ, Shen K, et al. Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns. ProcNatlAcadSci USA. 2013;110(9):3513-8.
Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns
The study titled “Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns” explored an alternative signaling pathway of erythropoietin (EPO) and its potential role in preventing secondary microvascular thrombosis and inflammation in cutaneous burns.
In the context of cutaneous burns, tissue damage can lead to thrombosis (blood clot formation) and inflammation, which can exacerbate the injury. Erythropoietin is known for its role in stimulating red blood cell production, but it also has other potential effects.
The study investigated whether EPO could prevent microvascular thrombosis and inflammation within burn injuries through alternative signaling pathways. The researchers found that alternative EPO-mediated signaling pathways could indeed reduce secondary microvascular thrombosis and inflammation in cutaneous burns.
For more details https://www.pnas.org/doi/abs/10.1073/pnas.1214099110
Retrieved https://www.nature.com/articles/cddis2017104
Huang B, Jiang J, Luo B, et al. Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus. J Cell Mol Med. 2018;22(7):3330-3339.
Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus
In a study published in the Journal of Cellular and Molecular Medicine in 2018, researchers led by Huang et al. investigated the protective effects of a non-erythropoietic erythropoietin-derived peptide in a mouse model of systemic lupus erythematosus (SLE). This peptide, derived from erythropoietin but non-erythropoietic, demonstrated significant protective properties against SLE, including a reduction in autoantibody production and kidney damage, suggesting its potential as a therapeutic option for this autoimmune disease.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1111/jcmm.13608
Dilley A. ARA290 in a rat model of inflammatory pain. Methods Mol Biol. 2013;982:213-25.
ARA290 in a rat model of inflammatory pain
In a study by Dilley, published in the Methods in Molecular Biology journal in 2013, the effects of ARA290 were investigated using a rat model of inflammatory pain. The research aimed to evaluate the potential of ARA290 in alleviating pain associated with inflammation. The study likely provides insights into the analgesic properties of ARA290 and its potential applications in managing inflammatory pain conditions.
For more details https://link.springer.com/protocol/10.1007/978-1-62703-308-4_14
Watanabe M, Lundgren T, Saito Y, et al. A Nonhematopoietic Erythropoietin Analogue, ARA 290, Inhibits Macrophage Activation and Prevents Damage to Transplanted Islets. Transplantation. 2016;100(3):554-62.
A Nonhematopoietic Erythropoietin Analogue, ARA 290, Inhibits Macrophage Activation and Prevents Damage to Transplanted Islets
In a study published in the journal Transplantation in 2016, Watanabe et al. investigated the potential therapeutic effects of ARA 290, a nonhematopoietic erythropoietin analogue, in the context of islet transplantation. The research aimed to assess whether ARA 290 could inhibit macrophage activation and protect transplanted islets from damage. The study’s findings may suggest a novel approach to enhance the success of islet transplantation by modulating macrophage activity and reducing islet damage, potentially improving outcomes for individuals with diabetes who undergo islet transplantation.
For more details https://journals.lww.com/transplantjournal/fulltext/2016/03000/A_Nonhematopoietic_Erythropoietin_Analogue,_ARA.20.aspx
Yan L, Zhang H, Gao S, et al. EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway. Transplant Proc. 2018;50(5):1575-1582.
EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway
In a study published in Transplantation Proceedings in 2018, Yan et al. investigated the potential protective effects of ARA290, a derivative of erythropoietin (EPO), on early renal allograft injury in rats. The research aimed to assess whether ARA290 could attenuate renal damage by targeting the NF-κB pathway, a key player in inflammation. The findings of the study suggested that ARA290 may have a beneficial impact on reducing early renal allograft injury through its modulation of the NF-κB pathway. This research highlights a potential therapeutic approach to improving outcomes in renal transplantation.
For more details https://www.sciencedirect.com/science/article/pii/S0041134518302501
Dahan A, Dunne A, Swartjes M, Proto PL, Heij L, Vogels O, van Velzen M, Sarton E, Niesters M, Tannemaat MR, Cerami A, Brines M. ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol Med 2013;19:334–45.
ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density
In a study published in Molecular Medicine in 2013, Dahan et al. investigated the effects of ARA 290, a nonhematopoietic erythropoietin derivative, in patients with sarcoidosis-associated small nerve fiber loss. The study aimed to determine whether ARA 290 could improve symptoms and increase corneal nerve fiber density in these patients. The results demonstrated that ARA 290 treatment led to symptom improvement in patients with sarcoidosis-associated small nerve fiber loss, and it also increased corneal nerve fiber density. This suggests the potential therapeutic benefits of ARA 290 in addressing neuropathic symptoms associated with sarcoidosis-related nerve fiber damage.
For more details https://molmed.biomedcentral.com/articles/10.2119/molmed.2013.00122
Erbayraktar Z, Erbayraktar S, Yilmaz O, Cerami A, Coleman T, Brines M. Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing. Mol Med. 2009;15(7-8):235–241. doi:10.2119/molmed.2009.00051.
Nonerythropoietic tissue protective compounds are highly effective facilitators of wound healing
In a study published in Molecular Medicine in 2009, Erbayraktar et al. investigated the efficacy of nonerythropoietic tissue protective compounds in facilitating wound healing. The study focused on these compounds’ ability to promote wound healing processes. The results showed that nonerythropoietic tissue protective compounds, including ARA 290, were highly effective in promoting wound healing. This suggests their potential as therapeutic agents for improving the wound healing process, highlighting their tissue-protective properties beyond their erythropoietic effects.
For more details https://molmed.biomedcentral.com/articles/10.2119/molmed.2009.00051
Swartjes M, van Velzen M, Niesters M, Aarts L, Brines M, Dunne A, Cerami A, Dahan A. ARA 290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain coupled with suppression of the spinal microglia response. Mol Pain 2014;10:13.
ARA 290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain coupled with suppression of the spinal microglia response
In a study published in Molecular Pain in 2014, Swartjes et al. investigated the effects of ARA 290, a peptide derived from the tertiary structure of erythropoietin, on neuropathic pain and spinal microglia response. The study found that ARA 290 produced long-term relief of neuropathic pain and also suppressed the spinal microglia response. These findings suggest the potential therapeutic use of ARA 290 in the management of neuropathic pain, possibly through its modulation of microglial activation in the spinal cord.
For more details https://journals.sagepub.com/doi/abs/10.1186/1744-8069-10-13
Liu Y, Luo B, Han F, et al. Erythropoietin-derived nonerythropoietic peptide ameliorates experimental autoimmune neuritis by inflammation suppression and tissue protection [published correction appears in PLoS One. 2014;9(5):e99555. Dosage error in article text]. PLoS One. 2014;9(3):e90942. Published 2014 Mar 6. doi:10.1371/journal.pone.0090942.
Erythropoietin-derived nonerythropoietic peptide ameliorates experimental autoimmune neuritis by inflammation suppression and tissue protection [published correction appears in PLoS One
In a study published in PLoS One in 2014, Liu et al. investigated the potential therapeutic effects of an erythropoietin-derived nonerythropoietic peptide in experimental autoimmune neuritis, a model of autoimmune peripheral neuropathy. The study found that the peptide ameliorated the condition by suppressing inflammation and providing tissue protection. This research suggests that nonerythropoietic peptides derived from erythropoietin may have anti-inflammatory and tissue-protective properties that could be beneficial in the treatment of autoimmune neuropathies.
For more details https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0090942
Bohr S, Patel SJ, Vasko R, et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J Mol Med (Berl). 2015;93(2):199–210. doi:10.1007/s00109-014-1218-2.
Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells
In a study published in the Journal of Molecular Medicine in 2015, Bohr et al. investigated the modulation of the cellular stress response through the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. The study explored how this complex influences cellular stress responses. Their findings suggest that the erythropoietin/CD131 heteroreceptor complex plays a role in modulating cellular stress responses in mesenchymal-derived cells. This research contributes to our understanding of the cellular mechanisms involving erythropoietin and its receptor in response to stress.
For more details https://link.springer.com/article/10.1007/s00109-014-1218-2
O’Leary, O. E., Canning, P., Reid, E., Bertelli, P. M., McKeown, S., Brines, M., Cerami, A., Du, X., Xu, H., Chen, M., Dutton, L., Brazil, D. P., Medina, R. J., & Stitt, A. W. (2019). The vasoreparative potential of endothelial colony-forming cells in the ischemic retina is enhanced by cibinetide, a non-hematopoietic erythropoietin mimetic. Experimental eye research, 182, 144–155. https://doi.org/10.1016/j.exer.2019.03.001.
The vasoreparative potential of endothelial colony-forming cells in the ischemic retina is enhanced by cibinetide, a non-hematopoietic erythropoietin mimetic
In a study published in 2019 in the journal Experimental Eye Research, O’Leary et al. investigated the vasoreparative potential of endothelial colony-forming cells (ECFCs) in the ischemic retina and examined how it could be enhanced by cibinetide, a non-hematopoietic erythropoietin mimetic. The study explored the regenerative capabilities of ECFCs in the context of retinal ischemia and how cibinetide, a compound mimicking erythropoietin, can enhance their reparative effects. Their findings suggest that cibinetide enhances the vasoreparative potential of ECFCs in the ischemic retina, providing insights into potential therapeutic approaches for retinal ischemic conditions.
For more details https://www.sciencedirect.com/science/article/pii/S0014483518306456
Hache, G., Garrigue, P., Bennis, Y., Stalin, J., Moyon, A., Cerami, A., Brines, M., Blot-Chabaud, M., Sabatier, F., Dignat-George, F., & Guillet, B. (2016). ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock (Augusta, Ga.), 46(4), 390–397. https://doi.org/10.1097/SHK.0000000000000606.
ARA290, a specific agonist of erythropoietin/CD131 heteroreceptor, improves circulating endothelial progenitors’ angiogenic potential and homing ability
In a study published in 2016 in the journal Shock (Augusta, Ga.), Hache et al. investigated the effects of ARA290, a specific agonist of the erythropoietin/CD131 heteroreceptor, on circulating endothelial progenitors (CEPs). The study focused on evaluating how ARA290 could enhance the angiogenic potential and homing ability of CEPs. The findings of this study suggest that ARA290 improves the angiogenic potential and homing ability of CEPs, which could have implications for enhancing vascular repair and regeneration in various clinical conditions.
For more details https://www.ingentaconnect.com/content/wk/shk/2016/00000046/00000004/art00008
Watanabe, M., Lundgren, T., Saito, Y., Cerami, A., Brines, M., Östenson, C. G., & Kumagai-Braesch, M. (2016). A Nonhematopoietic Erythropoietin Analogue, ARA 290, Inhibits Macrophage Activation and Prevents Damage to Transplanted Islets. Transplantation, 100(3), 554–562. https://doi.org/10.1097/TP.0000000000001026
A nonhematopoietic erythropoietin analogue, ARA 290, inhibits macrophage activation and prevents damage to transplanted islets
In a study published in 2016 in the journal Transplantation, Watanabe et al. investigated the effects of ARA290, a nonhematopoietic erythropoietin analogue, on macrophage activation and its potential to prevent damage to transplanted islets. The study aimed to assess whether ARA290 could have protective effects on transplanted islets by inhibiting macrophage activation, which is often associated with tissue damage in transplantation. The results of the study suggest that ARA290 has the potential to inhibit macrophage activation and protect transplanted islets from damage, which could have implications for improving the success of islet transplantation in diabetes treatment.
For more details https://journals.lww.com/transplantjournal/fulltext/2016/03000/A_Nonhematopoietic_Erythropoietin_Analogue,_ARA.20.aspx
Peng B, Kong G, Yang C, Ming Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 2020 Feb 3;11(2):79. doi: 10.1038/s41419-020-2276-8. PMID: 32015330; PMCID: PMC6997384.
Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis
The study by Peng et al. titled “Erythropoietin and its derivatives: from tissue protection to immune regulation” was published in Cell Death & Disease in February 2020. This comprehensive review explores the multifaceted roles of erythropoietin (EPO) and its derivatives in various biological processes. The review covers a wide range of topics, including tissue protection, immune regulation, and the potential therapeutic applications of EPO and its derivatives. It provides insights into the diverse functions of EPO beyond its classical role in erythropoiesis, highlighting its potential as a therapeutic agent in different medical contexts.
For more details https://www.nature.com/articles/s41419-020-2276-8
Bitto, A., Irrera, N., Pizzino, G., Pallio, G., Mannino, F., Vaccaro, M., Arcoraci, V., Aliquò, F., Minutoli, L., Colonna, M. R., Galeano, M. R., Brines, M., De Ponte, C., Collino, M., Squadrito, F., & Altavilla, D. (2018). Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. Biochimica et biophysica acta. Molecular basis of disease, 1864(2), 632–639. https://doi.org/10.1016/j.bbadis.2017.12.006.
Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes
The study conducted by Bitto et al. and titled “Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes” was published in Biochimica et Biophysica Acta: Molecular Basis of Disease in 2018. This research focused on the use of cibinetide, an agonist of the common receptor complex EPOR-β, as a potential therapeutic intervention for impaired wound healing in mice with genetic diabetes. The study found that cibinetide treatment improved wound healing in diabetic mice by enhancing tissue regeneration and reducing inflammation. It demonstrated the potential of cibinetide to address the impaired wound healing associated with diabetes, offering promise for future therapeutic strategies in this context.
For more details https://www.sciencedirect.com/science/article/pii/S0925443917304556
Zhang W, Yu G, Zhang M. ARA 290 relieves pathophysiological pain by targeting TRPV1 channel: Integration between immune system and nociception. Peptides. 2016;76:73-9.
ARA 290 relieves pathophysiological pain by targeting TRPV1 channel: Integration between the immune system and nociception
The study conducted by Zhang et al. and titled “ARA 290 relieves pathophysiological pain by targeting TRPV1 channel: Integration between the immune system and nociception” was published in the journal Peptides in 2016. This research investigated the potential pain-relieving effects of ARA 290 and its interaction with the TRPV1 channel, which is involved in nociception (the perception of pain). The study found that ARA 290 had a pain-relieving effect by modulating the TRPV1 channel. This suggests a connection between the immune system and pain perception, highlighting a potential mechanism for ARA 290 in relieving pathophysiological pain.
For more details https://www.sciencedirect.com/science/article/pii/S0196978116300031
Heij L, Niesters M, Swartjes M, et al. Safety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study. Mol Med. 2012;18:1430-6.
Safety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study
The study conducted by Heij et al. and titled “Safety and efficacy of ARA 290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double-blind pilot study” was published in the journal Molecular Medicine in 2012. This pilot study aimed to assess the safety and efficacy of ARA 290 in sarcoidosis patients who exhibited symptoms of small fiber neuropathy. The study was conducted as a randomized, double-blind trial. While the specific results and findings of the study were not provided, the research aimed to investigate whether ARA 290 could be a potential treatment for small fiber neuropathy in sarcoidosis patients, with a focus on safety and efficacy.
For more details https://link.springer.com/article/10.2119/molmed.2012.00332
Brines M, Dunne AN, Van velzen M, et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2015;20:658-66.
ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes
The study conducted by Brines et al. and titled “ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes” was published in the journal Molecular Medicine in 2015. This study aimed to investigate the effects of ARA 290, a nonerythropoietic peptide derived from erythropoietin, on metabolic control and neuropathic symptoms in patients with type 2 diabetes. The research found that ARA 290 led to improvements in both metabolic control and neuropathic symptoms in these patients, suggesting its potential as a therapeutic option for individuals with type 2 diabetes who experience neuropathy-related symptoms.
For more details https://molmed.biomedcentral.com/articles/10.2119/molmed.2014.00215
Available from https://www.tandfonline.com/doi/full/10.1517/21678707.2013.719289.
Niesters M, Swartjes M, Heij L, Brines M, Cerami A, Dunne A, Hoitsma E, Dahan A. The erythropoietin analog ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Expert Opin Orphan Drugs 2013;1:77–87.
The erythropoietin analog ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain
ARA 290 is an erythropoietin analog that has shown promise in the treatment of sarcoidosis-induced chronic neuropathic pain. This peptide, derived from erythropoietin, has been investigated for its potential to alleviate neuropathic pain symptoms in patients with sarcoidosis. Studies have demonstrated that ARA 290 may improve symptoms associated with small fiber neuropathy, a common complication of sarcoidosis. It appears to work by targeting specific mechanisms involved in pain perception and inflammation. Further research and clinical trials are needed to fully understand the effectiveness and safety of ARA 290 in the treatment of neuropathic pain associated with sarcoidosis.
For more details https://www.tandfonline.com/doi/abs/10.1517/21678707.2013.719289
Swartjes M, van Velzen M, Niesters M, Aarts L, Brines M, Dunne A, Cerami A, Dahan A. ARA 290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain coupled with suppression of the spinal microglia response. Mol Pain 2014;10:13.
van Velzen M, Heij L, Niesters M, Cerami A, Dunne A, Dahan A, Brines M. ARA 290 for treatment of small fiber neuropathy in sarcoidosis. Expert OpinInvestig Drugs 2014;23:541–50.
ARA 290 for treatment of small fiber neuropathy in sarcoidosis
The study by van Velzen et al. explored the use of ARA 290 for the treatment of small fiber neuropathy in patients with sarcoidosis. Small fiber neuropathy is a condition characterized by damage to the small nerve fibers, leading to various sensory symptoms. The research found that ARA 290 showed promise in improving the symptoms of small fiber neuropathy in sarcoidosis patients. This suggests that ARA 290 may have a therapeutic role in addressing the neuropathic symptoms associated with sarcoidosis-induced small fiber neuropathy. These findings provide valuable insights into potential treatment options for this condition.
For more details https://www.tandfonline.com/doi/abs/10.1517/13543784.2014.892072
Dahan A, Brines M, Niesters M, Cerami A, van Velzen M. Targeting the innate repair receptor to treat neuropathy. Pain Rep. 2016;1(1):e566. Published 2016 Aug 9. doi:10.1097/PR9.0000000000000566.
Targeting the innate repair receptor to treat neuropathy
The study by Dahan et al. discusses the concept of targeting the innate repair receptor as a potential approach to treat neuropathy. Specifically, it explores the use of ARA 290, a peptide derived from the structure of erythropoietin, which acts on the innate repair receptor. This approach aims to harness the body’s natural repair mechanisms to address neuropathic pain and other symptoms associated with neuropathy. The research suggests that targeting the innate repair receptor with ARA 290 may hold promise as a therapeutic strategy for neuropathic conditions. This study contributes to the understanding of innovative approaches to neuropathy treatment.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5741312/
Pulman KG, Smith M, Mengozzi M, Ghezzi P, Dilley A. The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model. Neuroscience 2013;233:174–83.
The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model
The study by Pulman et al. investigates the effects of the erythropoietin-derived peptide ARA290 in a neuritis model. Specifically, the research focuses on its ability to reverse mechanical allodynia, a condition characterized by pain in response to normally non-painful stimuli. The findings suggest that ARA290 has the potential to alleviate mechanical allodynia in the context of neuritis, which may have implications for the treatment of neuropathic pain. This study contributes to the understanding of ARA290 as a potential therapeutic agent for neuropathic conditions.
For more details https://www.sciencedirect.com/science/article/pii/S0306452212012031
Schmidt RE, Feng D, Wang Q, Green KG, Snipes LL, Yamin M, Brines M. Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuriticdystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia. ExpNeurol 2011;232:126–35.
Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuriticdystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia
The research conducted by Schmidt et al. investigates the effects of insulin and an erythropoietin-derived peptide called ARA290 on established neuritic dystrophy and neuronopathy in Akita diabetic mouse sympathetic ganglia. This study aims to understand the potential therapeutic benefits of these treatments in mitigating nerve damage associated with diabetes. The findings suggest that ARA290, in addition to insulin, has the potential to positively impact the neurological complications seen in diabetic mice. This research contributes to our understanding of potential interventions for diabetic neuropathy and related nerve disorders.
For more details https://www.sciencedirect.com/science/article/pii/S0014488611002743
Pulman KG, Smith M, Mengozzi M, Ghezzi P, Dilley A. The erythropoietin-derived peptide ARA290 reverses mechanical allodynia in the neuritis model. Neuroscience 2013;233:174–83.
Swartjes M, Morariu A, Niesters M, Brines M, Cerami A, Aarts L, Dahan A. ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and beta-common receptor knockout mice. Anesthesiology 2011;115:1084–92.
ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and beta-common receptor knockout mice
The study conducted by Swartjes et al. explores the potential of ARA290, a peptide derived from the tertiary structure of erythropoietin, in providing long-term relief from neuropathic pain. The research includes experiments conducted in both rats and beta-common receptor knockout mice. The findings indicate that ARA290 has the ability to produce long-lasting relief from neuropathic pain, highlighting its potential as a therapeutic agent for managing this type of chronic pain condition. This study contributes valuable insights into the potential applications of ARA290 in pain management.
For more details https://pubs.asahq.org/anesthesiology/article-abstract/115/5/1084/12842
Swartjes M, Niesters M, Heij L, Dunne A, Aarts L, Hand CC, Kim HS, Brines M, Cerami A, Dahan A. Ketamine does not produce relief of neuropathic pain in mice lacking the beta-common receptor (CD131). PLoS One 2013;8:e71326.
Ketamine does not produce relief of neuropathic pain in mice lacking the β-common receptor (CD131)
In the study conducted by Swartjes et al., the researchers investigated the effect of ketamine on neuropathic pain in mice lacking the beta-common receptor (CD131). The findings of this study revealed that ketamine did not produce relief of neuropathic pain in mice without the beta-common receptor. This suggests that the presence of the beta-common receptor may be necessary for ketamine to exert its analgesic effects in neuropathic pain conditions. This research contributes to our understanding of the mechanisms involved in ketamine’s action on neuropathic pain and highlights the importance of the beta-common receptor in mediating its effects.
For more details https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0071326
Takahashi T, Kinoshita M, Shono S, Habu Y, Ogura T, Seki S, Kazama T. The effect of ketamine anesthesia on the immune function of mice with postoperative septicemia. Anesth Analgesia 2010;111:1051–8.
The effect of ketamine anesthesia on the immune function of mice with postoperative septicemia
In the study conducted by Takahashi et al., the researchers investigated the effect of ketamine anesthesia on the immune function of mice with postoperative septicemia. The findings of this study revealed that ketamine anesthesia had a positive effect on the immune function of mice in the context of postoperative septicemia. Specifically, ketamine administration was associated with improved bacterial clearance and enhanced immune responses, leading to better outcomes in mice with septicemia following surgery. These results suggest that ketamine may have immunomodulatory properties that can be beneficial in the setting of postoperative infections and septicemia.
For more details https://journals.lww.com/anesthesia-analgesia/FullText/2010/10000/The_Effect_of_Ketamine_Anesthesia_on_the_Immune.36.aspx
Culver DA, Dahan A, Bajorunas D, et al. Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Invest Ophthalmol Vis Sci. 2017;58(6):BIO52-BIO60.
Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain
In the study titled “Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain” conducted by Culver et al., the researchers investigated the effects of cibinetide on corneal nerve fiber abundance in individuals with sarcoidosis who were experiencing small nerve fiber loss and neuropathic pain. The findings of this study revealed that treatment with cibinetide led to an improvement in corneal nerve fiber abundance in these patients. This improvement suggests a potential therapeutic benefit of cibinetide in alleviating neuropathic pain and addressing small nerve fiber loss associated with sarcoidosis. Corneal nerve fiber density is often used as an indicator of small nerve fiber health, and the observed enhancement in this study implies a positive impact of cibinetide on neuropathic symptoms in sarcoidosis patients.
For more details https://iovs.arvojournals.org/article.aspx?articleid=2625918
Retrieved from https://clinicaltrials.gov/ct2/show/NCT02039687.
Swartjes, M., Morariu, A., Niesters, M., Brines, M., Cerami, A., Aarts, L., & Dahan, A. (2011). ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and β-common receptor knockout mice. Anesthesiology, 115(5), 1084–1092. https://doi.org/10.1097/ALN.0b013e31822fcefd.
ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and β-common receptor knockout mice.
The study titled “ARA290, a peptide derived from the tertiary structure of erythropoietin, produces long-term relief of neuropathic pain: an experimental study in rats and β-common receptor knockout mice” was published in the journal Anesthesiology in 2011. The study investigated the potential of ARA290, a peptide derived from the structure of erythropoietin, to provide long-term relief for neuropathic pain.
The main findings of the study are as follows:
- ARA290, when administered to rats and mice with neuropathic pain, produced long-term relief of pain.
- The study involved both normal rats and mice as well as rats and mice lacking the beta-common receptor, which is involved in erythropoietin signaling.
- ARA290 was found to be effective in relieving neuropathic pain in both normal animals and animals lacking the beta-common receptor, suggesting that its mechanism of action may be independent of erythropoietin signaling.
- The study indicates that ARA290 has the potential to provide relief from neuropathic pain and may be a promising therapeutic option for this condition.
For more details https://pubs.asahq.org/anesthesiology/article-abstract/115/5/1084/12842
Retrieved from https://pdfs.semanticscholar.org/7d4b/9ec022053c196726a65c4fe1732736d6aa48.pdf
Retrieved from https://doloranimal.org/images/fdocum/targeting_the_innate_repair_receptor_to_treat.2.pdf
Retrieved from https://ichgcp.net/clinical-trials-registry/NCT01933529
Muller C, Yassin K, Li LS, et al. ARA290 Improves Insulin Release and Glucose Tolerance in Type 2 Diabetic Goto-Kakizaki Rats. Mol Med. 2016;21(1):969–978. doi:10.2119/molmed.2015.00267.
ARA290 Improves Insulin Release and Glucose Tolerance in Type 2 Diabetic Goto-Kakizaki Rats
In a study conducted by Müller et al., titled “ARA290 Improves Insulin Release and Glucose Tolerance in Type 2 Diabetic Goto-Kakizaki Rats,” the researchers investigated the effects of ARA290 on insulin release and glucose tolerance in Goto-Kakizaki rats, a model for type 2 diabetes. The study found that ARA290, a non-erythropoietic peptide derived from erythropoietin, improved insulin release and glucose tolerance in these diabetic rats. This suggests that ARA290 may have potential benefits in the management of type 2 diabetes by improving insulin function and glucose regulation.
For more details https://molmed.biomedcentral.com/articles/10.2119/molmed.2015.00267
Muller C, et al. (2013) Thenonhematopoietic erythropoietin analogue ARA 290 improves glucose tolerance by stimulating insulin secretion in spontaneously type 2 diabetic Goto-Kakizaki rats. Diabetologia. 56(Suppl 1):S268.
Thenonhematopoietic erythropoietin analogue ARA 290 improves glucose tolerance by stimulating insulin secretion in spontaneously type 2 diabetic Goto-Kakizaki rats
In the study conducted by Müller et al. titled “The nonhematopoietic erythropoietin analogue ARA 290 improves glucose tolerance by stimulating insulin secretion in spontaneously type 2 diabetic Goto-Kakizaki rats,” the researchers investigated the effects of ARA 290, a non-erythropoietic erythropoietin analogue, on glucose tolerance and insulin secretion in spontaneously type 2 diabetic Goto-Kakizaki rats. The study found that ARA 290 improved glucose tolerance by stimulating insulin secretion in these diabetic rats. This suggests that ARA 290 may have potential as a therapeutic agent for improving glucose regulation in type 2 diabetes by enhancing insulin secretion.
Schmidt RE, et al. Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia. Exp Neurol. 2011;232:126–35.
Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia
In the study conducted by Schmidt et al. titled “Effect of insulin and an erythropoietin-derived peptide (ARA290) on established neuritic dystrophy and neuronopathy in Akita (Ins2 Akita) diabetic mouse sympathetic ganglia,” the researchers investigated the impact of insulin and ARA290, an erythropoietin-derived peptide, on established neuritic dystrophy and neuronopathy in diabetic mice with the Akita mutation. The study found that both insulin and ARA290 had a positive effect in mitigating established neuritic dystrophy and neuronopathy in the sympathetic ganglia of diabetic mice. This suggests that ARA290 may have therapeutic potential in ameliorating neuropathic complications associated with diabetes, particularly in the sympathetic nervous system.
For more details https://www.sciencedirect.com/science/article/pii/S0014488611002743
Retrieved from https://clinicaltrials.gov/ct2/show/NCT01933529
-
Polgárová K, Lüthje P, Cerami A, Brauner A. The erythropoietin analogue ARA290 modulates the innate immune response and reduces Escherichia coli invasion into urothelial cells. FEMS Immunol Med Microbiol. 2011;62(2):190-6.
The erythropoietin analogue ARA290 modulates the innate immune response and reduces Escherichia coli invasion into urothelial cells
The study titled “The erythropoietin analogue ARA290 modulates the innate immune response and reduces Escherichia coli invasion into urothelial cells” investigated the immunomodulatory effects of ARA290, a non-erythropoietic peptide derived from erythropoietin, on the innate immune response and its ability to inhibit Escherichia coli (E. coli) invasion into urothelial cells. The research demonstrated that ARA290 effectively modulated the innate immune response, indicating its potential to influence the body’s initial defense against pathogens and inflammation. Furthermore, ARA290 exhibited a reduction in the invasion of E. coli bacteria into urothelial cells, suggesting its potential in protecting against urinary tract infections. These findings highlight ARA290’s immunomodulatory properties and its ability to enhance innate immune defenses and combat certain pathogenic invasions, making it a candidate for further therapeutic exploration in conditions related to the innate immune system or urothelial infections (Reference: Polgárová K, Lüthje P, Cerami A, Brauner A. FEMS Immunol Med Microbiol. 2011;62(2):190-6).
For more details https://academic.oup.com/femspd/article-abstract/62/2/190/534943
Macdougall I.C., Rossert J., Casadevall N., Stead R.B., Duliege A.M., Froissart M., Eckardt K.U. A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N. Engl. J. Med. 2009;361:1848–1855.
A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia
The study titled “A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia” by Macdougall et al. explored the development of a novel peptide-based erythropoietin-receptor agonist as a therapeutic approach for pure red-cell aplasia (PRCA). PRCA is a rare condition characterized by a severe deficiency of red blood cells due to the presence of neutralizing antibodies against endogenous erythropoietin. The researchers aimed to address this condition by developing a synthetic peptide that could activate the erythropoietin receptor independently of erythropoietin itself. The study demonstrated the efficacy of this peptide-based agonist in increasing red blood cell production and improving hemoglobin levels in PRCA patients, providing a promising alternative treatment option for this challenging medical condition (Reference: Macdougall I.C., Rossert J., Casadevall N., Stead R.B., Duliege A.M., Froissart M., Eckardt K.U. N. Engl. J. Med. 2009;361:1848–1855).
For more details https://www.nejm.org/doi/full/10.1056/NEJMoa074037
Wrighton N.C., Farrell F.X., Chang R., Kashyap A.K., Barbone F.P., Mulcahy L.S., Johnson D.L., Barrett R.W., Jolliffe L.K., Dower W.J. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–464.
Small peptides as potent mimetics of the protein hormone erythropoietin
In the study titled “Small peptides as potent mimetics of the protein hormone erythropoietin” by Wrighton et al., researchers aimed to develop small peptides that could mimic the activity of the protein hormone erythropoietin (EPO). EPO is a key regulator of red blood cell production. The study focused on identifying short peptide sequences that could bind to and activate the EPO receptor, leading to the production of red blood cells. The research successfully identified small peptides that displayed potent EPO-mimetic activity, demonstrating their potential as therapeutic agents for conditions associated with erythropoietin deficiency or dysfunction. This work laid the foundation for the development of peptide-based erythropoiesis-stimulating agents as an alternative to traditional EPO therapy (Reference: Wrighton N.C., Farrell F.X., Chang R., Kashyap A.K., Barbone F.P., Mulcahy L.S., Johnson D.L., Barrett R.W., Jolliffe L.K., Dower W.J. Science. 1996;273:458–464).
For more details https://www.science.org/doi/abs/10.1126/science.273.5274.458
Brines M., Patel N.S., Villa P., Brines C., Mennini T., De Paola M., Erbayraktar Z., Erbayraktar S., Sepodes B., Thiemermann C., Ghezzi P., Yamin M., Hand C.C., Xie Q.W., Coleman T., Cerami A. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. U S A. 2008;105:10925–10930.
Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin
In the study titled “Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin” by Brines et al., researchers investigated the therapeutic potential of nonerythropoietic peptides derived from the tertiary structure of erythropoietin (EPO). EPO is known for its role in red blood cell production, but this study focused on identifying EPO-derived peptides that had tissue-protective properties without stimulating red blood cell production. The research successfully identified such peptides, which were found to have protective effects on various tissues and organs, including the brain, heart, kidney, and more. These tissue-protective peptides offered potential therapeutic benefits for conditions involving tissue damage and injury. This study contributed to the exploration of novel therapeutic agents derived from EPO for tissue protection (Reference: Brines M., Patel N.S., Villa P., Brines C., Mennini T., De Paola M., Erbayraktar Z., Erbayraktar S., Sepodes B., Thiemermann C., Ghezzi P., Yamin M., Hand C.C., Xie Q.W., Coleman T., Cerami A. Proc. Natl. Acad. Sci. U S A. 2008;105:10925–10930).
For more details https://www.pnas.org/doi/abs/10.1073/pnas.0805594105
Nairz M, Haschka D, Dichtl S, et al. Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis. Sci Rep. 2017;7(1):13012.
Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis
In the study conducted by Nairz et al., titled “Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis,” the researchers investigated the potential therapeutic effects of cibinetide in experimental colitis. Colitis is characterized by inflammation of the colon and can lead to various gastrointestinal symptoms. Cibinetide is a peptide derived from erythropoietin that has been studied for its tissue-protective and anti-inflammatory properties. In this study, the researchers found that cibinetide was able to dampen the functions of innate immune cells, which are involved in the inflammatory response. As a result, cibinetide ameliorated the course of experimental colitis, suggesting its potential as a therapeutic agent for inflammatory bowel diseases. This research contributes to our understanding of the immunomodulatory effects of cibinetide in the context of colitis (Reference: Nairz M, Haschka D, Dichtl S, et al. Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis. Sci Rep. 2017;7(1):13012).
For more details https://www.nature.com/articles/s41598-017-13046-3
Hache G, Garrigue P, Bennis Y, et al. ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock. 2016;46(4):390-7.
ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability
In the study conducted by Hache et al., titled “ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability,” the researchers investigated the effects of ARA290, a specific agonist of the erythropoietin/CD131 heteroreceptor, on circulating endothelial progenitor cells (EPCs). EPCs play a crucial role in angiogenesis, the formation of new blood vessels, which is important for tissue repair and regeneration. The study aimed to determine whether ARA290 could enhance the angiogenic potential and homing ability of EPCs.
The researchers found that ARA290 treatment significantly improved the angiogenic potential of EPCs, as demonstrated by their increased ability to form new blood vessels in vitro. Additionally, ARA290 enhanced the homing ability of EPCs to ischemic tissues, suggesting its potential for improving tissue repair in conditions associated with impaired angiogenesis. This study contributes to our understanding of the therapeutic potential of ARA290 in promoting angiogenesis and tissue repair (Reference: Hache G, Garrigue P, Bennis Y, et al. ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock. 2016;46(4):390-7)
For more details https://www.ingentaconnect.com/content/wk/shk/2016/00000046/00000004/art00008
Chen H, Luo B, Yang X, et al. Therapeutic effects of nonerythropoietic erythropoietin analog ARA290 in experimental autoimmune encephalomyelitis rat. J Neuroimmunol. 2014;268(1-2):64-70.
Therapeutic effects of nonerythropoietic erythropoietin analog ARA290 in experimental autoimmune encephalomyelitis rat
In the study conducted by Chen et al., titled “Therapeutic effects of nonerythropoietic erythropoietin analog ARA290 in experimental autoimmune encephalomyelitis rat,” the researchers investigated the therapeutic potential of ARA290, a nonerythropoietic erythropoietin analog, in an experimental autoimmune encephalomyelitis (EAE) rat model. EAE is a widely used model for studying multiple sclerosis (MS), an autoimmune disease that affects the central nervous system.
The study aimed to assess whether ARA290 could ameliorate the symptoms of EAE and provide neuroprotective effects. The researchers found that ARA290 treatment resulted in several positive outcomes, including a reduction in clinical severity, decreased inflammatory markers, and improved histopathological changes in the spinal cord. These findings suggest that ARA290 has potential therapeutic effects in EAE, which may have implications for the treatment of MS.
For more details https://www.sciencedirect.com/science/article/pii/S0165572814000095
Ahmet I, et al. A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage. Mol Med. 2011;17:194–200.
A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage
In the study conducted by Ahmet et al. titled “A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage,” researchers investigated the cardioprotective properties of a small nonerythropoietic peptide derived from the structure of erythropoietin. The study focused on its potential to protect the heart from ischemic myocardial damage.
Ischemic myocardial damage occurs when the blood supply to the heart muscle is reduced or blocked, typically during a heart attack. The researchers found that the small peptide, which is structurally similar to erythropoietin, exhibited cardioprotective effects in a rat model of myocardial infarction (heart attack). This included reducing the size of the infarcted area and improving cardiac function.
For more details https://molmed.biomedcentral.com/articles/10.2119/molmed.2010.00235
Yan, L., Zhang, H., Gao, S., Zhu, G., Zhu, Q., Gu, Y., & Shao, F. (2018). EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway. Transplantation proceedings, 50(5), 1575–1582. https://doi.org/10.1016/j.transproceed.2018.03.015.
EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway
In the study conducted by Yan et al. titled “EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway,” the researchers investigated the potential of the EPO derivative ARA290 in attenuating early renal allograft (kidney transplant) injury in rats. They focused on the involvement of the NF-κB pathway, which plays a crucial role in inflammation and immune responses.
The study utilized a rat model of renal transplantation to assess the effects of ARA290 on graft survival and renal function. The results demonstrated that treatment with ARA290 led to a significant improvement in graft survival and a reduction in early renal allograft injury compared to control groups. This protective effect was attributed to the modulation of the NF-κB pathway.
For more details https://www.sciencedirect.com/science/article/pii/S0041134518302501
Nairz, M., Haschka, D., Dichtl, S., Sonnweber, T., Schroll, A., Aßhoff, M., Mindur, J. E., Moser, P. L., Wolf, D., Swirski, F. K., Theurl, I., Cerami, A., Brines, M., & Weiss, G. (2017). Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis. Scientific reports, 7(1), 13012. https://doi.org/10.1038/s41598-017-13046-3.
Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis
In the study conducted by Nairz et al. titled “Cibinetide Dampens Innate Immune Cell Functions Thus Ameliorating the Course of Experimental Colitis,” the researchers investigated the potential therapeutic effects of cibinetide, a compound derived from erythropoietin, in the context of experimental colitis. Colitis is a type of inflammatory bowel disease characterized by inflammation of the colon.
The study employed a mouse model of colitis to evaluate the impact of cibinetide on the progression of the disease. The results showed that treatment with cibinetide led to significant improvements in the course of experimental colitis. Specifically, cibinetide was found to dampen the functions of innate immune cells, which are involved in the inflammatory response.
For more details https://www.nature.com/articles/s41598-017-13046-3
Huang, B., Jiang, J., Luo, B., Zhu, W., Liu, Y., Wang, Z., & Zhang, Z. (2018). Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus. Journal of cellular and molecular medicine, 22(7), 3330–3339. https://doi.org/10.1111/jcmm.13608.
Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus
In the study conducted by Huang et al. titled “Non-erythropoietic Erythropoietin-Derived Peptide Protects Mice from Systemic Lupus Erythematosus,” the researchers investigated the potential therapeutic effects of a non-erythropoietic peptide derived from erythropoietin in a mouse model of systemic lupus erythematosus (SLE). SLE is an autoimmune disease characterized by chronic inflammation and damage to various tissues and organs.
The study aimed to evaluate whether this erythropoietin-derived peptide could mitigate the symptoms and pathology associated with SLE. The results demonstrated that treatment with the non-erythropoietic peptide had a protective effect in SLE mice. Specifically, it reduced disease severity, ameliorated kidney damage (a common feature of SLE), and modulated the immune response.
For more details https://onlinelibrary.wiley.com/doi/abs/10.1111/jcmm.13608
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

