Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Potential Health Benefits of Amlexanox
Amlexanox offers a range of benefits, including promoting weight loss, improving blood sugar levels, preventing bone loss, enhancing liver health, treating asthma, and effectively managing mouth ulcers, making it a versatile compound with potential therapeutic applications across multiple health conditions.
- Promotes weight loss [1-4]
- Improves blood sugar levels [1-3] [5]
- Prevents bone loss [6]
- Improves liver health [5] [7-10]
- Treats asthma [11-19]
- Treats mouth ulcers [20-27]
Key Takeaways of Amlexanox
- Amlexanox is primarily known as a treatment for aphthous ulcers (canker sores), with its primary indication being for the topical treatment of these ulcers in people with a normal immune system.
- It works as an anti-inflammatory and anti-allergic agent, suppressing the formation and release of inflammatory mediators.
- While it’s available in an oral paste form, it should not be intentionally swallowed. The paste is applied directly to the ulcer, typically 2 to 4 times daily until healing occurs.
- There’s ongoing research into other potential applications for amlexanox, including its role in obesity and metabolic dysfunction by targeting specific inflammatory enzymes.
- Amlexanox is available by prescription and is FDA-approved for the treatment of aphthous ulcers. Its brand name for the oral paste is Aphthasol.
What is Amlexanox?
Amlexanox is a medication with fat-burning properties. It promotes fat loss by inhibiting the production of a specific enzyme in the body that is responsible for fat storage and inflammation. Amlexanox is known to protect against bone disorders and liver disease. It can also help treat certain medical conditions like asthma due to its anti-allergic properties. Furthermore, it has traditionally been used to treat ulcers due to its anti-inflammatory effects.
Amlexanox Mechanism of Action
Amlexanox works by inhibiting the release of histamine and leukotrienes – both of which are involved in multiple mechanisms such as inflammation and allergic reaction. This medication also helps reduce weight loss by inhibiting TBK1 (TANK-binding kinase 1), an enzyme involved in fat storage and inflammation.
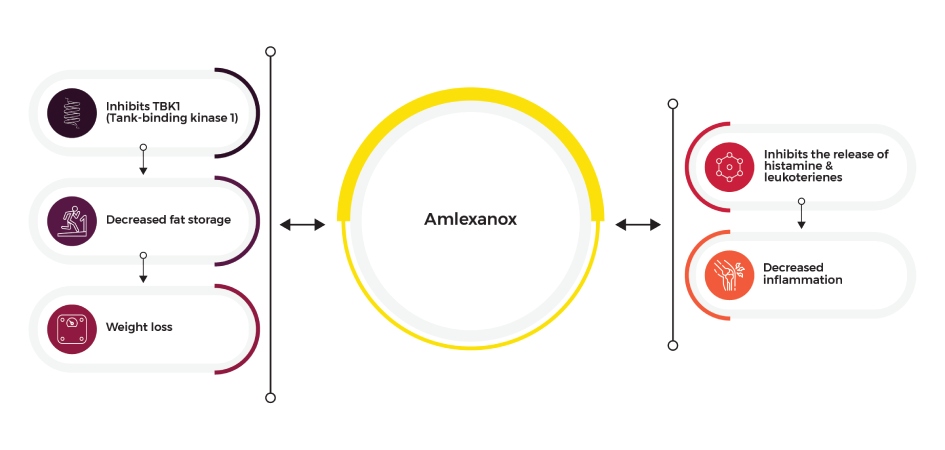 Amlexanox Infographic
Amlexanox Infographic
Chemical Structure of Amlexanox
Research on Amlexanox
A. Promotes Weight Loss

Amlexanox promotes reversible weight loss by influencing multiple mechanisms involved in metabolism and appetite regulation. It has been found to affect the expression of genes associated with fat storage and metabolism, contributing to reversible weight loss through reduced fat accumulation.
Moreover, amlexanox may influence energy expenditure through its potential effects on metabolic processes and energy homeostasis. Research suggests that amlexanox can impact energy expenditure by modulating certain signaling pathways within cells, potentially leading to increased energy expenditure. These actions on energy expenditure are of interest in understanding how amlexanox may contribute to metabolic regulation and overall metabolic health.
Additionally, amlexanox may suppress appetite and reduce food intake, which can further support reversible weight loss efforts. These combined effects make amlexanox a potential candidate for managing reversible weight loss and are of interest to those exploring amlexanox weight loss strategies.
The primary benefit of amlexanox is supported by a number of high-quality studies:
- A study found that amlexanox can help induce fat loss by increasing the energy expenditure of fat cells. [1]
- In obese mice, amlexanox improved obesity-related metabolic dysfunctions by increasing thermogenesis (dissipation of energy through the production of heat), improving insulin sensitivity, and decreasing steatosis (fat build-up in the liver). [2]
- A study found a reduction in both percent total body fat and percent truncal fat in a group of patients treated with amlexanox compared to the non-treated subjects. [3]
- A study reported that amlexanox can help promote weight loss by inhibiting TBK1 (TANK-binding kinase 1), an enzyme involved in the storage of fat and lipid metabolism in fat cells. [4]
B. Improves Blood Sugar Levels

Amlexanox improves blood sugar levels primarily by targeting insulin resistance, a key factor in type 2 diabetes and metabolic disease. It enhances insulin sensitivity, allowing cells to respond more effectively to insulin signals and facilitating glucose uptake. This improved insulin sensitivity, promoted by amlexanox, results in better blood sugar control.
Additionally, amlexanox’s capacity to combat insulin resistance is a crucial aspect of its mechanism for enhancing blood sugar management. Through its effects on insulin resistance and improved insulin sensitivity, amlexanox contributes significantly to the regulation of blood sugar levels in individuals with type 2 diabetes and metabolic disease.
Studies show that amlexanox exerts its beneficial effects on blood sugar levels by targeting and mitigating insulin resistance, ultimately leading to improved insulin sensitivity in individuals with type 2 diabetes:
- In obese mice, acute amlexanox treatment suppressed blood sugar production in the liver which in turn reduced insulin resistance. [1]
- In obese mice, amlexanox decreased blood sugar levels which in turn led to improved insulin sensitivity. [2]
- In obese patients with type 2 diabetes and nonalcoholic fatty liver disease, treatment with amlexanox produced a statistically significant reduction in Hemoglobin A1c, a measure of blood sugar, resulting in improved glucose control. [3]
- In mice fed with a high-fat diet, amlexanox administration for 18 weeks produced substantial results in terms of reversing blood sugar and lipid metabolic disturbance. [5]
C. Prevents Bone Loss
Amlexanox can prevent bone loss through multiple mechanisms by inhibiting the activity of osteoclasts, the cells responsible for breaking down bone tissue. By suppressing osteoclast function, amlexanox helps maintain bone density and prevent bone loss, making it a potential therapeutic option for conditions associated with bone deterioration such as osteoporosis. In addition, amlexanox may also be used as an anti-inflammatory agent for the treatment of bone disorders by inhibiting inflammation.
In one study, researchers investigated the effects of amlexanox on mice with bone loss caused by surgical removal of the ovaries. [6] Results showed that amlexanox inhibited the formation of osteoclasts, which are specialized cells that absorb and remove bone to allow the development of new bones and the maintenance of bone strength. Moreover, amlexanox enhanced the production of bone-forming cells known as osteoblasts. These results suggest that amlexanox may be considered a new therapeutic candidate for bone disorders like osteoporosis and rheumatoid arthritis.
D. Improves Liver Health
Amlexanox improves liver health by inhibiting inflammation and oxidative stress within the liver. It has been shown to inhibit certain inflammatory pathways and decrease the production of pro-inflammatory molecules. This significant effect helps protect liver cells from damage.
Additionally, amlexanox may enhance the body’s antioxidant defenses, further reducing oxidative stress in the liver. These combined actions contribute to improved liver health and make amlexanox a potential candidate for the management of liver conditions like non-alcoholic fatty liver disease (NAFLD) and inflammation-related liver disorders.
A number of studies suggest that amlexanox can protect against various types of liver disease:
- In mice fed with a high-fat diet, amlexanox produced substantial results in terms of reversing fat build-up in the liver. [5]
- In mice, amlexanox ameliorated acetaminophen-induced acute liver injury by reducing oxidative stress. [7]
- In male mice fed with a high-fat diet, amlexanox alleviated steatohepatitis (liver inflammation caused by fat build-up) by inhibiting inflammation. [8]
- In mouse models with metabolic dysfunctions due to obesity, amlexanox mitigated palmitic acid-induced liver toxicity. [9]
- In mice, oral administration with amlexanox (25, 50, and 100 mg/kg) attenuated liver scarring caused by the liver toxin carbon tetrachloride. [10]
E. Treats Asthma
Amlexanox treats asthma by inhibiting histamine release from mast cells, a key contributor to bronchoconstriction and airway inflammation in asthmatic individuals. By reducing histamine release, amlexanox helps to alleviate airway constriction and inflammation, thereby providing relief from asthma symptoms. This action makes amlexanox a potential candidate for asthma management, particularly in cases where histamine release from mast cells plays a significant role in symptom exacerbation.
Amlexanox also exhibits its potential in treating asthma as an anti-allergic compound, acting on multiple fronts to mitigate allergic responses. It operates as an anti-allergic compound by inhibiting histamine release, which is a hallmark of allergic reactions and a trigger for bronchoconstriction and airway inflammation in asthma patients.
Furthermore, this anti-allergic compound modulates other inflammatory pathways and immune responses, collectively contributing to its potential as an effective therapeutic option in managing asthma and reducing healing time.
Evidence supports the anti-asthma properties of amlexanox:
- In patients with aspirin-induced asthma, amlexanox significantly improved measures of lung function. [11-12]
- Studies found that amlexanox can effectively treat allergic asthma and rhinitis by reducing the levels of inflammatory substances such as LTB4, LTC4, LTD4, and LTE4 and by inhibiting histamine release. [13-14]
- In mice, intranasal and oral administration of amlexanox decreased lung allergic inflammation. [15]
- In rats and guinea pigs, amlexanox inhibited experimental asthma by decreasing airway constriction. [16-17]
- In mice with lung inflammation, amlexanox improved lung function and structure. [18-19]
F. Treats Mouth Ulcers
-
Amlexanox is a medication specifically formulated as an anti-inflammatory treatment to treat ulcers, particularly canker sores, also known as mouth ulcers. Canker sores are painful, small lesions that can develop inside the mouth, on the lips, or along the gumline.
Amlexanox functions as an anti-inflammatory medication by reducing the inflammation and irritation associated with canker sores. It does this by inhibiting the release of inflammatory mediators and suppressing the immune response in the affected area. By targeting the underlying inflammation, amlexanox provides effective relief from the discomfort and pain of canker sores.
Amlexanox is applied topically as a gel or ointment directly to the ulcerated area. This localized treatment helps speed up the healing time and reduces the duration of the canker sore. Amlexanox is a valuable option for individuals who suffer from recurrent canker sores, as it not only alleviates symptoms but also promotes faster healing time, improving overall oral health and comfort. Therefore, it is an effective canker sore treatment.
Studies show that amlexanox can safely and effectively treat ulcers such as canker sores:
- In patients with canker sores, the application of 5% amlexanox topical paste was shown to consistently and significantly speed up complete ulcer healing time and the time to pain resolution. [20]
- In patients diagnosed with canker sores, the application of 5% amlexanox oral paste directly on the ulcer 4 times a day for 6 days resulted in a marked reduction in canker sore ulcer size and pain scores, thus reducing healing time. [21]
- The administration of OraDisc (active component 2 mg amlexanox) in patients with canker sores resulted in significant improvement in erythema (skin rash caused by inflamed blood capillaries) score, canker sore ulcer size, and pain scores. [22]
- In patients with recurrent aphthous stomatitis (mouth sores or canker sores), amlexanox oral adhesive pellicles significantly reduced canker sore ulcer size and alleviated ulcer pain, thus reducing healing time. [23]
- A clinical trial conducted on 100 patients with canker sores found that amlexanox 5% can reduce the frequency, duration, and symptoms of the condition with no adverse side effects. [24]
- In patients with recurrent oral ulcerations who were enrolled in a placebo-controlled study, the application of 5% amlexanox paste on the ulcers of the treatment group resulted in a faster healing time of canker sores compared to the placebo-treated group. [25]
- When applied directly to the lesion of canker sores, 5% amlexanox topical paste at a dose of 100 mg was found to be safe, effective, and well-tolerated. [26]
- In immunocompetent patients with mild to moderate canker sores, the application of amlexanox oral paste (Aphthasol) was shown to achieve complete healing of canker sore ulcers and resolution of pain in just 3 days. [27]
Associated Side Effects of Amlexanox
Amlexanox side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on amlexanox. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of amlexanox. Despite this, it was listed as a side effect associated with amlexanox even though these associated side effects are very uncommon.
Side effects associated with amlexanox may include the following:
- Rashes
- Itching
- Difficulty breathing
- Tightness in the chest
- Swelling of the mouth, face, lips, or tongue
- Inflammation of the lining of the mouth
- Slight pain, stinging, or burning of the skin
- Nausea
- Diarrhea
Amlexanox Before and After
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
FAQ
Is amlexanox over the counter?
No, amlexanox is typically available by prescription from a healthcare provider, not over the counter.
Can we swallow amlexanox oral paste?
Amlexanox oral paste is intended for topical application to aphthous ulcers and should not be intentionally swallowed. However, small amounts that may be inadvertently swallowed due to saliva movement are generally not harmful.
How do you use amlexanox?
Amlexanox tablets or amlexanox capsules (amlexanox 40 mg capsule) are taken orally, typically as directed by your healthcare provider. Amlexanox oral paste, on the other hand, is applied directly to the aphthous ulcer (canker sore) and is usually administered 2 to 4 times daily until the ulcer heals. Consult with your healthcare provider for guidance on the proper use of either form of amlexanox.
How does amlexanox work for weight loss?
Research has shown that amlexanox for weight loss targets specific inflammatory enzymes that play a role in obesity and metabolic dysfunction. Consult with your healthcare provider to learn more about the potential applications of amlexanox in your health management.
What class of drug is amlexanox?
Amlexanox is classified as an anti-inflammatory and anti-allergic agent.
Is amlexanox FDA-approved?
Yes, amlexanox is FDA-approved for aphthous ulcer treatment.
Is Amlexanox a steroid?
No, amlexanox is not a steroid.
What is the solubility of amlexanox?
Amlexanox is sparingly soluble in water, slightly soluble in ethanol, and practically insoluble in methylene chloride.
What is the brand name for amlexanox oral paste?
The brand name for amlexanox oral paste is Aphthasol. If you have any questions about using Aphthasol, consult your healthcare provider for guidance on its appropriate use.
How long does it take an amlexanox to work for weight loss?
There is no established timeframe for amlexanox’s potential efficacy in weight loss. It depends on factors such as your current weight and metabolism.
What are amlexanox and lidocaine HCL paste used for?
A combination of amlexanox and lidocaine HCL would provide both anti-inflammatory effects (from the amlexanox) and local anesthetic effects (from the lidocaine). It could be used to treat painful oral conditions. However, specifics would depend on the product’s intended indication and FDA approval.
Is Amlexanox available in the US?
Yes, amlexanox is available in the US, primarily by prescription for the treatment of aphthous ulcers.
When should I see my healthcare provider?
You should call your healthcare provider if you have canker sores that:
- Begin to spread
- Are unusually large
- Last longer than two weeks
- Interfere with eating, drinking, or other daily routines
- Are accompanied by a high fever
Reference
Reilly SM, Abu-Odeh M, Ameka M, DeLuca JH, Naber MC, Dadpey B, Ebadat N, Gomez AV, Peng X, Poirier B, Walk E, Potthoff MJ, Saltiel AR. FGF21 is required for the metabolic benefits of IKKε/TBK1 inhibition. J Clin Invest. 2021 May 17;131(10):e145546. doi: 10.1172/JCI145546. PMID: 33822771; PMCID: PMC8121507.
FGF21 is required for the metabolic benefits of IKKε/TBK1 inhibition
In mouse models of obesity, the protein kinases IKKε and TBK1 become activated in the liver and fat tissues. Previous research showed that treating obese animals and patients with the IKKε/TBK1 inhibitor amlexanox resulted in weight loss and improved insulin resistance. While amlexanox initially reduced food intake, long-term weight loss occurred due to increased energy expenditure through FGF21-dependent beiging of white adipose tissue (WAT). Amlexanox boosted FGF21 production and release in various tissues, and notably, adipocyte-secreted FGF21 seemed to act as an autocrine factor that triggered adipose tissue browning and weight loss in obese mice. Additionally, increased energy expenditure played a crucial role in enhancing insulin sensitivity with amlexanox treatment, while immediate reductions in fasting blood glucose resulted from the suppression of hepatic glucose production through adipocyte-secreted IL-6 activation of hepatic STAT3. These findings highlight the mechanisms through which amlexanox improves metabolic health by affecting FGF21 in adipocytes and IL-6 in regulating glucose metabolism.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8121507/
Reilly SM, Chiang SH, Decker SJ, et al. An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313-321. doi:10.1038/nm.3082.
An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice
Recent research points to inflammation as a crucial link between obesity and insulin resistance. In response to a high-fat diet, the noncanonical IκB kinases IKK-ɛ and TANK-binding kinase 1 (TBK1) become activated in the liver and fat due to NF-κB activation, leading to a counterinflammatory program that preserves energy storage. A study reveals that amlexanox, an approved therapeutic for conditions like aphthous ulcers and asthma, is an inhibitor of these kinases. Administering amlexanox to obese mice increases energy expenditure by boosting thermogenesis, resulting in weight loss, improved insulin sensitivity, and reduced steatosis. Given its safety record in patients, amlexanox holds promise for clinical evaluation in treating obesity and related disorders.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594079/
Oral EA, Reilly SM, Gomez AV, et al. Inhibition of IKKɛ and TBK1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes. Cell Metab. 2017;26(1):157-170.e7. doi:10.1016/j.cmet.2017.06.006.
Inhibition of IKKɛ and TBK1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes
Numerous studies have highlighted the inflammatory connection between obesity and type 2 diabetes. In obese individuals, the inflammatory kinases IKKɛ and TBK1 are elevated, and inhibiting them in obese mice has been shown to reduce weight, insulin resistance, fatty liver, and inflammation. In a proof-of-concept randomized, double-blind, placebo-controlled study involving 42 obese patients with type 2 diabetes and nonalcoholic fatty liver disease, the use of amlexanox, an inhibitor of IKKɛ and TBK1, resulted in a statistically significant reduction in Hemoglobin A1c and fructosamine levels. Interestingly, a subgroup of patients who responded positively to the drug also showed improvements in insulin sensitivity and hepatic steatosis. This subgroup had a unique inflammatory gene expression profile in their subcutaneous fat at baseline and exhibited distinctive gene expression changes in response to amlexanox, indicating enhanced energy expenditure. These findings suggest that dual-specificity inhibitors of IKKɛ and TBK1 may be effective treatments for metabolic diseases in a specific subset of patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5663294/.
Beyett TS, Gan X, Reilly SM, Chang L, Gomez AV, Saltiel AR, et al. Carboxylic Acid Derivatives of Amlexanox Display Enhanced Potency toward TBK1 and IKKε and Reveal Mechanisms for Selective Inhibition. Mol Pharmacol. 2018. October;94(4):1210–9. 10.1124/mol.118.112185.
Carboxylic Acid Derivatives of Amlexanox Display Enhanced Potency toward TBK1 and IKKε and Reveal Mechanisms for Selective Inhibition
Chronic low-grade inflammation is a characteristic feature of obesity, a risk factor for type 2 diabetes. The drug amlexanox inhibits IκB kinase ε (IKKε) and TANK binding kinase 1 (TBK1), promoting energy expenditure and improving insulin sensitivity. Clinical studies have shown efficacy in certain diabetic patients with underlying adipose tissue inflammation, but with only moderate potency, necessitating the development of better analogs. Crystal structures of TBK1 in complex with amlexanox and modified analogs that alter its carboxylic acid moiety were examined. Removal of the carboxylic acid or mutation of the adjacent Thr156 residue reduced potency toward TBK1, while conversion to a short amide or ester nearly abolished inhibitory effects. IKKε was less affected, possibly due to variation in its hinge region, allowing greater conformational flexibility. The introduction of a tetrazole carboxylic acid bioisostere improved potency to 200 and 400 nM for IKKε and TBK1, respectively. Despite enhanced in vitro potency, none of the analogs outperformed amlexanox in adipocytes, potentially due to altered absorption and distribution. The described structure-activity relationships and cocrystal structures will guide the future development of inhibitors using the amlexanox pharmacophore for obesity and type 2 diabetes treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6128810/
He Q, Xia X, Yao K, Zeng J, Wang W, Wu Q, Tang R, Zou X. Amlexanox reversed non-alcoholic fatty liver disease through IKKε inhibition of hepatic stellate cell. Life Sci. 2019 Dec 15;239:117010. doi: 10.1016/j.lfs.2019.117010. Epub 2019 Oct 28. PMID: 31672578.
Amlexanox reversed non-alcoholic fatty liver disease through IKKε inhibition of hepatic stellate cell
Aims: This study aimed to investigate the molecular mechanisms underlying the role of amlexanox, an inhibitor of nuclear factor κB kinase epsilon (IKKε) and TANK-binding kinase 1(TBK1), in non-alcoholic fatty liver disease (NAFLD). Methods: NAFLD mouse models were established using a high-fat diet (HFD) and lipopolysaccharide (LPS). Amlexanox or vehicle was administered to the mice, and various parameters were measured. Main findings: The study showed that amlexanox improved glucose and lipid metabolism and reduced hepatic steatosis in NAFLD mice. IKKε was specifically expressed in hepatic stellate cells (HSCs) rather than hepatocytes. Furthermore, amlexanox enhanced insulin signaling in hepatocytes by inhibiting inflammation in HSCs. Significance: These findings confirm that IKKε is expressed in HSCs, and the inhibition of activated HSCs contributes to amlexanox’s effects on NAFLD, including the improvement of insulin signaling in hepatocytes.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0024320519309373?via%3Dihub.
Zhang Y, Guan H, Li J, Fang Z, Chen W, Li F. Amlexanox Suppresses Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss. Sci Rep. 2015;5:13575. Published 2015 Sep 4. doi:10.1038/srep13575.
Amlexanox Suppresses Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss
The protein kinases IKK-ε and TANK-binding kinase 1 (TBK1) is known to play a role in inflammatory diseases, and amlexanox, an inhibitor of these kinases, is used clinically to treat conditions like ulcers, allergic rhinitis, and asthma. This study explores the potential of amlexanox for treating osteoclast-related diseases, often associated with low-grade systemic inflammation. In both in vitro and in vivo experiments, amlexanox inhibited osteoclast formation, bone resorption, and the expression of osteoclast-specific genes, while promoting osteoblast differentiation. In an ovariectomy-induced bone loss mouse model, amlexanox prevented bone loss by suppressing osteoclast activity. These findings suggest that amlexanox may serve as a therapeutic candidate for osteoclast-related diseases such as osteoporosis and rheumatoid arthritis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4559750/
Qi J, Zhou Z, Lim CW, Kim JW, Kim B. Amlexanox ameliorates acetaminophen-induced acute liver injury by reducing oxidative stress in mice. Toxicol Appl Pharmacol. 2019 Dec 15;385:114767. doi: 10.1016/j.taap.2019.114767. Epub 2019 Nov 5. PMID: 31697998.
Amlexanox ameliorates acetaminophen-induced acute liver injury by reducing oxidative stress in mice
Amlexanox, a clinically approved drug used to treat conditions like allergic rhinitis, ulcers, and asthma, has been investigated for its protective mechanisms in acetaminophen (APAP)-induced acute liver injury (ALI). In this study, mice with APAP-induced ALI were administered amlexanox, which inhibits IKKε and TBK1. The treatment reduced liver injury as evidenced by decreased enzyme levels in the blood, reduced hepatocellular apoptosis, and lower oxidative stress. Additionally, amlexanox increased the expression of genes associated with antioxidant pathways through the activation of AMP-activated protein kinase (AMPK) and nuclear factor erythroid 2-related factor 2 (Nrf2). These findings suggest that amlexanox exerts its protective effects against APAP-induced liver injury through the AMPK/Nrf2 pathway.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0041008X19303758?via%3Dihub.
He Q, Zeng J, Yao K, Wang W, Wu Q, Tang R, Xia X, Zou X. Long-term subcutaneous injection of lipopolysaccharides and high-fat diet-induced non-alcoholic fatty liver disease through IKKε/ NF-κB signaling. BiochemBiophys Res Commun. 2020 Nov 12;532(3):362-369. doi: 10.1016/j.bbrc.2020.08.036. Epub 2020 Aug 31. PMID: 32883523.
Long-term subcutaneous injection of lipopolysaccharides and high-fat diet-induced non-alcoholic fatty liver disease through IKKε/ NF-κB signaling
This study aimed to investigate the role of IKKε/NF-κB signaling in the development of non-alcoholic fatty liver disease (NAFLD) induced by lipopolysaccharides (LPS) and a high-fat diet (HFD). Male C57BL/6 mice were subjected to an 18-week regimen of HFD combined with low-dose LPS injections, exacerbating steatosis and inflammation compared to HFD alone or high-dose LPS + HFD. Inhibiting IKKε/NF-κB signaling with amlexanox significantly mitigated metabolic disorders and hepatic steatosis induced by HFD + LPS, resulting in the downregulation of LPS-upregulated genes associated with glucolipid metabolism. These findings suggest that IKKε/NF-κB signaling plays a key role in the development of NAFLD induced by the combined effects of low-dose LPS and HFD.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0006291X20316077?via%3Dihub
Zhou Z, Qi J, Lim CW, Kim JW, Kim B. Dual TBK1/IKKε inhibitor amlexanox mitigates palmitic acid-induced hepatotoxicity and lipoapoptosis in vitro. Toxicology. 2020 Nov;444:152579. doi: 10.1016/j.tox.2020.152579. Epub 2020 Sep 6. PMID: 32905826.
Dual TBK1/IKKε inhibitor amlexanox mitigates palmitic acid-induced hepatotoxicity and lipoapoptosis in vitro
This study aimed to investigate the effects of amlexanox, a dual inhibitor of TBK1 and IKKε, in an in vitro nonalcoholic steatohepatitis (NASH) model induced by palmitic acid (PA). The PA treatment significantly increased phosphorylation levels of TBK1 and IKKε in hepatocytes and Kupffer cells (KCs), indicating their potential involvement in PA-induced NASH progression. Amlexanox treatment reduced phosphorylation of TBK1 and IKKε, mitigated hepatotoxicity, and reversed PA-induced inflammation and lipotoxic cell death in hepatocytes. Additionally, amlexanox inhibited KC activation and induced M2 polarization, leading to decreased phosphorylation of NF-κB in both hepatocytes and KCs. These findings suggest that amlexanox attenuated PA-induced hepatotoxicity and lipoapoptosis by inhibiting the TBK1/IKKε-NF-κB and/or IRF3 pathways in hepatocytes and KCs.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0300483X20302183?via%3Dihub
Zhou Z, Qi J, Zhao J, Lim CW, Kim JW, Kim B. Dual TBK1/IKKɛ inhibitor amlexanox attenuates the severity of hepatotoxin-induced liver fibrosis and biliary fibrosis in mice. J Cell Mol Med. 2020 Jan;24(2):1383-1398. doi: 10.1111/jcmm.14817. Epub 2019 Dec 10. PMID: 31821710; PMCID: PMC6991653.
This study aimed to investigate the effects of amlexanox, a dual inhibitor of TBK1 and IKKε, in an in vitro nonalcoholic steatohepatitis (NASH) model induced by palmitic acid (PA). The PA treatment significantly increased phosphorylation levels of TBK1 and IKKε in hepatocytes and Kupffer cells (KCs), indicating their potential involvement in PA-induced NASH progression. Amlexanox treatment reduced phosphorylation of TBK1 and IKKε, mitigated hepatotoxicity, and reversed PA-induced inflammation and lipotoxic cell death in hepatocytes. Additionally, amlexanox inhibited KC activation and induced M2 polarization, leading to decreased phosphorylation of NF-κB in both hepatocytes and KCs. These findings suggest that amlexanox attenuated PA-induced hepatotoxicity and lipoapoptosis by inhibiting the TBK1/IKKε-NF-κB and/or IRF3 pathways in hepatocytes and KCs.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0300483X20302183?via%3Dihub
Imokawa S, Satou A, Taniguchi M, Toyoshima M, Nakazawa K, Hayakawa H, Chida K. [Amlexanox has an acute bronchodilator effect in patients with aspirin-induced asthma (AIA)]. Nihon KyobuShikkan Gakkai Zasshi. 1993 Aug;31(8):976-82. Japanese. PMID: 8230896.
Amlexanox has an acute bronchodilator effect in patients with aspirin-induced asthma (AIA)
In a recent study, researchers investigated the acute bronchodilator effect of orally administered amlexanox in adult asthmatics, with a specific focus on comparing patients with aspirin-induced asthma (AIA) and those without AIA. Fifteen patients participated, with 8 having AIA and 7 without. Spirometry measurements were taken at various time intervals after administering either amlexanox or a placebo in a randomized double-blind manner. The results showed that amlexanox significantly improved FEV1 in the AIA group, while FEV1 significantly decreased after placebo administration. In contrast, the non-AIA group did not experience significant changes in FEV1 after receiving either amlexanox or a placebo. These findings suggest that amlexanox has an acute bronchodilator effect specifically in AIA patients.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/8230896/.
Inagaki M, Michimata H, Minato K, Sunaga Y, Kobayashi S, Tani G, Nakazawa T. [Inhibitory effect of amlexanox on asthmatic attacks in an aspirin-sensitive asthmatic]. Nihon KyobuShikkan Gakkai Zasshi. 1992 Jun;30(6):1180-5. Japanese. PMID: 1507696.
Inhibitory effect of amlexanox on asthmatic attacks in an aspirin-sensitive asthmatic
Amlexanox is known for its anti-allergic properties, attributed to its ability to inhibit the release of LTC4, LTD4, and histamine, as well as its antagonistic action on leukotrienes. An 18-year-old female with a history of bronchial asthma since age fifteen, diagnosed with aspirin-sensitive asthma, experienced a severe asthmatic attack with syncope after taking an analgesic for a common cold at seventeen. Additionally, she displayed sensitivity to toothpaste. An inhalation challenge with incremental concentrations of sulpyrine triggered simultaneous increases in LTC4, LTD4, and histamine in her blood. After starting daily oral administration of 150 mg of amlexanox, she remained free from attacks for approximately 8 months. In a subsequent inhalation test, premedication with amlexanox raised the sulpyrine threshold and suppressed the release of LTC4, LTD4, and histamine. This case report suggests that amlexanox effectively controlled asthmatic attacks in this aspirin-sensitive asthmatic patient.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/1507696/
Kohno S, Shimizu T, Mizuta J, Ogino K, Yamamura H, Ohata K, Kawai M, Shindo T, Wada H, Hitomi S. [Inhibitory effect of amlexanox (AA-673) on the immunological and non-immunological release of histamine or leukotrienes]. Arerugi. 1989 Nov;38(11):1236-45. Japanese. PMID: 2483310.
Inhibitory effect of amlexanox (AA-673) on the immunological and non-immunological release of histamine or leukotrienes
Amlexanox’s impact on histamine and leukotriene (LT) release, whether immunological or non-immunological, from passively sensitized human lung fragments and atopic human leukocytes were examined and compared with AA-861, tranilast, azelastine, and disodium cromoglycate. 1) At concentrations of 10(-7) to 10(-4) M, amlexanox demonstrated a concentration-dependent inhibition of histamine, LTB4, LTC4, LTD4, and LTE4 release from passively sensitized human lung fragments. AA-861, a selective competitive inhibitor of 5-lipoxygenase activity, moderately affected histamine release and significantly suppressed LT release at 10(-7) and 10(-6) M concentrations. Other antiallergic drugs like tranilast and disodium cromoglycate also inhibited this chemical mediator release, although with slightly weaker potency than amlexanox. 2) While amlexanox slightly enhanced Ca ionophore A23187-induced LTB4 and LTC4 release from atopic human leukocytes up to 10(-6) M, it strongly reduced both LT releases at 10(-4) M. These findings suggest that amlexanox is a clinically effective drug for atopic diseases, particularly allergic asthma and rhinitis.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/2483310/.
Urisu A, Iimi K, Kondo Y, Horiba F, Masuda S, Tsuruta M, Yazaki T, Torii S. [Inhibitory action amlexanox on interleukin-3-induced enhancement of histamine releasability of human leukocytes]. Arerugi. 1990 Oct;39(10):1448-54. Japanese. PMID: 1701989.
Inhibitory action amlexanox on interleukin-3-induced enhancement of histamine releasability of human leukocytes
Amlexanox, an anti-allergic drug, exhibited concentration-dependent inhibition of hrIL-3-induced histamine release from human leukocytes stimulated by anti-IgE in vitro. Notably, amlexanox displayed significant inhibitory effects at concentrations of 10(-5) M and 10(-4) M in one out of nine and six out of nine allergic subjects, respectively, indicating variable responses among patients. Both post-treatment and simultaneous treatment with amlexanox demonstrated inhibitory actions on hrIL-3-induced enhancement, suggesting the reversibility of this process. Other substances like AA-861, OKY-046, superoxide dismutase, and prostaglandin E2 had no impact on hrIL-3-induced histamine release enhancement. The precise details of amlexanox’s novel anti-allergic mechanism targeting hrIL-3-induced histamine releasability remain unclear
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/1701989/.
Ozasa K, Temizoz B, Kusakabe T, Kobari S, Momota M, Coban C, Ito S, Kobiyama K, Kuroda E, Ishii KJ. Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor. Front Immunol. 2019 Sep 26;10:2212. doi: 10.3389/fimmu.2019.02212. PMID: 31616416; PMCID: PMC6775192.
Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor
This study explored the role of cyclic GMP-AMP (cGAMP), generated upon immune recognition of extracellular host-derived DNA, in allergic asthma. The researchers intranasally sensitized mice with cGAMP and house dust mite antigen (HDM), leading to increased HDM-specific allergic asthma characterized by elevated antibody levels and eosinophil infiltration in the lungs. cGAMP stimulated IL-33 production in lung fibroblast cells, and mice lacking components of the cGAMP-mediated innate immune activation failed to develop enhanced asthma. Amlexanox, a TBK1 inhibitor, reduced cGAMP-induced lung inflammation, highlighting the potential of targeting the STING/TBK1/IRF3/7 signaling pathway and the IL-33/ST2 pathway as a novel therapeutic approach for allergic asthma.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6775192/
Iwama T, Komatsu N, Shikada K, Tanaka S. Reversing effect of anti-asthmatic drugs on bronchoconstriction induced by antigen challenge and histamine in anesthetized guinea pigs. Jpn J Pharmacol. 1992 Jan;58(1):19-25. doi: 10.1254/jjp.58.19. PMID: 1640659.
Reversing effect of anti-asthmatic drugs on bronchoconstriction induced by antigen challenge and histamine in anesthetized guinea pigs
In anesthetized guinea pigs, we conducted an in vivo assessment of bronchodilation using a model of antigen-induced bronchoconstriction, comparing the results to histamine-induced responses. Leukotriene (LT) D4 antagonists, FPL55712 and LY171883, gradually reduced antigen-induced responses, while the lipoxygenase inhibitor phenidone did not. The bronchodilators theophylline and forskolin rapidly reduced antigen-induced responses, while several other drugs, including nifedipine, cromakalim, amlexanox, disodium cromoglycate (DSCG), OKY-046, and dapsone, had no effect. Theophylline, salbutamol (a beta-adrenoceptor agonist), and pyrilamine (a histamine H1-blocker) had only a minor effect on histamine-induced bronchoconstriction. These findings suggest that antigen-induced bronchoconstriction in guinea pigs under anesthesia provides a valuable in vivo model for assessing the bronchodilatory effects of anti-asthmatic drugs, particularly for antigens.
You can read the abstract of the article at https://www.jstage.jst.go.jp/article/jphs1951/58/1/58_1_19/_article.
Makino E, Ohashi T, Takahashi H, Kato H, Ito Y, Nagai H, Koda A, Azuma H. Inhibitory effect of HSR-6071, a new anti-allergic agent, on experimental asthma in rats and guinea-pigs. J Pharm Pharmacol. 1990 Apr;42(4):236-41. doi: 10.1111/j.2042-7158.1990.tb05399.x. PMID: 1974289.
Inhibitory effect of HSR-6071, a new anti-allergic agent, on experimental asthma in rats and guinea pigs
HSR-6071, a compound (6-(1-pyrrolidinyl)-N-(1H-tetrazol-5-yl)-2-pyrazinecarboxamide), effectively inhibited experimental asthma induced by IgE antibody in rats in a dose-dependent manner. Its inhibitory potency exceeded that of disodium cromoglycate and ketotifen, and was comparable to amlexanox. HSR-6071 also prevented bronchoconstriction mediated by IgE or IgG antibodies in guinea pigs, along with amlexanox and ketotifen, but not disodium cromoglycate. HSR-6071 suppressed antigen-induced histamine and SRS-A release from guinea pig lung tissues, with a stronger inhibition of SRS-A release than histamine. Moreover, HSR-6071 inhibited LTD4-induced bronchoconstriction, while having minimal impact on histamine- or acetylcholine-induced bronchoconstriction. Additionally, HSR-6071 inhibited cyclic AMP phosphodiesterase activity and induced relaxation of guinea pig isolated trachea. These multifaceted pharmacological actions contribute to the anti-allergic effect of HSR-6071.
You can read the abstract of the article at https://academic.oup.com/jpp/article-abstract/42/4/236/6164382?redirectedFrom=fulltext&login=false
Costa R, Wagner DE, Doryab A, De Santis MM, Schorpp K, Rothenaigner I, Lehmann M, Baarsma HA, Liu X, Schmid O, Campillos M, Yildirim AÖ, Hadian K, Königshoff M. A drug screen with approved compounds identifies amlexanox as a novel Wnt/β-catenin activator inducing lung epithelial organoid formation. Br J Pharmacol. 2021 Jun 5. doi: 10.1111/bph.15581. Epub ahead of print. PMID: 34089180.
A drug screen with approved compounds identifies amlexanox as a novel Wnt/β-catenin activator inducing lung epithelial organoid formation
In the quest to find a treatment for emphysema, a debilitating lung disease, researchers conducted a screening of 1216 human-approved compounds to identify substances that could activate Wnt/β-catenin signaling, a pathway known to improve lung function and structure. They discovered 16 compounds that effectively boosted Wnt/β-catenin activity without causing harm to cells. Two of these compounds were found to promote the formation of lung organoids, which are miniature lung tissue structures. Further evaluation using amlexanox in a mouse model of emphysema demonstrated enhanced lung function and structure, along with increased expression of a pro-regenerative marker and reduced expression of a disease marker. This suggests that amlexanox holds promise as a potential therapeutic agent for emphysema.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8965750/
Ozasa K, Temizoz B, Kusakabe T, et al. Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor. Front Immunol. 2019;10:2212. Published 2019 Sep 26. doi:10.3389/fimmu.2019.02212.
Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor
This study explored the role of extracellular host-derived DNA, a damage-associated molecular pattern (DAMP), in allergic type 2 immune responses, particularly in asthma. The researchers used cGAMP, a second messenger generated upon immune recognition of such DNA, as an adjuvant in a mouse model. They found that cGAMP promoted allergic asthma characterized by increased IgG1 and total IgE, along with eosinophil infiltration. This effect was associated with the production of IL-33 in lung fibroblast cells. Mice deficient in IL-33 or its receptor ST2 did not exhibit enhanced asthma symptoms when exposed to cGAMP. Additionally, mice lacking key components of the cGAMP-mediated innate immune pathway failed to show increased eosinophils. Importantly, treatment with amlexanox, a TBK1 inhibitor, reduced cGAMP-induced lung allergic inflammation. This suggests that cGAMP acts as a type 2 adjuvant in the lung, potentially offering a novel therapeutic target for allergic asthma through the STING/TBK1/IRF3/7 signaling pathway and the IL-33-ST2 intercellular signaling pathway.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6775192/
Khandwala A, Van Inwegen RG, Alfano MC. 5% amlexanox oral paste, a new treatment for recurrent minor aphthous ulcers: I. Clinical demonstration of acceleration of healing and resolution of pain. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 1997 Feb;83(2):222-30. doi: 10.1016/s1079-2104(97)90009-3. PMID: 9117754.
5% amlexanox oral paste, a new treatment for recurrent minor aphthous ulcers: I. Clinical demonstration of acceleration of healing and resolution of pain
In four double-blind clinical studies involving 1335 participants with 1 to 3 aphthous ulcers, 5% Amlexanox oral paste (Aphthasol) was compared to vehicle-controlled groups. Subjects applied the study paste directly to the ulcers four times a day. The primary measure of effectiveness was the percentage of subjects experiencing complete healing of ulcers and the resolution of ulcer pain. While the vehicle had limited beneficial effects, 5% Amlexanox oral paste consistently and significantly accelerated the resolution of pain and the healing of aphthous ulcers when compared to both the vehicle and no-treatment groups.
You can read the abstract of the article at https://www.oooojournal.net/article/S1079-2104(97)90009-3/pdf.
Bhat S, Sujatha D. A clinical evaluation of 5% amlexanox oral paste in the treatment of minor recurrent aphthous ulcers and comparison with the placebo paste: a randomized, vehicle-controlled, parallel, single-center clinical trial. Indian J Dent Res. 2013 Sep-Oct;24(5):593-8. doi: 10.4103/0970-9290.123382. PMID: 24355961.
A clinical evaluation of 5% amlexanox oral paste in the treatment of minor recurrent aphthous ulcers and comparison with the placebo paste: a randomized, vehicle-controlled, parallel, single-center clinical trial
The main objective of this study was to assess the efficacy and safety of 5% amlexanox oral paste in treating recurrent minor aphthous ulcers, with a secondary objective of comparing it to a placebo. A total of 100 patients with recurrent minor aphthous ulcers participated, with 50 using amlexanox oral paste and 50 using a placebo for six days. The amlexanox group exhibited significant reductions in ulcer size, pain, erythema, and exudation compared to the placebo group after six days. While the recurrence of ulcers was notably reduced for up to six months, the rate gradually increased thereafter. Overall, 5% amlexanox oral paste demonstrated clinical benefits in alleviating ulcer-related symptoms over six days, but its long-term effect on ulcer recurrence over one year remains inconclusive.
You can read the full article at https://www.ijdr.in/article.asp?issn=0970-9290;year=2013;volume=24;issue=5;spage=593;epage=598;aulast=Bhat.
Murray B, Biagioni PA, Lamey PJ. The efficacy of amlexanoxOraDisc on the prevention of recurrent minor aphthous ulceration. J Oral Pathol Med. 2006 Feb;35(2):117-22. doi: 10.1111/j.1600-0714.2006.00379.x. PMID: 16430743.
The efficacy of amlexanoxOraDisc on the prevention of recurrent minor aphthous ulceration
This study aimed to evaluate the effectiveness of OraDisc, containing 2 mg amlexanox, in preventing aphthous ulcers when applied at the prodromal stage. Fifty-two patients were randomly assigned to receive OraDisc or vehicle patches applied four times a day for 72 hours over the prodromal area. The results indicated that 50% of those in the OraDisc group developed an ulcer by day 4, compared to 69% in the vehicle group. Additionally, various factors such as erythema score, ulcer size, pain scores, and thermographic measures showed trends toward healing in the OraDisc group. In conclusion, OraDisc demonstrated effectiveness in preventing the development of ulcers compared to the vehicle patch.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0714.2006.00379.x.
Meng W, Dong Y, Liu J, Wang Z, Zhong X, Chen R, Zhou H, Lin M, Jiang L, Gao F, Xu T, Chen Q, Zeng X. A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: a randomized, placebo-controlled, blinded, multicenter clinical trial. Trials. 2009 May 6;10:30. doi: 10.1186/1745-6215-10-30. PMID: 19419555; PMCID: PMC2690593
A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: a randomized, placebo-controlled, blinded, multicenter clinical trial
In a randomized, blinded, placebo-controlled study involving 216 patients with minor recurrent aphthous ulcers (MiRAU), the effectiveness of amlexanox oral adhesive pellicles was explored and compared to amlexanox oral adhesive tablets. Both forms of amlexanox significantly reduced ulcer size and alleviated ulcer pain, with no significant difference in treatment effectiveness between the pellicles and tablets. These findings suggest that amlexanox oral adhesive pellicles are as effective and safe as tablets for treating MiRAU in Chinese patients, with the added advantage of greater user comfort. Therefore, pellicles may be a preferable option in clinical practice for individuals with recurrent aphthous stomatitis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2690593/
Darshan DD, Kumar CN, Kumar AD, Manikantan NS, Balakrishnan D, Uthkal MP. Clinical study to know the efficacy of Amlexanox 5% with other topical Antiseptic, Analgesic and Anesthetic agents in treating minor RAS. J Int Oral Health. 2014 Feb;6(1):5-11. Epub 2014 Feb 26. PMID: 24653596; PMCID: PMC3959130.
Clinical study to know the efficacy of Amlexanox 5% with other topical Antiseptic, Analgesic and Anesthetic agents in treating minor RAS
Amlexanox, a 5 percent topical oral paste, has been used for treating recurrent aphthous stomatitis (RAS) in most European countries but is not available in China. This study aimed to assess the effectiveness of amlexanox oral adhesive pellicles in treating minor recurrent aphthous ulcers (MiRAU) and compare the results with amlexanox oral adhesive tablets. In a randomized, blinded, placebo-controlled clinical study involving 216 patients, both forms of amlexanox significantly reduced ulcer size and alleviated ulcer pain. There was no significant difference in treatment effectiveness between the two forms, but pellicles were found to be more comfortable for use. Thus, amlexanox oral adhesive pellicles may be a preferable choice for RAS patients in clinical practice.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2690593/.
Greer RO Jr, Lindenmuth JE, Juarez T, Khandwala A. A double-blind study of topically applied 5% amlexanox in the treatment of aphthous ulcers. J Oral Maxillofac Surg. 1993 Mar;51(3):243-8; discussion 248-9. doi: 10.1016/s0278-2391(10)80164-8. PMID: 8445464.
A double-blind study of topically applied 5% amlexanox in the treatment of aphthous ulcers
In a double-blind trial involving 32 patients with recurrent oral aphthous ulcers, the efficacy of amlexanox (C16H14N2O4) was evaluated. Over a 3-day treatment period, patients received either a placebo topical paste or 5% amlexanox paste, applied twice a day for 3 days and once on the fourth day. Efficacy was assessed based on pain, erythema, ulcer size, and an investigator’s improvement scale. Patients receiving amlexanox showed superior outcomes in all effectiveness criteria, with statistically significant group differences for all parameters except pain reduction (P < .05). No side effects were reported, leading to the conclusion that amlexanox effectively reduces aphthous ulcer erythema, pain, and lesional size.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/8445464/
Bell J. Amlexanox for the treatment of recurrent aphthous ulcers. Clin Drug Investig. 2005;25(9):555-66. doi: 10.2165/00044011-200525090-00001. PMID: 17532700.
Amlexanox for the treatment of recurrent aphthous ulcers
Recurrent aphthous ulcer (RAU) is a common oral mucosal condition, and amlexanox, a novel anti-inflammatory and anti-allergic agent, has been assessed for RAU treatment in clinical trials. When applied topically as a 5% paste, amlexanox demonstrated significant and consistent acceleration of ulcer healing and pain resolution across multiple studies. It achieved steady-state concentrations within a week, and adverse effects were generally mild, with low incidence rates, making 5% amlexanox topical paste a well-tolerated and effective option for RAU treatment.
You can read the abstract of the article at https://link.springer.com/article/10.2165/00044011-200525090-00001
Binnie WH, Curro FA, Khandwala A, Van Inwegan RG. Amlexanox oral paste: a novel treatment that accelerates the healing of aphthous ulcers. Compend Contin Educ Dent. 1997 Nov;18(11):1116-8, 1120-2, 1124 passim. PMID: 9533345.
Amlexanox oral paste: a novel treatment that accelerates the healing of aphthous ulcers
Five percent Amlexanox oral paste, a novel treatment for aphthous ulcers, was evaluated in three controlled clinical studies involving 1,124 immunocompetent patients with mild to moderate aphthous ulcers. These studies demonstrated that 5% Amlexanox oral paste (Aphthasol) significantly expedited the healing of these ulcers, reducing the median time to ulcer healing and pain resolution when compared to both a vehicle and no treatment. After three days, the paste led to complete ulcer healing for 21% of patients (compared to 8% with no treatment) and complete pain resolution for 44% of patients (compared to 20% with no treatment).
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/9533345/#:~:text=Treatment%20with%20Aphthasol%20reduced%20the,was%20compared%20to%20no%20treatment
Other Peptides
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








