GENEMEDICS APP
GENEMEDICS NUTRITION
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Tirzepatide
- Key Takeaways of Tirzepatide Guide 2023
- What is Tirzepatide?
- How Tirzepatide Works
- Chemical Structure of Tirzepatide
- Research/Clinical Trials on Tirzepatide
- Tirzepatide and Metabolic Health
- Tirzepatide's Impact on Body Weight
- Mechanisms and Implications
- Potential Candidates for Treatment
- Tirzepatide Brand Name
- Tirzepatide Side Effects
- Tirzepatide Dosage
- Semaglutide vs Tirzepatide
- What is Compounded Tirzepatide?
- Tirzepatide Cost
- Tirzepatide Before and After Results
- FAQ
- Blog
- Reference
Book a Free Consultation
Table of Contents
- Potential Health Benefits of Tirzepatide
- Key Takeaways of Tirzepatide Guide 2023
- What is Tirzepatide?
- How Tirzepatide Works
- Chemical Structure of Tirzepatide
- Research/Clinical Trials on Tirzepatide
- Tirzepatide and Metabolic Health
- Tirzepatide's Impact on Body Weight
- Mechanisms and Implications
- Potential Candidates for Treatment
- Tirzepatide Brand Name
- Tirzepatide Side Effects
- Tirzepatide Dosage
- Semaglutide vs Tirzepatide
- What is Compounded Tirzepatide?
- Tirzepatide Cost
- Tirzepatide Before and After Results
- FAQ
- Blog
- Reference
Potential Health Benefits of Tirzepatide
- Lowers blood sugar levels [1-26]
- Promotes weight loss [1-4, 7-8, 13-15, 18, 27-31]
- Lowers the risk of heart disease [32-36]
- Improves liver health [37-41]
Key Takeaways of Tirzepatide Guide 2023
- Tirzepatide is a once-weekly injectable drug that is used to treat type 2 diabetes and obesity. It works by mimicking the effects of two natural hormones, GLP-1 and GIP. These hormones help to regulate blood sugar levels and promote weight loss.
- In clinical trials, tirzepatide was shown to be effective in lowering blood sugar levels and promoting weight loss in people with type 2 diabetes and obesity. In one trial, tirzepatide was shown to reduce A1C levels by up to 2.4% and to cause weight loss of up to 20%.
- Tirzepatide is generally well-tolerated, with the most common side effects being nausea, vomiting, diarrhea, and constipation. These side effects are usually mild and go away on their own.
- Tirzepatide is currently approved by the Food and Drug Administration (FDA) for the treatment of type 2 diabetes.
What is Tirzepatide?
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (RA) that is manufactured by Eli Lilly and Company. This medication can help treat medical conditions such as type 2 diabetes (T2D), obesity, and non-alcoholic fatty liver disease. In 2021, tirzepatide has completed phase 3 trials.
Tirzepatide injection is administered into the subcutaneous tissue, which is the fatty area just beneath the skin. It comes in a pre-filled pen or syringe, making it relatively easy for individuals to self-administer once they’ve been trained on how to properly do so. The exact dosing regimen and instructions for administration will be provided by a healthcare provider based on the patient’s individual needs and the specific formulation of tirzepatide.
The American Diabetes Association (ADA) has approved tirzepatide as a treatment for adults with type 2 diabetes and obesity. In May 2022, tirzepatide also gained FDA approval for the treatment of adults with type 2 diabetes and obesity.
Researchers are also exploring tirzepatide’s potential as a treatment for conditions like heart failure with preserved ejection fraction (HFpEF), obstructive sleep apnea (OSA), and non-alcoholic steatohepatitis (NASH). They’re also planning studies to investigate how tirzepatide might help with chronic kidney disease and with understanding how it impacts health in people with obesity.
How Tirzepatide Works?
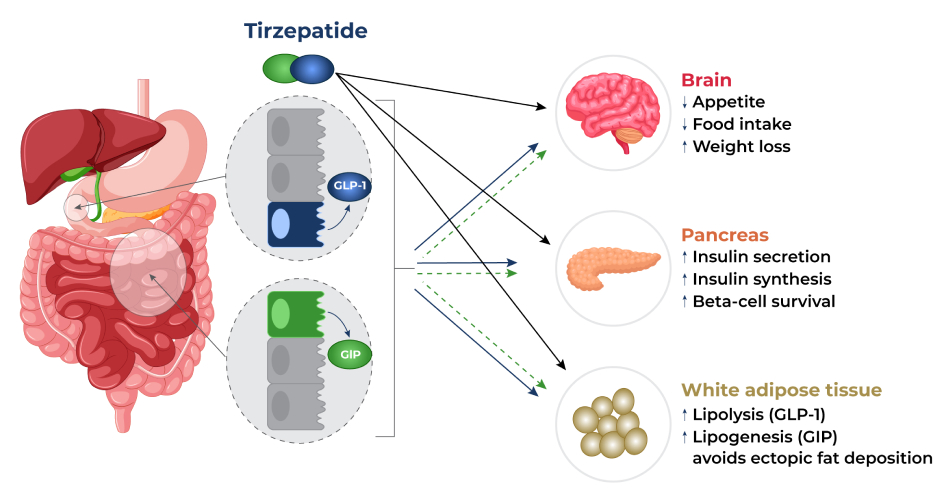
Eli Lilly and Company’s tirzepatide works by activating both the GLP-1 and GIP receptors in your body. This prompts the body to release insulin from your pancreas that blocks glucagon, a hormone that increases blood sugar levels. As a result, your blood sugar levels can be controlled effectively, especially after a meal. In addition, tirzepatide affects certain chemicals in the brain which in turn decreases appetite, increases energy expenditure, and prevents ectopic fat deposition (abnormal fat accumulation in body parts that normally contains small amounts of fat). These effects result in significant weight reductions.
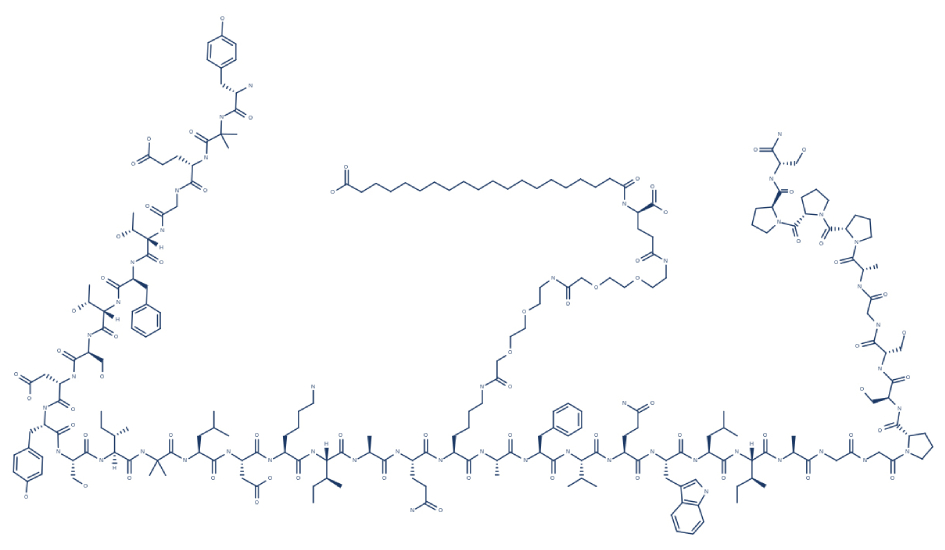
5. Chemical Structure of Tirzepatide
6. Research/Clinical Trials on Tirzepatide
A. Lowers Blood Sugar Levels
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1 and GIP are hormones involved in the control of blood sugar levels. By acting on these receptors, it produces synergistic effects like increased insulin production and release. This makes tirzepatide more effective than strict GLP-1 agonists that are already approved for the treatment of type 2 diabetes.
Tirzepatide works by stimulating the release of insulin in a glucose-dependent manner, meaning that it is more effective at lowering blood sugar levels when blood sugar levels are high. In other words, tirzepatide is more effective at helping people with type 2 diabetes control their blood sugar levels when they have eaten a meal or snack that has caused their blood sugar levels to rise. This is because tirzepatide is designed to mimic the action of two gut hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are released in response to food intake and help to regulate blood sugar levels.
By increasing insulin secretion and blocking glucagon, tirzepatide can help lower blood sugar levels. Research indicates that this effect is beneficial in patients with type 2 diabetes (T2D):
- In T2D patients, tirzepatide administration resulted in reduced body weight and improved glycemic (blood sugar) control. [1]
- A study showed that tirzepatide produced potent glucose and weight-lowering effects in obese diabetic patients. [2]
- In diabetic patients, tirzepatide was shown to be the most effective treatment for T2D because of its benefits on glucose and weight control. [3]
- In T2D patients, tirzepatide induced greater reductions in overall blood sugar levels and body weight and was associated with a lower risk of hypoglycemia (abnormally low blood sugar levels). [4]
- In T2D patients, tirzepatide was able to safely and effectively improve blood sugar levels and body weight. [5]
- In T2D patients, once weekly subcutaneous injection of tirzepatide resulted in a significant reduction in overall blood sugar levels with a lower risk of adverse events compared to placebo treatment. [6]
- In T2D patients, tirzepatide improved markers of insulin sensitivity and beta-cell function. [7]
- In adult T2D patients, tirzepatide showed significant improvements in glycemic control and body weight without an increased risk of hypoglycemia. [8]
- In patients with T2D and elevated cardiovascular risk, tirzepatide offered a greater glucose-reduction effect compared to glargine (a type of insulin). [9]
- In patients with type 2 diabetes and inadequate control of blood sugar levels, the administration of tirzepatide in addition to other diabetes medicines, such as insulin glargine, resulted in improved blood sugar control after 40 weeks. [10]
- In T2D patients, tirzepatide was superior to semaglutide when it comes to the mean change in blood sugar levels. [11]
- A study showed that tirzepatide is a good candidate for the development of pharmacotherapies in treating obesity, diabetes, and neurodegenerative disorders. [12]
- In T2D patients, tirzepatide showed significantly better efficacy with regard to glucose control and weight loss compared to dulaglutide, an anti-diabetic medication. [13]
- In T2D patients, tirzepatide treatment resulted in significant improvement in the control of blood sugar levels and body weight. [14]
- In adults with T2D and inadequate control of blood sugar levels despite treatment with insulin glargine, additional treatment with tirzepatide resulted in significant improvements in the control of blood sugar levels. [15]
- In T2D patients, treatment with tirzepatide greatly improved insulin sensitivity (the body’s response to the effects of insulin). [16]
- In Japanese patients with T2D, tirzepatide administration was well-tolerated and resulted in decreased blood sugar and body weight as well as lower adverse events. [17]
- A 72-week trial in obese participants found that 5 mg, 10 mg, or 15 mg of tirzepatide once weekly resulted in substantial and sustained body weight reductions. [18]
- In five clinical trials involving individuals with type 2 diabetes (SURPASS 1-5), tirzepatide administered at 5 mg, 10 mg, or 15 mg per week demonstrated unprecedented reductions in HbA1c (1.24 to 2.58%) and body weight (5.4-11.7 kg). A notable percentage of patients (23.0 to 62.4%) achieved an HbA1c below 5.7%, indicating normoglycemia (normal blood sugar levels), and a substantial portion (20.7 to 68.4%) experienced more than 10% baseline body weight loss. Notably, tirzepatide exhibited superior efficacy in decreasing HbA1c and body weight compared to selective GLP-1 RA semaglutide (1.0 mg per week) and titrated basal insulin. It was also observed that patients receiving insulin without tirzepatide tended to gain weight during the study. [19]
- Studies suggest that tirzepatide administration in patients with type 2 diabetes can improve insulin sensitivity and insulin secretory responses to a greater extent compared with other antidiabetic medications. [20-26]
B. Helps Lose Weight

Mounjaro tirzepatide is approved as a weight loss medication for overweight adults with at least one associated health problem. The FDA approved tirzepatide on June 7, 2022, for this indication.
In clinical trials, tirzepatide was shown to be effective in helping overweight adults with at least one associated health problem lose weight. In one trial, tirzepatide was shown to cause weight loss of up to 22% in people with a BMI of 30 or higher and at least one associated health problem.
Like other weight loss drugs, tirzepatide promotes weight loss by affecting certain brain chemicals involved in food intake, energy expenditure, and fat deposition. It does this by acting on the glucagon-like peptide-1 (GLP-1) receptor, which reduces fat cells and enhances fat breakdown. Evidence suggests that these mechanisms are the key to weight loss:
- The SURMOUNT-2 trial for tirzepatide (phase 3 trial) found that participants taking tirzepatide 5 mg, 10 mg, or 15 mg once weekly for 72 weeks achieved average weight reductions of 16.4%, 20.9%, and 22.5% for the 5 mg, 10 mg, and 15 mg tirzepatide groups compared to 2.4% for the placebo group. The rates of treatment discontinuation because of adverse events stood at 3.8% (10 mg), 7.4% (15 mg), and 3.8% (placebo). [27]
- In patients with T2D, tirzepatide treatment induced clinically significant weight loss. [1-4, 7-8, 13-15,18]
- In T2D patients, tirzepatide administration resulted in greater body weight reductions compared to semaglutide, an anti-diabetic medication. Gastrointestinal symptoms (nausea, vomiting, and diarrhea) were the most frequently reported adverse events, and they were mostly of mild to moderate intensity in both the tirzepatide and semaglutide groups. [28]
- Weekly administration of tirzepatide and semaglutide resulted in significant weight reduction in patients with T2D. [29]
- In T2D patients, tirzepatide treatment regimen resulted in a strong weight loss (greater than 5%). [30]
- In obese/overweight adults, tirzepatide treatment resulted in successful weight loss. [31]
C. Lowers the Risk of Heart Disease

The beneficial effects of tirzepatide on body composition and blood sugar levels can all contribute to a healthy heart. Specifically, tirzepatide increases adiponectin, a protein hormone that regulates the breakdown of fatty acids. Increased adiponectin levels are associated with a lower risk of cardiovascular disease. [32] In addition, aside from blood sugar, tirzepatide can also protect against heart problems by lowering the levels of triglycerides (blood fat). [33]
Studies suggest that tirzepatide can lower the risk of cardiovascular disease and improve cardiovascular outcomes:
- In participants with T2D, tirzepatide treatment regimen (5 mg, 10 mg, and 15 mg) was safe and did not increase the risk of major cardiovascular events. [34]
- In patients with T2D, tirzepatide successfully decreased biomarkers associated with cardiovascular risks such as YKL-40 (also known as chitinase-3 like-protein-1), intercellular adhesion molecule 1 (ICAM-1), leptin, and growth differentiation factor 15. [35]
- Tirzepatide can lower the risk of cardiovascular disease by acting on the GLP-1 receptor, which in turn reduces cardiovascular risk factors such as hypertension, high lipid levels, obesity, increased inflammation, and endothelial cell dysfunction (constriction of the blood vessels of the heart). [36]
D. Improves Liver Health

GIP and GLP-1 are natural incretin hormones that play a role in liver function. They can help to protect the liver from damage, promote the regeneration of liver cells, and improve liver function. Here are some specific ways that GIP and GLP-1 contribute to improving liver health:
- Protect the liver from damage: GIP and GLP-1 can help to protect the liver from damage caused by toxins, such as alcohol and fat. They do this by increasing the production of antioxidants and by reducing the production of free radicals. Free radicals are unstable molecules that can damage cells, including liver cells.
- Promote the regeneration of liver cells: GIP and GLP-1 can help to promote the regeneration of liver cells after injury. They do this by stimulating the production of growth factors, which are proteins that help cells to grow and repair themselves.
- Improve liver function: GIP and GLP-1 can improve liver function by increasing the production of bile and by reducing the production of liver enzymes. Bile is a fluid that helps to digest fats and remove toxins from the body. Liver enzymes are proteins that help the liver metabolize drugs and toxins.
Tirzepatide also has the ability to improve liver health by reducing liver fat. A number of studies backs this beneficial effect:
- In T2D patients, high doses of tirzepatide significantly decreased nonalcoholic steatohepatitis-related biomarkers while increasing adiponectin, a protein hormone involved in fatty acid breakdown. [37]
- In adult patients with T2D, tirzepatide treatment resulted in reduced liver fat. [38]
- In patients with liver abnormalities, tirzepatide significantly improved several liver biomarkers such as liver fat. [39]
- In patients with metabolic-associated fatty liver disease (MAFLD), treatment with tirzepatide resulted in a significant reduction in nonalcoholic steatohepatitis-related biomarkers such as liver enzymes. [40]
- In patients with non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH), tirzepatide treatment resulted in a better reduction in liver fat content compared to dulaglutide, an anti-diabetic medication. [41]
E. Improves Kidney Function
Tirzepatide has shown a potential to improve kidney function through its multifaceted mechanism of action. By promoting glucose control, reducing insulin resistance, and mitigating inflammation, tirzepatide can help alleviate the metabolic stress on the kidneys. Additionally, its GLP-1 receptor agonist component has been associated with vasodilation and decreased glomerular hyperfiltration, which could contribute to preserving kidney function.
Studies support the beneficial effects of tirzepatide on overall kidney function:
- In the SURPASS-4 trial, the dual GIP and GLP-1 receptor agonist tirzepatide exhibited superior effects over titrated daily insulin glargine in people with type 2 diabetes and high cardiovascular risk. This trial showcased that tirzepatide not only reduced HbA1c concentrations, body weight, and blood pressure more effectively, but it also demonstrated a notable impact on kidney parameters.A post-hoc analysis of the trial revealed that tirzepatide led to a slower rate of estimated glomerular filtration rate (eGFR) decline and significantly reduced urine albumin-creatinine ratio (UACR) when compared to insulin glargine. This effect was also observed in participants who did not fulfill the CKD criteria outlined by either eGFR or albuminuria according to KDIGO guidelines. These findings highlight the potential of tirzepatide to positively influence kidney function in individuals with type 2 diabetes and heightened cardiovascular risk. [42]
- A study reported that tirzepatide could pave the way for a new type of diabetes and weight management medication. [43] Beyond its action on certain receptors, there’s ongoing research on even more powerful versions that could help control weight and boost energy usage. These potential effects on kidney health could help prevent and treat CKD.
7. Tirzepatide and Metabolic Health
Tirzepatide is a groundbreaking medication that has the potential to significantly improve metabolic health for people dealing with conditions like diabetes and weight issues. This new drug is like a superhero for your body’s metabolic health, working to make things better and more balanced.
Think of metabolic health as your body’s way of managing the energy it gets from food. When metabolic health is good, your body can use the energy efficiently, and everything runs smoothly. But when metabolic health is off-track, it can lead to problems like high blood sugar levels and excess weight.
Tirzepatide steps in to save the day by helping your body use energy better and keep things in check. It’s like giving your metabolism a boost, helping it work more effectively. This can lead to improvements in blood sugar level control and weight management, which are crucial for overall metabolic health. So, with tirzepatide on your side, you’re giving your metabolic health a helping hand and taking a big step toward feeling better and more balanced.
8. Tirzepatide’s Impact on Body Weight
Tirzepatide, a novel medical intervention, has been gaining attention for its significant impact on body weight. This innovative treatment has shown promising results in addressing weight management, making it a potential game-changer in the field of obesity and metabolic health.
Clinical studies have demonstrated that tirzepatide not only effectively manages blood sugar levels but also leads to substantial weight loss in individuals who are overweight or obese. Unlike many traditional treatments, tirzepatide targets multiple metabolic pathways, harnessing its potential to influence body weight through various mechanisms.
Tirzepatide mechanism of action is thought to involve the activation of specific receptors in the brain that regulate appetite and metabolism. By modulating these receptors, tirzepatide can help individuals consume fewer calories and experience increased feelings of fullness, contributing to the reduction in body weight.
Furthermore, tirzepatide’s impact on body weight appears to be dose-dependent, with higher doses often resulting in more pronounced weight loss. This dose-response relationship underscores the potential of tirzepatide to be tailored to individual needs, optimizing both glycemic control and weight management.
It’s important to note that tirzepatide is not a standalone solution. A holistic approach that includes a balanced diet, regular physical activity, and lifestyle modifications remains crucial for achieving and sustaining healthy weight loss. However, tirzepatide’s unique ability to address both blood sugar levels and body weight offers a promising avenue for individuals struggling with obesity-related complications, such as type 2 diabetes.
In conclusion, tirzepatide presents a compelling case as a breakthrough treatment for addressing body weight concerns in conjunction with metabolic health. As research continues and more data accumulates, this innovative therapy could redefine how we approach weight management and its associated health benefits.
9. Tirzepatide’s Role in Facilitating Weight Loss: Mechanisms and Implications
The efficacy of tirzepatide weight loss is evident in its ability to significantly reduce body weight in individuals with type 2 diabetes, offering a promising approach to both glycemic control and weight management. Eli Lilly weight loss drug tirzepatide mechanisms and implications are explored through the following:
Mechanisms:
- Dual Agonism: Tirzepatide targets both the glucagon-like peptide-1 (GLP-1) and glucagon receptors, triggering a dual agonist effect. This dual action stimulates various physiological responses that contribute to weight loss.
- Enhanced Satiety: Activation of GLP-1 receptors leads to increased feelings of satiety and reduced appetite. This effect translates into decreased caloric intake, aiding in weight reduction.
- Appetite Regulation: Tirzepatide’s influence on the central nervous system helps regulate appetite by affecting brain regions associated with hunger and reward pathways, further promoting controlled eating habits.
- Energy Expenditure: Engagement with the glucagon receptor is linked to heightened energy expenditure and increased fat oxidation. These metabolic changes contribute to a negative energy balance, crucial for sustained weight loss.
Implications:
- Obesity Management: The unexpected weight loss effects of tirzepatide offer a potential new approach to combating obesity. Its dual action not only addresses glycemic control in diabetes but also serves as a valuable tool in the broader context of obesity management.
- Multi-Factorial Benefits: Tirzepatide’s mechanisms encompass a range of physiological responses, making it a versatile intervention that tackles multiple aspects of weight gain. This multi-faceted approach enhances its potential effectiveness.
- Holistic Health: The potential implications extend beyond weight loss alone. As obesity is associated with various health conditions, tirzepatide’s role in weight management could have positive ripple effects on overall health and quality of life.
10. Potential Candidates for Tirzepatide Peptide Treatment
Eli Lilly and Company’s tirzepatide is used to help control type 2 diabetes in adults. It’s meant to be used alongside healthy eating and exercise. The main goal is to improve how well your blood sugar levels are managed. This treatment is for people who haven’t been able to reach their blood sugar goals with other diabetes medications and need extra help to get their levels where they should be.
Contraindications for tirzepatide include hypersensitivity to the drug, personal or family history of medullary thyroid carcinoma, multiple endocrine neoplasia syndrome type 2, and severe gastrointestinal disease.
11. Tirzepatide Brand Name
Mounjaro is the brand name for tirzepatide, which is a prescription medication used to treat type 2 diabetes and obesity. It is a once-weekly injectable medication that works by mimicking the effects of two natural hormones, GLP-1 and GIP. These hormones help to regulate blood sugar levels and decrease food intake leading to weight loss.
Mounjaro tirzepatide is not right for everyone. It should not be used by people with a history of pancreatitis, medullary thyroid cancer, or diabetic retinopathy (a diabetes-related eye disease that damages the blood vessels in the retina).
If you are considering using Mounjaro tirzepatide, it is important to talk to your doctor about the risks and benefits of this medication. They can help you to determine if Mounjaro tirzepatide is right for you and can provide you with guidance on how to use it safely.
12. Tirzepatide Side Effects
Tirzepatide side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on tirzepatide. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of tirzepatide. Despite this, it was listed as a side effect associated with tirzepatide even though these associated side effects are very uncommon.
Side effects associated with tirzepatide may include the following:
- Constipation
- Decreased appetite
- Diarrhea
- Nausea
- Upper abdominal discomfort and abdominal pain
- Vomiting
13. Tirzepatide Dosage
Tirzepatide is a once-weekly injectable drug that is used to treat adults with type 2 diabetes and obesity. The initial dose of tirzepatide is 0.25 mg once weekly. The dose is then gradually increased over a 12-week period to reach a maximum dose of 1.0 mg once weekly.
The dose escalation period is important for tirzepatide because it allows the doctor to determine the optimal dosage for each individual patient. The dosage of tirzepatide can be adjusted up or down depending on the patient’s individual response to the drug.
The dose escalation period is also important because it allows the doctor to monitor for any side effects of tirzepatide. If the patient experiences any serious side effects, the doctor can stop the drug or reduce the dosage.
It is important to note that these are just the recommended tirzepatide doses. The doctor may adjust the dosage up or down depending on the individual patient’s response to the drug.
14. Semaglutide vs Tirzepatide
As a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (RA), tirzepatide produces synergistic effects like increased insulin production and release, which gives it an edge over strict GLP-1 receptor agonist such as semaglutide (Ozempic).
An overwhelming body of clinical evidence suggests that tirzepatide is superior to the anti-diabetic medication semaglutide with regards to improving blood sugar levels and reducing body weight:
- In T2D patients, tirzepatide was superior to semaglutide when it comes to the mean change in blood sugar levels. [11]
- In T2D patients, tirzepatide treatment for 28 weeks resulted in significant improvements in total insulin secretion rate and insulin sensitivity compared with semaglutide. [16]
- In T2D patients, tirzepatide administration resulted in greater weight reduction compared with semaglutide. [20]
- Weekly administration of tirzepatide in diabetic patients resulted in significant weight reduction than semaglutide. [34]
- The SURPASS-2 study involving patients with type 2 diabetes found that tirzepatide is superior to semaglutide in improving HbA1c levels, body weight, fasting blood sugar, and self-monitored blood sugar levels. [44]
- Available data from 2 short-term randomized trials suggest that tirzepatide is more effective than semaglutide in reducing HbA1c levels and body weight in patients with type 2 diabetes and obesity. [45]
- In participants with T2D who were taking metformin, once weekly subcutaneous injection of tirzepatide significantly reduced HbA1c levels and body weight compared with semaglutide. [46]
- In patients with T2D, the administration of tirzepatide 5/10/15 mg for 40 weeks produced greater reductions in HbA1c and weight compared with semaglutide 2 mg. [47]
- In patients with T2D, tirzepatide was superior to semaglutide with regard to improvement in HbA1c levels and resulted in greater weight reductions with similar overall side effects. [48]
- In patients with T2D who have abnormal blood sugar levels, tirzepatide 5 mg, 10 mg, and 15 mg demonstrated superiority versus semaglutide 1 mg and titrated insulin degludec (iDeg) in improving HbA1c levels at 40 weeks and 52 weeks. [49]
- In patients with T2D who were on metformin, higher doses of tirzepatide (10 mg and 15 mg) resulted in greater improvements in health-related quality of life and weight-related quality of life outcomes compared with semaglutide. [50]
- In people with T2D, treatment with tirzepatide 15 mg substantially improved blood sugar levels and insulin sensitivity at week 28 compared with 1 mg semaglutide. [51]
- In people with T2D, treatment with tirzepatide 5 mg and 15 mg significantly improved markers of islet cell function and insulin sensitivity compared with 1 mg semaglutide. [52]
- The administration of tirzepatide in diabetic patients produced greater weight loss and improvements in blood sugar levels compared with semaglutide. [53]
- In patients with T2D, tirzepatide significantly reduced HbA1c, a marker of blood sugar levels, compared with semaglutide. [54]
The comparison between Tirzepatide vs Ozempic highlights two distinct approaches to managing type 2 diabetes and weight, each with its own unique mechanisms and potential benefits.
15. What is Compounded Tirzepatide?
Compounded tirzepatide is a medication that is made by a compounding pharmacy. Compounding pharmacies are licensed to create custom medications that are not available as commercially manufactured drugs. A tirzepatide compounding pharmacy can create custom formulations of tirzepatide that are tailored to the individual needs of each patient.
Compounded tirzepatide is made by combining tirzepatide with other medications or ingredients. This can be done to make the medication more effective, to improve its taste, or to make it more affordable.
Here are some of the potential risks of using compounded tirzepatide:
- The medication may not be as effective as commercially manufactured tirzepatide.
- The medication may not be safe.
- The medication may not be consistent from batch to batch.
- The medication may not be covered by insurance.
If you are considering using compounded tirzepatide, it is important to talk to your doctor. Your doctor can help you decide if compounded tirzepatide is right for you. It is also important to talk to your doctor about the risks of using compounded medications.
16. Tirzepatide Cost
The cost of tirzepatide can vary depending on several factors. Factors such as the specific healthcare provider, insurance coverage, dosage, and duration of treatment play a role in determining the overall cost.
It’s important to consult with your healthcare provider and your insurance company to get a better understanding of how these factors will impact tirzepatide price for your individual situation. Keep in mind that your healthcare team can provide guidance on potential financial assistance programs or options that may be available to help manage the cost of the medication.
17. Tirzepatide Before and After Results
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
FAQ
Can you take tirzepatide if you are not diabetic?
No, Eli Lilly and Company’s tirzepatide is currently only approved by the FDA for the treatment of adults with type 2 diabetes and obesity. It is not recommended or prescribed for individuals who do not have diabetes. If you do not have diabetes and are interested in weight loss or other health concerns, talk to your doctor about suitable treatment options or lifestyle changes that may be appropriate for you. Always follow your doctor’s advice and avoid using medications that are not specifically prescribed for your condition.
Is tirzepatide harmful to the kidneys?
Tirzepatide, a drug manufactured by Eli Lilly and Company, is generally considered safe for the kidneys. In clinical trials, there were no significant harmful effects on kidney function reported. However, as with any medication, individual responses can vary, and some people may be more sensitive to certain drugs.
If you have pre-existing kidney problems or concerns about how tirzepatide may affect your kidneys, it’s essential to discuss this with your doctor. They can assess your kidney function and determine if tirzepatide is a suitable treatment option for you.
Do you have to take tirzepatide forever?
The duration of taking tirzepatide depends on your individual health condition and the recommendation of your healthcare provider. For many people with type 2 diabetes, tirzepatide may be used as a long-term treatment option to help manage their condition effectively.
Tirzepatide is not a cure for diabetes, but it can be beneficial in controlling blood sugar levels and promoting weight loss. Some individuals may need to take tirzepatide indefinitely, while others may use it for a specific period based on their response to the medication and their overall health.
Is tirzepatide safe for heart patients?
Tirzepatide has shown positive effects on cardiovascular health in clinical trials and is generally considered safe for heart patients when used as prescribed by their healthcare provider. It has been associated with a reduced risk of heart-related issues, such as heart attacks and strokes, in people with type type 2 diabetes
However, as with any medication, individual responses can vary, and there may be specific factors to consider for each person. If you have a history of heart conditions or other cardiovascular issues, it’s essential to discuss this with your doctor before starting tirzepatide.
Can I take tirzepatide for weight loss?
Yes, tirzepatide can be used for weight loss in addition to its approved use for type 2 diabetes. In clinical trials, this FDA approved medication has shown promising results in helping people lose weight, even in those without diabetes.
However, before considering tirzepatide for weight loss, it’s essential to talk to your healthcare provider. They can assess your individual health status, discuss your weight loss goals, and determine if tirzepatide is a suitable option for you.
Tirzepatide is typically prescribed for people with a body mass index (BMI) above a certain level or those who have other health concerns related to obesity. Your doctor will consider your medical history, potential risks, and benefits to ensure that tirzepatide is a safe and effective choice for your weight loss journey.
Can you take metformin and tirzepatide together?
Yes, in many cases, metformin and tirzepatide can be taken together. Metformin is a common medication used to treat treat type 2 diabetes, and tirzepatide is also used for the management of type 2 diabetes. These two medications have different mechanisms of action and can complement each other to help control control blood sugar levels
Does tirzepatide cause hypoglycemia?
Tirzepatide itself does not usually cause hypoglycemia (low blood sugar) when used alone. However, when tirzepatide is taken together with certain other diabetes medications, it may increase the risk of hypoglycemia.
Does tirzepatide affect the liver?
Tirzepatide has not been associated with significant harmful effects on the liver in clinical trials. In studies conducted so far, no significant liver-related issues have been reported in individuals using tirzepatide.
Does tirzepatide decrease appetite?
Yes, tirzepatide has been shown to decrease appetite in some people. It belongs to a class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which can help regulate appetite and reduce feelings of hunger.
Does tirzepatide lower cholesterol?
Yes, tirzepatide has been shown to lower cholesterol levelsin some individuals. In clinical trials, tirzepatide has demonstrated a positive impact on lipid profiles, including reductions in total cholesterol and low-density lipoprotein cholesterol (LDL-C), often referred to as “bad” cholesterol.
By improving cholesterol levels, tirzepatide may help reduce the risk of cardiovascular events, such as heart attacks and strokes. However, it’s important to remember that individual responses to medications can vary, and not everyone may experience the same degree of cholesterol reduction with tirzepatide.
How fast does tirzepatide start working?
The onset of action for tirzepatide can vary from person to person, but it generally starts to work relatively quickly after starting the treatment. In clinical trials, some participants experienced improvements in their blood sugar levels within the first few weeks of taking tirzepatide.
Does tirzepatide slow gastric emptying?
Yes, tirzepatide has been shown to slow gastric emptying in clinical studies. Gastric emptying refers to how quickly food leaves the stomach and enters the small intestine for further digestion and absorption.
Tirzepatide, a glucagon-like peptide-1 receptor agonist GLP-1 RA affects the gastrointestinal system by slowing down the emptying of the stomach. This can help you feel fuller for longer after eating, leading to reduced feelings of hunger and potentially aiding in weight loss.
How quickly does tirzepatide work for weight loss?
Like other weight loss drugs, the speed at which tirzepatide works for weight loss can vary from person to person. Some individuals may start to notice weight loss within a few weeks of starting the medication, while others may take longer to see significant results.
Tirzepatide helps with weight loss by regulating appetite, slowing gastric emptying, and promoting feelings of fullness after meals. However, it’s important to remember that weight loss is a gradual process and can be influenced by various factors, including diet, exercise, metabolism, and individual response to the medication.
Does tirzepatide make you sleepy?
Tirzepatide itself is not known to cause drowsiness or sleepiness as a direct side effect. However, some people may experience fatigue or tiredness when they first start taking the medication. This is because the body is adjusting to the changes brought about by tirzepatide, such as improved blood sugar levels and potential weight loss.
How is tirzepatide eliminated?
Tirzepatide is eliminated from the body through a process called metabolism. The body breaks down tirzepatide into smaller components, known as metabolites, which are then excreted primarily through urine and feces.
Does tirzepatide cause constipation?
Yes, constipation is a possible side effect of tirzepatide for some individuals. Constipation refers to having difficulty passing stools or experiencing infrequent bowel movements. While not everyone will experience constipation while taking tirzepatide, it can occur in some cases.
What is the alternative to tirzepatide?
The alternative to tirzepatide would depend on the specific medical condition it is being used to treat. For type 2 diabetes, there are several other medications available that work in different ways to help control blood sugar levels. Some common alternatives to tirzepatide for type 2 diabetes include:
Metformin: A widely used oral medication that helps lower blood sugar levels by reducing the production of glucose in the liver and improving insulin sensitivity.
Sulfonylureas: Another type of oral medication that stimulates the pancreas to release more insulin, helping to lower blood sugar levels.
DPP-4 inhibitors: Oral medications that help increase insulin production and decrease glucose production in the liver.
SGLT-2 inhibitors: Oral medications that work by helping the kidneys remove excess glucose from the body through urine.
GLP-1 receptor agonists: Similar to tirzepatide, these injectable weight loss drugs called GLP-1 inhibitors have been found to be highly effective in lowering blood sugar levels by increasing insulin production and decreasing glucagon release from the pancreas.
How effective is tirzepatide?
Tirzepatide is considered to be quite effective for its intended purposes. It has shown positive results in both managing type 2 diabetes and promoting weight loss. For type 2 diabetes, tirzepatide has been found to help lower blood sugar levels, improve control of blood sugar levels, and reduce the risk of complications related to diabetes.
In terms of weight loss, tirzepatide has demonstrated promising results, helping people lose significant amounts of weight when used as part of a comprehensive weight management plan. In addition, this medication shows potential in addressing weight-related complication by promoting significant weight loss in individuals struggling with obesity. Furthermore, tirzepatide’s efficacy in promoting weight loss raises the prospect of providing an alternative option to bariatric surgery for individuals seeking effective interventions against obesity.
The effectiveness of tirzepatide can vary from person to person, and it’s important to remember that individual responses may differ. Overall, it has shown to be a beneficial medication for those with type 2 diabetes and those seeking to lose weight. However, it should always be used under the guidance of a healthcare provider to ensure its safety and appropriate use for each individual’s health needs.
Does tirzepatide cause hair loss?
There is no evidence to suggest that tirzepatide causes hair loss as a direct side effect. Hair loss is not a commonly reported side effect of tirzepatide in clinical trials or medical literature.
Can tirzepatide be taken with insulin?
Yes, tirzepatide can be taken with insulin, but it is essential to do so under the guidance and supervision of a healthcare provider. When using tirzepatide with insulin, the dosages of both medications may need to be adjusted to improve blood sugar control and avoid the risk of lower blood sugar levels
Tirzepatide and insulin work in different ways to lower blood sugar levels, and combining them may provide additional benefits for people with type 2 diabetes who need better blood sugar management. Your healthcare provider will determine the appropriate dosages and schedule for taking both medications based on your individual health needs and treatment goals.
Can you take semaglutide and tirzepatide together?
No, you should not take semaglutide and tirzepatide together. Semaglutide and tirzepatide are both glucagon-like peptide-1 GLP-1 receptor agonists, which means that they work in the same way to reduce appetite and regulate blood sugar levels. Taking both medications together could increase the risk of side effects, such as nausea, vomiting, diarrhea, and constipation.
If you are taking semaglutide and are considering adding tirzepatide, you should talk to your doctor. Your doctor can help you decide if tirzepatide is right for you and can prescribe it to you if it is. It is important to talk to your doctor about the risks and benefits of taking both medications together.
Blog
Tirzepatide’s Impact on Blood Sugar Levels: Promoting Glycemic Control
Introduction:
Managing blood sugar levels is crucial for individuals living with diabetes. With advancements in medical research, new therapies have emerged to address the challenges faced by patients. One such innovative treatment option is tirzepatide. This groundbreaking medication has shown promising results in promoting glycemic control and improving the quality of life for people with diabetes.
The Power of Tirzepatide:
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. By simultaneously targeting two key hormones involved in regulating blood sugar levels, tirzepatide provides a unique advantage over traditional medications.
Efficacy and Clinical Trials:
Clinical trials have demonstrated the efficacy of tirzepatide in reducing blood sugar levels significantly. In a recent study, tirzepatide achieved greater reductions in HbA1c levels compared to other commonly used diabetes medications. Additionally, the treatment has shown the potential to aid in weight loss, with participants experiencing notable decreases in body weight during trials.
Improved Safety Profile:
Tirzepatide has also displayed a favorable safety profile. Common side effects, such as nausea, are generally mild and transient. The medication has exhibited minimal risk of hypoglycemia, a common concern with certain diabetes treatments. This enhanced safety profile allows individuals to manage their condition effectively without compromising their overall well-being.
Benefits Beyond Glycemic Control:
Apart from its impact on blood sugar levels, tirzepatide has shown other positive health benefits. Studies suggest that the medication may help reduce the risk of cardiovascular events, making it an attractive option for individuals with diabetes and cardiovascular comorbidities.
Conclusion:
Tirzepatide represents a significant advancement in diabetes management, offering patients a promising solution for achieving glycemic control. With its unique dual-action mechanism, the medication has the potential to transform the lives of individuals with diabetes, enhancing their overall well-being and reducing the risk of complications. Consultation with a healthcare professional is crucial to determine the suitability of tirzepatide as a treatment option and to ensure the best possible outcomes for patients managing diabetes.

Managing Type 2 Diabetes with Tirzepatide: Balancing Blood Sugar and Weight Loss
Introduction:
Type 2 diabetes is a complex condition that requires careful management to maintain healthy blood sugar levels and mitigate associated risks. In recent years, tirzepatide has emerged as a promising treatment option for individuals living with this chronic disease. Not only does it help regulate blood sugar levels, but it also offers the added benefit of weight loss, making it a compelling choice for those looking to improve their overall health.
Controlling Blood Sugar Levels:
Tirzepatide belongs to a class of medications known as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. By targeting multiple hormonal pathways, it helps the body regulate blood sugar more effectively. Clinical trials have demonstrated that tirzepatide consistently reduces HbA1c levels, an important marker of long-term blood sugar control, leading to improved glycemic management in individuals with type 2 diabetes.
Promoting Weight Loss:
One of the remarkable aspects of tirzepatide is its ability to support weight loss in patients with type 2 diabetes. Excessive weight often exacerbates diabetes symptoms and increases the risk of complications. Studies have shown that tirzepatide not only helps patients shed unwanted pounds but also improves body composition by reducing visceral fat, the harmful fat that surrounds organs. This dual benefit of blood sugar control and weight loss can significantly improve the overall health of individuals managing type 2 diabetes.
Enhanced Safety Profile:
Safety is a crucial consideration when choosing diabetes treatments. Tirzepatide has exhibited a favorable safety profile in clinical trials. Common side effects include mild gastrointestinal symptoms like nausea, which typically subside over time. Moreover, tirzepatide carries a lower risk of hypoglycemia compared to certain other diabetes medications, minimizing the potential for dangerous drops in blood sugar levels.
Holistic Approach to Diabetes Management:
Tirzepatide goes beyond traditional approaches to diabetes management by addressing both blood sugar control and weight management. By targeting multiple pathways, it offers a holistic approach to disease management. This comprehensive strategy has the potential to improve not only glycemic control but also overall cardiovascular health, as excessive weight and high blood sugar are risk factors for heart disease.
Conclusion:
Tirzepatide presents a groundbreaking option for individuals with type 2 diabetes, offering the dual benefits of blood sugar control and weight loss. By effectively managing blood glucose levels and promoting healthy weight reduction, tirzepatide empowers patients to take charge of their diabetes management and improve their overall well-being. As always, it is important to consult with a healthcare professional to determine the suitability of tirzepatide as part of an individualized treatment plan. With the right guidance and support, individuals with type 2 diabetes can strive for a healthier future with the help of this innovative medication.
Improving Heart Health with Tirzepatide: Lowering the Risk of Cardiovascular Disease
Introduction:
Cardiovascular disease is a significant concern for individuals living with diabetes, as they face an increased risk of heart-related complications. However, recent advancements in diabetes treatment have highlighted the potential of tirzepatide in not only managing blood sugar levels but also improving heart health. This dual benefit makes tirzepatide a promising option for individuals seeking to reduce their risk of cardiovascular disease.
Addressing Multiple Factors:
Tirzepatide belongs to a class of medications known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. By simultaneously targeting multiple hormonal pathways, it offers a comprehensive approach to diabetes management. This mechanism of action has shown promising results in improving heart health in individuals with diabetes.
Reducing Cardiovascular Risk Factors:
Clinical trials have demonstrated that tirzepatide may reduce the risk of cardiovascular events, such as heart attacks and strokes. In addition to its glycemic control benefits, tirzepatide has been associated with improvements in several cardiovascular risk factors. These include reducing blood pressure levels, improving lipid profiles by lowering LDL cholesterol and triglycerides, and decreasing markers of inflammation and arterial stiffness.
Potential for Cardiovascular Protection:
The ability of tirzepatide to positively impact various cardiovascular risk factors suggests its potential for long-term cardiovascular protection. By managing blood sugar levels and addressing underlying risk factors, tirzepatide may help individuals with diabetes reduce their overall cardiovascular risk and improve their heart health.
Collaborative Approach:
Managing heart health and diabetes requires a collaborative effort between patients and healthcare professionals. It is crucial to work closely with a healthcare provider to determine the suitability of tirzepatide and to establish an individualized treatment plan. Regular monitoring of cardiovascular health markers, such as blood pressure and lipid profiles, is essential to assess the effectiveness of tirzepatide and make any necessary adjustments.
Conclusion:
Tirzepatide offers a promising avenue for individuals with diabetes to improve both glycemic control and heart health. By addressing multiple factors that contribute to cardiovascular disease, this innovative medication has the potential to reduce the risk of heart-related complications in individuals with diabetes. However, it is important to consult with a healthcare professional to determine the suitability of tirzepatide as part of an individualized treatment plan. Together with lifestyle modifications and regular monitoring, tirzepatide can play a vital role in promoting better heart health for those living with diabetes.

Tirzepatide: A Promising Treatment for Diabetes and Weight Management
Introduction:
Managing diabetes and achieving weight loss can be challenging for individuals living with the condition. However, recent advancements in medical research have brought about new possibilities, and tirzepatide is emerging as a promising treatment option for both diabetes control and weight management. This innovative medication offers a unique approach that addresses the dual challenges of diabetes and weight simultaneously.
Combating Diabetes:
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. By targeting multiple hormonal pathways, it enhances the body’s ability to regulate blood sugar levels. Clinical trials have demonstrated that tirzepatide effectively lowers HbA1c levels, resulting in improved glycemic control for individuals with diabetes.
Unlocking Weight Loss Potential:
One of the remarkable aspects of tirzepatide is its ability to promote weight loss. Excess weight is not only a risk factor for diabetes but also contributes to its progression and associated complications. Studies have shown that tirzepatide helps individuals with diabetes achieve significant weight loss by reducing appetite, enhancing feelings of satiety, and increasing energy expenditure. This dual action on diabetes control and weight management makes tirzepatide an attractive treatment option for individuals aiming to improve their overall health.
Supporting a Healthy Lifestyle:
While tirzepatide can have a profound impact on diabetes control and weight loss, it is important to remember that it is not a standalone solution. A holistic approach to diabetes management, including a healthy diet and regular exercise, is crucial for optimal results. Tirzepatide can complement these lifestyle modifications, providing individuals with the tools to achieve sustainable weight loss and maintain long-term glycemic control.
Consultation with Healthcare Professionals:
Before considering tirzepatide or any other medication, it is essential to consult with a healthcare professional. They can evaluate the individual’s specific health condition, medical history, and goals to determine the suitability of tirzepatide as part of a personalized treatment plan. Regular monitoring and follow-up visits will ensure the medication’s effectiveness and make any necessary adjustments.
Conclusion:
Tirzepatide offers new hope for individuals seeking effective management of both diabetes and weight loss. With its dual action on glycemic control and weight management, this innovative medication provides a comprehensive approach to addressing the challenges faced by individuals with diabetes. However, it is crucial to work closely with healthcare professionals and adopt a healthy lifestyle to maximize the benefits of tirzepatide. By incorporating tirzepatide into a holistic treatment plan, individuals can strive for better diabetes control, achieve weight loss, and improve their overall health and well-being.
Tirzepatide and Liver Health: A Promising Treatment for Non-Alcoholic Fatty Liver Disease
Introduction:
Non-alcoholic fatty liver disease (NAFLD) is a prevalent condition characterized by the accumulation of fat in the liver, which can lead to inflammation and liver damage. As the incidence of NAFLD rises, finding effective treatments becomes crucial. Tirzepatide, a novel medication known for its benefits in diabetes management, has shown promising potential as a treatment for NAFLD, offering hope for individuals struggling with this condition.
The Link between Tirzepatide and NAFLD:
Tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, targets multiple hormonal pathways involved in metabolic regulation. These pathways play a significant role in NAFLD development and progression. By addressing insulin resistance, promoting weight loss, and reducing inflammation, tirzepatide has the potential to improve liver health and mitigate the impact of NAFLD.
Reducing Liver Fat and Inflammation:
Clinical studies have indicated that tirzepatide can effectively reduce liver fat content in individuals with NAFLD. The medication’s ability to improve insulin sensitivity and promote weight loss contributes to the reduction of liver fat accumulation. Moreover, tirzepatide has demonstrated anti-inflammatory effects, which are crucial in reducing liver inflammation and preventing disease progression.
Potential Benefits for NAFLD Patients:
Tirzepatide offers several potential benefits for individuals with NAFLD. Besides reducing liver fat and inflammation, it may improve liver enzyme levels, indicating enhanced liver function. Additionally, tirzepatide’s impact on weight loss and glycemic control can help address associated metabolic risk factors and improve overall metabolic health.
A Collaborative Approach:
While tirzepatide shows promise as a treatment for NAFLD, it is essential to adopt a comprehensive approach to liver health management. Lifestyle modifications, including a healthy diet and regular exercise, are key components of NAFLD management. Consultation with healthcare professionals is vital to determine the suitability of tirzepatide and to develop an individualized treatment plan that addresses the specific needs of each patient.
Conclusion:
Tirzepatide represents a promising treatment option for individuals with NAFLD, offering the potential to improve liver health and mitigate the effects of this increasingly prevalent condition. By addressing underlying metabolic dysregulation, reducing liver fat, and alleviating inflammation, tirzepatide shows potential in enhancing liver function and slowing the progression of NAFLD. However, it is important to collaborate with healthcare professionals to determine the appropriateness of tirzepatide and to develop a holistic approach that includes lifestyle modifications. With the right guidance and treatment, individuals with NAFLD can take positive steps towards improving their liver health and overall well-being.
References
- Pirro V, Roth KD, Lin Y, Willency JA, Milligan PL, Wilson JM, Ruotolo G, Haupt A, Newgard CB, Duffin KL. Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes. J Clin Endocrinol Metab. 2022 Jan 18;107(2):363-378. doi: 10.1210/clinem/dgab722. PMID: 34608929.
Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes
In this study, researchers aimed to evaluate the effects of tirzepatide, a medication used to treat type 2 diabetes (T2D), on the plasma metabolome. The study compared the effects of tirzepatide with the glucagon-like peptide 1 receptor agonist dulaglutide and a placebo. The trial involved 259 participants with T2D who were randomly assigned to receive tirzepatide, dulaglutide, or placebo for 26 weeks. The results showed that tirzepatide, particularly at higher doses, had a significant impact on a cluster of metabolites and lipids associated with insulin resistance, obesity, and future risk of T2D. Certain metabolites such as branched-chain amino acids, glutamate, 3-hydroxyisobutyrate, branched-chain ketoacids, and 2-hydroxybutyrate decreased compared to baseline and placebo. These changes were more pronounced with tirzepatide compared to dulaglutide and were directly related to improvements in glycemic control, insulin resistance indices, and proinsulin levels. Additionally, tirzepatide led to significant reductions in triglycerides and diglycerides compared to baseline, dulaglutide, and placebo, with a preference for shorter and highly saturated species. The findings suggest that tirzepatide not only helps with weight loss and glycemic control but also has a unique effect on metabolites associated with T2D risk and metabolic dysregulation, indicating improved metabolic health.
You can read the abstract of this article at https://academic.oup.com/jcem/article/107/2/363/6381509?login=false.
- Min T, Bain SC. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021 Jan;12(1):143-157. doi: 10.1007/s13300-020-00981-0. Epub 2020 Dec 15. PMID: 33325008; PMCID: PMC7843845.
The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials.
GLucagon-like peptide 1 (GLP-1)-based therapy is an established treatment option for type 2 diabetes mellitus (T2DM) due to its effectiveness in reducing blood glucose levels, promoting weight loss and providing favorable cardiovascular outcomes. In contrast, glucose-dependent insulinotropic polypeptide (GIP) was previously believed to have no potential as a glucose-lowering therapy based on studies that showed no insulin-stimulating effect when administered at high levels to individuals with T2DM. However, recent evidence has demonstrated that the simultaneous administration of GLP-1 and GIP has a synergistic effect, resulting in significantly increased insulin and glucagon responses compared to administering each hormone separately. These findings led to the development of a dual GIP/GLP-1 receptor agonist, commonly referred to as a “twincretin.” Tirzepatide is a novel dual GIP/GLP-1 receptor agonist designed as a synthetic peptide consisting of 39 amino acids, based on the natural GIP sequence. Preclinical and early clinical trials (phase 1 and 2) have shown that tirzepatide effectively lowers blood glucose levels and induces weight loss, with side effects similar to established GLP-1 receptor agonists. The long-term efficacy, safety, and cardiovascular outcomes of tirzepatide will be further investigated in the SURPASS phase 3 clinical trial program. This paper aims to review the preclinical and early clinical trials of tirzepatide for the management of T2DM and provide an overview of the upcoming SURPASS clinical trials.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7843845/. - Frías JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2020 Nov;15(6):379-394. doi: 10.1080/17446651.2020.1830759. Epub 2020 Oct 8. PMID: 33030356.
Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabete
Glucagon-like peptide-1 (GLP-1) receptor agonists have become increasingly important in the treatment of type 2 diabetes (T2D) due to their positive effects on blood sugar control, weight management, and cardiovascular and renal health. However, there is still a need for more effective therapeutics to address the ongoing challenges in achieving metabolic goals for many T2D patients.Tirzepatide is a unique dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist currently under development for the treatment of T2D. This review provides an overview of tirzepatide’s key characteristics and summarizes the efficacy and safety results from Phase 1 and Phase 2 clinical trials. It also gives a brief overview of the ongoing Phase 3 clinical trial program for T2D, as well as recently initiated studies in patients with obesity and nonalcoholic steatohepatitis. The information in this review is primarily sourced from published clinical trials, the manufacturer’s websites, and ClinicalTrials.gov. Based on the data from Phase 2 trials, tirzepatide shows great potential as a highly effective therapy for T2D, particularly in terms of glucose control and weight management. The results from the ongoing Phase 3 clinical trial program, expected to be available in late 2020, will play a crucial role in determining the future prospects of this promising therapeutic agent.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/33030356/. - Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, Bray R, Rodríguez Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021 Aug 14;398(10300):583-598. doi: 10.1016/S0140-6736(21)01443-4. Epub 2021 Aug 6. PMID: 34370970.
Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial.
Tirzepatide, a novel dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist, is currently being developed for the treatment of type 2 diabetes. The objective of this study was to compare the efficacy and safety of tirzepatide with titrated insulin degludec in individuals with type 2 diabetes who had inadequate glycemic control despite treatment with metformin alone or in combination with SGLT2 inhibitors.In this phase 3 study, participants aged 18 years or older, with a baseline glycated hemoglobin (HbA1c) of 7.0-10.5%, body mass index of at least 25 kg/m2, stable weight, and no prior insulin treatment, were enrolled across multiple centers in 13 countries. They were randomly assigned in a 1:1:1:1 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg, 10 mg, or 15 mg) or once-daily subcutaneous injections of titrated insulin degludec. The primary efficacy endpoint was the non-inferiority of tirzepatide (10 mg or 15 mg) compared to insulin degludec in terms of the mean change from baseline in HbA1c at week 52. Key secondary endpoints included non-inferiority of tirzepatide (5 mg) compared to insulin degludec in HbA1c reduction, superiority of all tirzepatide doses over insulin degludec in HbA1c reduction and weight loss, and the proportion of participants achieving an HbA1c level below 7.0% at week 52. The study also assessed safety outcomes. Data from the modified intention-to-treat population, which included participants who received at least one dose of the study drug, were analyzed.Between April and November 2019, 1,444 participants were randomly assigned to treatment, with 1,437 included in the modified intention-to-treat population. The results showed that tirzepatide, at all doses, achieved superior reductions in HbA1c compared to insulin degludec at week 52, meeting the non-inferiority criteria. The estimated treatment difference for tirzepatide versus insulin degludec ranged from -0.59% to -1.04%, with all tirzepatide doses demonstrating statistical significance. Additionally, a higher proportion of participants in the tirzepatide groups achieved an HbA1c level below 7.0% compared to the insulin degludec group. Moreover, all three doses of tirzepatide resulted in significant weight loss, while insulin degludec led to weight gain. The safety profile of tirzepatide was similar to that of GLP-1 receptor agonists, with gastrointestinal events being the most common adverse events. Nausea, diarrhea, decreased appetite, and vomiting were reported more frequently in the tirzepatide groups compared to the insulin degludec group. Hypoglycemia was less common with tirzepatide than with insulin degludec. There were no deaths considered related to the study treatment.In conclusion, tirzepatide demonstrated superior efficacy compared to titrated insulin degludec in individuals with type 2 diabetes, as evidenced by greater reductions in HbA1c and body weight at week 52 and a lower risk of hypoglycemia. The safety profile of tirzepatide was consistent with GLP-1 receptor agonists.https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01443-4/fulltext. - Bhagavathula AS, Vidyasagar K, Tesfaye W. Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials. Pharmaceuticals (Basel). 2021;14(10):991. Published 2021 Sep 28. doi:10.3390/ph14100991.
Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials.
Diabetes mellitus affects a substantial number of individuals globally, with estimates indicating that the number of affected people will continue to rise significantly in the coming years. Type 2 diabetes mellitus (T2DM) is associated with severe microvascular and macrovascular complications, including cardiovascular issues, kidney failure, and blindness, which contribute to high mortality rates. Effective management of T2DM is crucial for preventing or delaying the progression of these complications. Various classes of medications are available for T2DM management, each with its own benefits. However, the ideal treatment should effectively reduce blood glucose levels without increasing the risk of hypoglycemia or weight gain, while also providing additional benefits for cardiovascular and renal outcomes. Emerging treatments like glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 inhibitors have shown promise in providing these additional benefits alongside glycemic control. Tirzepatide, a novel weekly dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist, is currently being studied for its efficacy and safety in people with T2DM. To assess the effectiveness of tirzepatide, a systematic review and meta-analysis was conducted using data from four randomized controlled trials involving a total of 2,783 patients. The analysis demonstrated that tirzepatide treatment led to significant reductions in glycated hemoglobin (HbA1c), fasting serum glucose, and body weight compared to placebo or selective GLP-1 receptor agonists. The improvements in HbA1c levels were sustained over the long term. The safety profile of tirzepatide was acceptable, with a low incidence of hypoglycemia and serious adverse events. The discontinuation rate was also within an acceptable range. These findings indicate that tirzepatide is an effective therapeutic option for lowering glucose levels and managing body weight in patients with T2DM.
You can read the abstract of this article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8537322/. - Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, Urva S, Haupt A, Robins DA. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020 Jun;22(6):938-946. doi: 10.1111/dom.13979. Epub 2020 Feb 11. PMID: 31984598; PMCID: PMC7318331.
Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens.
The aim of this study was to evaluate the effectiveness and tolerability of tirzepatide treatment using three different dose-escalation regimens in patients with type 2 diabetes. In a double-blind, placebo-controlled trial, participants were randomly assigned in a 1:1:1:1 ratio to receive once-weekly subcutaneous tirzepatide or placebo. The tirzepatide dose groups and regimens were as follows: 12 mg (4 mg for weeks 0-3, 8 mg for weeks 4-7, and 12 mg for weeks 8-11), 15 mg-1 (2.5 mg for weeks 0-1, 5 mg for weeks 2-3, 10 mg for weeks 4-7, and 15 mg for weeks 8-11), and 15 mg-2 (2.5 mg for weeks 0-3, 7.5 mg for weeks 4-7, and 15 mg for weeks 8-11). The primary objective was to compare tirzepatide with placebo in terms of the change in glycated hemoglobin (HbA1c) from baseline at 12 weeks.The study included a total of 111 randomized patients: placebo (26), tirzepatide 12 mg (29), tirzepatide 15 mg-1 (28), and tirzepatide 15 mg-2 (28). The mean age of the participants was 57.4 years, with a baseline HbA1c of 8.4% and a body mass index of 31.9 kg/m2. At week 12, the absolute change in HbA1c from baseline was significantly greater in the tirzepatide treatment groups compared to the placebo group (placebo: +0.2% [0.21]; 12 mg: -1.7% [0.19]; 15 mg-1: -2.0% [0.20]; 15 mg-2: -1.8% [0.19]). The incidence of nausea was as follows: placebo: 7.7%; 12 mg group: 24.1%; 15 mg-1 group: 39.3%; 15 mg-2 group: 35.7%. Three patients discontinued treatment due to adverse events, with one patient each from the placebo, 12 mg, and 15 mg-1 groups.
In conclusion, a 12-week tirzepatide treatment resulted in clinically significant reductions in HbA1c levels. This suggests that starting with lower doses and smaller dose increments is associated with a more favorable side effect profile.
Retrieved full article from:-https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7318331/. - Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021 Jan 23;106(2):388-396. doi: 10.1210/clinem/dgaa863. PMID: 33236115; PMCID: PMC7823251.
Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes.
The novel dual receptor agonist tirzepatide, which targets both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 GLP-1 receptor agonist demonstrated superior glucose control and weight loss compared to the selective GLP-1 receptor agonist dulaglutide. This study aimed to analyze biomarkers related to beta-cell function and insulin resistance (IR) and evaluate the contribution of weight loss to improvements in IR over a 26-week period.The results showed that homeostatic model assessment (HOMA) 2-B, an indicator of beta-cell function, significantly increased with dulaglutide and all doses of tirzepatide (5 mg, 10 mg, and 15 mg) compared to placebo. The ratios of proinsulin to insulin and proinsulin to C-peptide significantly decreased with tirzepatide (10 mg and 15 mg) compared to both placebo and dulaglutide. Fasting insulin levels significantly decreased with tirzepatide (10 mg and 15 mg), and HOMA2-IR, a measure of insulin resistance, significantly decreased with tirzepatide (10 mg) compared to both placebo and dulaglutide. Several markers of improved insulin sensitivity, including adiponectin, IGFBP-1, and IGFBP-2, significantly increased with one or more doses of tirzepatide.
To determine the impact of weight loss on improvements in insulin resistance, multiple linear regression analysis was conducted, taking into account potential confounding variables such as age, sex, metformin use, triglyceride levels, and glycated hemoglobin A1c. The analysis revealed that weight loss accounted for only 13% and 21% of the improvement in HOMA2-IR with tirzepatide (10 mg and 15 mg, respectively), indicating that weight loss was only partially responsible for the observed improvements in insulin resistance.
In conclusion, tirzepatide exhibited greater effects on markers of insulin sensitivity and beta-cell function compared to dulaglutide. The improvements in insulin sensitivity attributed to tirzepatide were only partly explained by weight loss, suggesting that the dual receptor agonism of tirzepatide provides distinct mechanisms for glycemic control beyond weight reduction.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7823251/. - Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-155. doi: 10.1016/S0140-6736(21)01324-6. Epub 2021 Jun 27. Erratum in: Lancet. 2021 Jul 17;398(10296):212. PMID: 34186022.
Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial
Despite advancements in diabetes care, many individuals with type 2 diabetes fail to achieve treatment goals. As a result, there is a need for the development of new therapies. The objective of this study was to evaluate the effectiveness, safety, and tolerance of a novel treatment, tirzepatide, which combines glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist as a monotherapy for people with type 2 diabetes who have not adequately controlled their condition through diet and exercise alone. A 40-week, double-blind, randomized, placebo-controlled phase 3 trial called SURPASS-1 was conducted at 52 medical research centers and hospitals in India, Japan, Mexico, and the USA. The study included adult participants aged 18 or above with type 2 diabetes who had not achieved sufficient control of their condition through diet and exercise alone and had not previously received injectable diabetes therapy. Participants were randomly assigned to receive tirzepatide once a week at different doses (5 mg, 10 mg, or 15 mg) or a placebo, using a computer-generated random sequence. Both the participants and the researchers involved in the study were unaware of the treatment assignment. The primary measure of the study was the average change in glycated hemoglobin (HbA1c) from baseline at the 40-week mark. Between June 3, 2019, and October 28, 2020, a total of 705 individuals were assessed for eligibility, out of which 478 were randomly assigned to receive tirzepatide (at doses of 5 mg, 10 mg, or 15 mg) or a placebo. The participants had a mean baseline HbA1c of 7.9% (63 mmol/mol), with an average age of 54.1 years, and 48% were women. The average duration of diabetes was 4.7 years, and the average body mass index was 31.9 kg/m2. During the study, 14% of participants discontinued the use of the study drug, and 10% withdrew from the study prematurely. At the end of the 40-week period, all doses of tirzepatide demonstrated superior results compared to the placebo in terms of reducing HbA1c, fasting serum glucose, body weight, and achieving HbA1c targets of less than 7.0% (<53 mmol/mol) and less than 5.7% (<39 mmol/mol). The mean decrease in HbA1c levels from baseline was 1.87% (20 mmol/mol) with tirzepatide 5 mg, 1.89% (21 mmol/mol) with tirzepatide 10 mg, and 2.07% (23 mmol/mol) with tirzepatide 15 mg, compared to an increase of 0.04% with the placebo. The estimated treatment differences versus the placebo were -1.91% (-21 mmol/mol) for tirzepatide 5 mg, -1.93% (-21 mmol/mol) for tirzepatide 10 mg, and -2.11% (-23 mmol/mol) for tirzepatide 15 mg (all p<0.0001). A significantly higher percentage of participants receiving tirzepatide achieved HbA1c targets of less than 7.0% (<53 mmol/mol; 87-92% vs 20%), 6.5% or less (≤48 mmol/mol; 81-86% vs 10%), and less than 5.7% (<39 mmol/mol; 31-52% for tirzepatide, 1% for placebo). Furthermore, tirzepatide led to dose-dependent weight loss, ranging from 7.0 to 9.5 kg. The most common adverse events associated with tirzepatide were mild to moderate and temporary gastrointestinal problems, including nausea (12-18%), diarrhea (12-14%), and vomiting (2-6%). No clinically significant or severe hypoglycemia (blood glucose levels below 54 mg/dL [<3 mmol/L]) related to tirzepatide use were reported. There was one reported death in the placebo group. You can read the full article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01324-6/fulltext. - Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ; SURPASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-1824. doi: 10.1016/S0140-6736(21)02188-7. Epub 2021 Oct 18. PMID: 34672967.
Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial.
The objective was to evaluate the effectiveness and safety, with a specific focus on cardiovascular safety, of tirzepatide, a novel dual GIP and GLP-1 receptor agonist, compared to insulin glargine in adults with type 2 diabetes and a high risk of cardiovascular events who have not achieved adequate control with oral glucose-lowering medications.This phase 3 study was conducted at 187 sites across 14 countries on five continents. Eligible participants were 18 years or older and had type 2 diabetes treated with a combination of metformin, sulfonylurea, or sodium-glucose co-transporter-2 inhibitor. They had a baseline glycated hemoglobin (HbA1c) level between 7.5% and 10.5% (58-91 mmol/mol), a body-mass index of 25 kg/m2 or greater, and established cardiovascular disease or a high risk of cardiovascular events. Participants were randomly assigned in a 1:1:1:3 ratio to receive subcutaneous injections of tirzepatide once per week at doses of 5 mg, 10 mg, or 15 mg, or insulin glargine (100 U/mL). The aim was to reach a fasting blood glucose level below 100 mg/dL. The primary endpoint was to demonstrate non-inferiority (with a non-inferiority margin of 0.3%) of tirzepatide 10 mg or 15 mg, or both, compared to glargine in terms of the change in HbA1c from baseline to 52 weeks. All participants received treatment for at least 52 weeks, with a maximum treatment duration of 104 weeks or until the study was completed to collect and evaluate major adverse cardiovascular events (MACE). Safety measures were assessed throughout the study. In individuals with type 2 diabetes and a heightened risk of cardiovascular events, tirzepatide demonstrated greater and clinically significant reduction in HbA1c compared to glargine at week 52. Tirzepatide treatment was associated with a lower incidence of hypoglycemia and did not pose an increased cardiovascular risk. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02188-7/fulltext. - Available at https://jamanetwork.com/journals/jama/article-abstract/2788781.
Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes
The researcher wanted to see how effective and safe tirzepatide is when added to insulin glargine for treating type 2 diabetes.The goal was to assess the effectiveness and safety of tirzepatide as an addition to insulin glargine in patients with type 2 diabetes who had poor control of their blood sugar levels. The resaercher conducted a randomized clinical trial involving 475 adults with type 2 diabetes from 45 medical research centers and hospitals in 8 countries. These participants had inadequate control of their blood sugar levels despite using once-daily insulin glargine with or without metformin.The participants were randomly assigned to receive weekly injections of tirzepatide at doses of 5 mg, 10 mg, or 15 mg, or a placebo over a period of 40 weeks. The tirzepatide dose was gradually increased every 4 weeks until the assigned dose was reached.The primary measure we looked at was the average change in glycated hemoglobin A1c (HbA1c) from the baseline at week 40. We also assessed other measures such as changes in body weight and the percentage of participants achieving specific HbA1c targets. Among the 475 participants, most completed the trial (94.9%). At week 40, there was a significant decrease in HbA1c levels with tirzepatide compared to the placebo. The 10-mg tirzepatide group showed a decrease of -2.40% in HbA1c, the 15-mg group had a decrease of -2.34%, while the placebo group had a decrease of -0.86%. The participants who received tirzepatide also experienced significant weight loss compared to those on the placeboAdding subcutaneous tirzepatide to titrated insulin glargine resulted in significant improvements in glycemic control for patients with type 2 diabetes who had inadequate control with insulin glargine alone. The addition of tirzepatide also led to weight loss.You can read the full article at https://jamanetwork.com/journals/jama/article-abstract/2788781.
- Available at https://www.nejm.org/doi/full/10.1056/NEJMoa2107519.
Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes
Tirzepatide is a medication being developed for the treatment of type 2 diabetes. It is a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist. However, its effectiveness and safety in comparison to semaglutide, another type 2 diabetes medication, have not been studied.
In a 40-week phase 3 trial, 1879 patients were randomly assigned to receive tirzepatide at doses of 5 mg, 10 mg, or 15 mg, or semaglutide at a dose of 1 mg. The patients had an average glycated hemoglobin (HbA1c) level of 8.28% at the beginning of the trial. The primary measure was the change in HbA1c level from baseline to 40 weeks. The estimated mean change in HbA1c level was -2.01%, -2.24%, and -2.30% with tirzepatide at doses of 5 mg, 10 mg, and 15 mg respectively, compared to -1.86% with semaglutide. The differences between the tirzepatide groups and the semaglutide group were statistically significant, showing that tirzepatide was both noninferior and superior to semaglutide. Patients who received tirzepatide also experienced greater reductions in body weight compared to those on semaglutide. The most common side effects were gastrointestinal issues such as nausea, diarrhea, and vomiting, which were generally mild to moderate. Hypoglycemia (low blood sugar) was reported in a small percentage of patients in the tirzepatide and semaglutide groups. Serious adverse events were reported in a slightly higher percentage of patients in the tirzepatide groups compared to the semaglutide group. In patients with type 2 diabetes, tirzepatide was shown to be noninferior and superior to semaglutide in terms of reducing HbA1c levels over a 40-week period.
You can read the full article at https://www.nejm.org/doi/full/10.1056/NEJMoa2107519.
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019 Dec;30:72-130. doi: 10.1016/j.molmet.2019.09.010. Epub 2019 Sep 30. PMID: 31767182; PMCID: PMC6812410.
Glucagon-like peptide 1 (GLP-1)
The hormone glucagon-like peptide-1 (GLP-1) has various effects on the body and has shown potential for pharmacological use. It stimulates insulin secretion in a glucose-dependent manner, slows down digestion, reduces food intake, increases urine production, and affects the growth of beta cells in rodents. GLP-1 also has protective effects on the heart and brain, reduces inflammation and cell death, and plays a role in learning, memory, and behavior related to rewards and taste. Modified versions of GLP-1 have been developed to be more potent and have longer-lasting effects, and they are currently used in clinical practice to treat type-2 diabetes. Additionally, GLP-1-based treatments are being studied for their potential in managing obesity. This review provides a comprehensive overview of the various functions of GLP-1 and its pharmacological properties. It explores the potential therapeutic applications of GLP-1 in different diseases.GLP-1 has proven to be a versatile hormone with multiple metabolic functions that extend beyond its initial recognition as an incretin hormone. Its many beneficial effects make it an intriguing candidate for the development of pharmacotherapies for obesity, diabetes, and neurodegenerative disorders.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6812410/.
- Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, Haupt A. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018 Nov 17;392(10160):2180-2193. doi: 10.1016/S0140-6736(18)32260-8. Epub 2018 Oct 4. PMID: 30293770.
Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial
The drug LY3298176 is a new type of medication being developed for treating type 2 diabetes. It works by stimulating two receptors in the body, known as the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. This study aimed to compare the effectiveness and safety of LY3298176 with a placebo and with dulaglutide, a drug that stimulates only the GLP-1 receptor.
The study was conducted over a period of 26 weeks and involved patients with poorly controlled type 2 diabetes. The participants were divided into six groups and received different treatments: LY3298176 at varying doses (1 mg, 5 mg, 10 mg, or 15 mg), dulaglutide (1.5 mg), or a placebo. The primary measure of effectiveness was the change in glycated hemoglobin A1c (HbA1c) levels, a marker of blood glucose control, from the start of the study to the end of the 26-week period.
The results showed that LY3298176 had a dose-dependent effect on reducing HbA1c levels, and the reduction was greater than that achieved with dulaglutide or the placebo. A significant percentage of patients treated with LY3298176 reached the target HbA1c levels of less than 7% and at least 6.5%. Additionally, LY3298176 led to weight loss, with a higher proportion of patients achieving the weight loss targets compared to dulaglutide and the placebo. There were also improvements in fasting plasma glucose, waist circumference, and total cholesterol levels with LY3298176.
In terms of safety, there were some adverse events reported, with gastrointestinal issues such as nausea, diarrhea, and vomiting being the most common. However, most of these events were mild to moderate and temporary. Decreased appetite was the second most common adverse event. Severe hypoglycemia was not reported, and only one unrelated death occurred during the study.
Based on these findings, LY3298176, the dual GIP and GLP-1 receptor agonist, demonstrated superior efficacy in glucose control and weight loss compared to dulaglutide. The drug showed an acceptable safety profile. The study suggests that simultaneous stimulation of GIP and GLP-1 receptors may offer a promising new treatment approach for type 2 diabetes.
You can read the abstract of this article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32260-8/fulltext.
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, Kuchibhotla U, Moyers JS, Benson CT, Gimeno RE, D’Alessio DA, Haupt A. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018 Dec;18:3-14. doi: 10.1016/j.molmet.2018.09.009. Epub 2018 Oct 3. PMID: 30473097; PMCID: PMC6308032.
LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept
A novel dual GIP and GLP-1 receptor agonist, LY3298176, was developed to determine whether the metabolic action of GIP adds to the established clinical benefits of selective GLP-1 receptor agonists in type 2 diabetes mellitus (T2DM).
Methods: LY3298176 is a fatty acid modified peptide with dual GIP and GLP-1 receptor agonist activity designed for once-weekly subcutaneous administration. LY3298176 was characterised in vitro, using signaling and functional assays in cell lines expressing recombinant or endogenous incretin receptors, and in vivo using body weight, food intake, insulin secretion and glycemic profiles in mice. A Phase 1, randomised, placebo-controlled, double-blind study was comprised of three parts: a single-ascending dose (SAD; doses 0.25-8 mg) and 4-week multiple-ascending dose (MAD; doses 0.5-10 mg) studies in healthy subjects (HS), followed by a 4-week multiple-dose Phase 1 b proof-of-concept (POC; doses 0.5-15 mg) in patients with T2DM (ClinicalTrials.gov no. NCT02759107). Doses higher than 5 mg were attained by titration, dulaglutide (DU) was used as a positive control. The primary objective was to investigate safety and tolerability of LY3298176. LY3298176 activated both GIP and GLP-1 receptor signaling in vitro and showed glucose-dependent insulin secretion and improved glucose tolerance by acting on both GIP and GLP-1 receptors in mice. With chronic administration to mice, LY3298176 potently decreased body weight and food intake; these effects were significantly greater than the effects of a GLP-1 receptor agonist. A total of 142 human subjects received at least 1 dose of LY3298176, dulaglutide, or placebo. The PK profile of LY3298176 was investigated over a wide dose range (0.25-15 mg) and supports once-weekly administration. In the Phase 1 b trial of diabetic subjects, LY3298176 doses of 10 mg and 15 mg significantly reduced fasting serum glucose compared to placebo (least square mean [LSM] difference [95% CI]: -49.12 mg/dL [-78.14, -20.12] and -43.15 mg/dL [-73.06, -13.21], respectively). Reductions in body weight were significantly greater with the LY3298176 1.5 mg, 4.5 mg and 10 mg doses versus placebo in MAD HS (LSM difference [95% CI]: -1.75 kg [-3.38, -0.12], -5.09 kg [-6.72, -3.46] and -4.61 kg [-6.21, -3.01], respectively) and doses of 10 mg and 15 mg had a relevant effect in T2DM patients (LSM difference [95% CI]: -2.62 kg [-3.79, -1.45] and -2.07 kg [-3.25, -0.88], respectively. The most frequent side effects reported with LY3298176 were gastrointestinal (vomiting, nausea, decreased appetite, diarrhoea, and abdominal distension) in both HS and patients with T2DM; all were dose-dependent and considered mild to moderate in severity. Based on these results, the pharmacology of LY3298176 translates from preclinical to clinical studies. LY3298176 has the potential to deliver clinically meaningful improvement in glycaemic control and body weight. The data warrant further clinical evaluation of LY3298176 for the treatment of T2DM and potentially obesity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6308032/.
- Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022 Feb 8;327(6):534-545. doi: 10.1001/jama.2022.0078. PMID: 35133415; PMCID: PMC8826179.
Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial
Little is known about the effects of tirzepatide, a medication that stimulates both the glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptors, when added to insulin glargine for treating type 2 diabetes. This study aimed to evaluate the effectiveness and safety of adding tirzepatide to insulin glargine in patients with type 2 diabetes who had inadequate control of blood sugar levels. This randomized phase 3 clinical trial took place at 45 medical research centers and hospitals in 8 countries. The study enrolled 475 adults with type 2 diabetes and inadequate blood sugar control while receiving once-daily insulin glargine, with or without metformin. The enrollment period lasted from August 30, 2019, to March 20, 2020, and follow-up was completed on January 13, 2021.The participants were divided into four groups in a 1:1:1:1 ratio. They received once-weekly subcutaneous injections of either 5 mg, 10 mg, or 15 mg of tirzepatide or a placebo over a period of 40 weeks. The dosage of tirzepatide was gradually increased every 4 weeks until the assigned dose was reached. The primary outcome measured was the average change in glycated hemoglobin A1c (HbA1c) levels from the baseline at week 40. The study also assessed secondary outcomes, including changes in body weight and the percentage of patients achieving specific HbA1c levels. Out of the 475 participants randomized in the study, 451 (94.9%) completed the trial. The discontinuation rates were 10% for the 5-mg tirzepatide group, 12% for the 10-mg tirzepatide group, 18% for the 15-mg tirzepatide group, and 3% for the placebo group. At week 40, the average change in HbA1c levels from the baseline was -2.40% for the 10-mg tirzepatide group and -2.34% for the 15-mg tirzepatide group, compared to -0.86% for the placebo group. The differences between the tirzepatide groups and the placebo group were statistically significant. The changes in body weight were also notable, with the tirzepatide groups showing significant reductions compared to the placebo group. A higher percentage of patients treated with tirzepatide achieved an HbA1c level below 7% compared to those treated with the placebo. The most common side effects reported in the tirzepatide groups were diarrhea and nausea.In patients with type 2 diabetes who had inadequate control of blood sugar levels despite treatment with insulin glargine, adding subcutaneous tirzepatide to titrated insulin glargine led to significant improvements in glycemic control after 40 weeks, along with reductions in body weight.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8826179/.
- Heise T, Mari A, DeVries JH, Urva S, Li J, Pratt EJ, Coskun T, Thomas MK, Mather KJ, Haupt A, Milicevic Z. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022 Apr 22:S2213-8587(22)00085-7. doi: 10.1016/S2213-8587(22)00085-7. Epub ahead of print. PMID: 35468322.
Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial
This study investigated the effects of tirzepatide, a medication used to treat type 2 diabetes, on the body’s physiological mechanisms. The study involved 117 participants with type 2 diabetes who were randomly assigned to receive tirzepatide, another medication called semaglutide, or a placebo. The primary goal was to assess the impact of tirzepatide compared to the placebo on the change in clamp disposition index, which combines measures of insulin secretion and sensitivity. The study found that tirzepatide significantly increased the clamp disposition index, indicating improved insulin secretion and sensitivity, compared to the placebo. Tirzepatide also outperformed semaglutide in these aspects. Additionally, tirzepatide effectively reduced glucose levels and had a similar safety profile to semaglutide. The study concluded that tirzepatide’s ability to lower blood glucose in type 2 diabetes is attributed to its positive effects on insulin secretion, insulin sensitivity, and glucagon secretion, which are key factors in diabetes.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00085-7/fulltext.
- Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes Obes Metab. 2022 Feb;24(2):239-246. doi: 10.1111/dom.14572. Epub 2021 Nov 18. PMID: 34647404.
A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes
This study aimed to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of tirzepatide in Japanese individuals with type 2 diabetes (T2D). The study was a double-blind, placebo-controlled trial with participants receiving once-weekly subcutaneous doses of tirzepatide or a placebo. Different dosage levels of tirzepatide were tested. The results showed that tirzepatide was well tolerated, with the most common side effects being decreased appetite and mild gastrointestinal issues, which were generally dose-dependent. The half-life of tirzepatide in the bloodstream was approximately 5 days. After 8 weeks of treatment, tirzepatide significantly reduced fasting plasma glucose levels, HbA1c levels (a measure of long-term blood sugar control), and body weight compared to the placebo. These findings support the further investigation of once-weekly dosing of tirzepatide in Japanese individuals with T2D.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9299227/.
- Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., Stefanski, A., & SURMOUNT-1 Investigators (2022). Tirzepatide Once Weekly for the Treatment of Obesity. The New England journal of medicine, 387(3), 205–216. https://doi.org/10.1056/NEJMoa2206038.
Tirzepatide Once Weekly for the Treatment of Obesity
This phase 3 trial aimed to evaluate the efficacy and safety of tirzepatide, a novel medication, in treating obesity. The study included 2539 adults with a body mass index (BMI) of 30 or higher or 27 or higher with weight-related complications. Participants were randomly assigned to receive once-weekly subcutaneous doses of tirzepatide (5 mg, 10 mg, or 15 mg) or a placebo for 72 weeks. The primary outcomes measured were the percentage change in weight from baseline and achieving a weight reduction of 5% or more. The results showed that tirzepatide led to significant and sustained reductions in body weight. At week 72, the percentage change in weight was -15.0% with 5 mg of tirzepatide, -19.5% with 10 mg, and -20.9% with 15 mg, compared to -3.1% with the placebo. Additionally, a high percentage of participants achieved a weight reduction of 5% or more (85% with 5 mg, 89% with 10 mg, and 91% with 15 mg of tirzepatide). Tirzepatide also demonstrated improvements in cardiometabolic measures. The most common adverse events were gastrointestinal and generally mild to moderate. The study concluded that once-weekly doses of tirzepatide provided substantial and sustained reductions in body weight over the 72-week period.
You can read the full article at https://www.nejm.org/doi/full/10.1056/NEJMoa2206038.
- Nauck, M. A., & D’Alessio, D. A. (2022). Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovascular diabetology, 21(1), 169. https://doi.org/10.1186/s12933-022-01604-7.
Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction
Tirzepatide is a recently approved medication for the treatment of type 2 diabetes. It is the first dual GIP/GLP-1 receptor co-agonist that activates key receptors involved in insulin secretion and food intake regulation. Clinical trials have shown that tirzepatide effectively reduces HbA1c levels (1.24% to 2.58%) and body weight (5.4 kg to 11.7 kg) in type 2 diabetic patients, surpassing the effects of other agents. A significant proportion of patients achieved normal HbA1c levels and substantial weight loss. Tirzepatide demonstrated superior efficacy compared to semaglutide and basal insulin. Common adverse events were similar to those reported for selective GLP-1RA, including nausea, vomiting, diarrhea, and constipation, which were more frequent at higher doses. Cardiovascular events tended to be reduced with tirzepatide, meeting the criteria for cardiovascular safety. The drug improved insulin sensitivity, insulin secretion, and prandial insulin and glucagon concentrations. It also caused appetite reduction and greater weight loss compared to semaglutide. However, questions remain regarding the mechanism of action and the effects of GIP in humans. Overall, tirzepatide’s efficacy and unique profile have generated interest in the therapeutic potential of GIP in type 2 diabetes, obesity, and related conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9438179/.
- Inagaki, N., Takeuchi, M., Oura, T., Imaoka, T., & Seino, Y. (2022). Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. The lancet. Diabetes & endocrinology, 10(9), 623–633. https://doi.org/10.1016/S2213-8587(22)00188-7.
Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial
In a phase 3 trial conducted in Japan, the efficacy and safety of tirzepatide, a new medication for type 2 diabetes, were compared to dulaglutide. The trial involved 636 participants who were randomly assigned to receive tirzepatide (5 mg, 10 mg, or 15 mg) or dulaglutide (0.75 mg) once a week for 52 weeks. The primary endpoint was the change in HbA1c levels from baseline. At week 52, tirzepatide demonstrated superior results compared to dulaglutide, with significant reductions in HbA1c levels. The mean treatment differences in HbA1c reduction between tirzepatide and dulaglutide were -1.1% for 5 mg, -1.3% for 10 mg, and -1.5% for 15 mg. Tirzepatide also resulted in dose-dependent reductions in body weight, with significant weight loss compared to dulaglutide. The most common adverse events associated with tirzepatide were nausea, constipation, and nasopharyngitis, with a low frequency of gastrointestinal events. The study concluded that tirzepatide was more effective than dulaglutide in improving glycemic control and reducing body weight. The safety profile of tirzepatide was consistent with other GLP-1 receptor agonists, indicating its potential as a treatment option for Japanese patients with type 2 diabetes.
You can read the abstract of this article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587%2822%2900188-7/fulltext.
- Nowak, M., Nowak, W., & Grzeszczak, W. (2022). Tirzepatide – a dual GIP/GLP-1 receptor agonist – a new antidiabetic drug with potential metabolic activity in the treatment of type 2 diabetes. Endokrynologia Polska, 73(4), 745–755. https://doi.org/10.5603/EP.a2022.0029.
Tirzepatide – a dual GIP/GLP-1 receptor agonist – a new antidiabetic drug with potential metabolic activity in the treatment of type 2 diabetes
The hormones GLP-1 and GIP play a significant role in insulin secretion after meals. Tirzepatide, developed by Eli Lilly, is a synthetic peptide that activates both GLP-1 and GIP receptors. It can be dosed once a week due to its ability to bind to albumin using acylation technology. This article reviews the characteristics and pharmacokinetics of tirzepatide. The authors summarize the findings from phase 1, 2, and 3 clinical trials, which demonstrated that tirzepatide effectively controls blood sugar levels, improves insulin sensitivity, aids in weight loss, and positively influences lipid metabolism in patients with type 2 diabetes. Weekly subcutaneous injections of tirzepatide show promise in treating both type 2 diabetes and cardiometabolic disorders. The mechanism of action and safety profile of tirzepatide address important gaps in the current treatment of type 2 diabetes.
You can read the abstract of this article at https://journals.viamedica.pl/endokrynologia_polska/article/view/87859.
- Battelino, T., Bergenstal, R. M., Rodríguez, A., Fernández Landó, L., Bray, R., Tong, Z., & Brown, K. (2022). Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. The lancet. Diabetes & endocrinology, 10(6), 407–417. https://doi.org/10.1016/S2213-8587(22)00077-8.
Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial
In this study, the researchers compared the effects of tirzepatide, a novel treatment for type 2 diabetes, with insulin degludec using continuous glucose monitoring (CGM). The study involved participants with type 2 diabetes who were on metformin with or without an SGLT2 inhibitor. The primary outcome was to assess the proportion of time CGM values were in the target glucose range (71-140 mg/dL) at 52 weeks. The secondary outcomes included comparing the proportion and duration of time spent in the target range at 24 and 52 weeks. The results showed that patients receiving once-weekly tirzepatide had a higher proportion of time within the target range compared to those receiving insulin degludec. At 52 weeks, tirzepatide-treated participants spent significantly more time in the target range compared to the insulin group. At 24 weeks, the 10 mg and 15 mg tirzepatide groups also spent more time in the target range compared to the insulin group. These findings indicate that tirzepatide provides superior glycemic control without increasing the risk of hypoglycemia compared to insulin degludec.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587%2822%2900077-8/fulltext.
- Bhagavathula, A. S., Vidyasagar, K., & Tesfaye, W. (2021). Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials. Pharmaceuticals (Basel, Switzerland), 14(10), 991. https://doi.org/10.3390/ph14100991.
Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Phase II/III Trials
Tirzepatide is a new medication being studied for its effectiveness and safety in treating type 2 diabetes. A review and analysis of multiple trials were conducted to assess how tirzepatide impacts glycemic control (HbA1c levels and fasting serum glucose) and body weight in patients with uncontrolled type 2 diabetes. The analysis included four trials with a total of 2,783 patients. The results showed that tirzepatide led to greater reductions in HbA1c levels, fasting serum glucose, and body weight compared to placebo or a selective GLP-1 receptor agonist. The improvements in HbA1c levels were sustained over the long-term trials. The safety profile of tirzepatide was also favorable, with a low incidence of hypoglycemia and serious adverse events. The discontinuation rate of tirzepatide was relatively low. Overall, tirzepatide was found to effectively improve glycemic control and reduce body weight, making it a promising treatment option for type 2 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8537322/.
- Kadowaki, T., Chin, R., Ozeki, A., Imaoka, T., & Ogawa, Y. (2022). Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. The lancet. Diabetes & endocrinology, 10(9), 634–644. https://doi.org/10.1016/S2213-8587(22)00187-5.
Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial
In order to assess the safety and effectiveness of tirzepatide as an additional treatment for Japanese patients with type 2 diabetes, a phase 3 trial was conducted. The trial involved 443 participants who had inadequate glycemic control despite being on stable doses of oral antihyperglycemic medications. Participants were randomly assigned to receive 5 mg, 10 mg, or 15 mg of tirzepatide once a week for 52 weeks. The primary goal was to evaluate the safety and tolerability of tirzepatide over the treatment period. The results showed that tirzepatide was well-tolerated, with most participants experiencing mild to moderate adverse events such as nasopharyngitis, nausea, constipation, diarrhea, and decreased appetite. Significant reductions in body weight and HbA1c levels were observed across all three tirzepatide dosage groups. These findings suggest that tirzepatide could be a promising treatment option for Japanese patients with type 2 diabetes who have inadequate glycemic control with a single oral antihyperglycemic medication.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00187-5/fulltext.
- Kaneko S. (2022). Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. TouchREVIEWS in endocrinology, 18(1), 10–19. https://doi.org/10.17925/EE.2022.18.1.10.
Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes
Gastrointestinal hormones are currently used in the treatment of type 2 diabetes mellitus (T2D). Incretin therapies that target gastric inhibitory polypeptide (GIP) or glucagon-like peptide-1 (GLP-1) offer new ways to manage blood glucose levels, body weight, and lipid metabolism. GIP, an incretin hormone, was not previously considered effective for diabetes treatment. However, recent research has shown that GIP plays a crucial role in regulating glucagon and insulin secretion even under normal blood sugar conditions. Combining GIP with GLP-1 and glucagon signaling has a more powerful effect than GLP-1 stimulation alone. Scientists are currently developing a new type of medication, a GIP/GLP-1 receptor dual agonist, which can target both GIP and GLP-1 receptors simultaneously. This medication, known as tirzepatide, is undergoing clinical trials and is expected to be available for patients soon. Tirzepatide acts on metabolism through various organs and the central nervous system. The SURPASS phase III clinical trials for tirzepatide involve more than 13,000 patients with T2D and aim to gain a better understanding of its efficacy and safety (ClinicalTrials.gov Identifier: NCT04255433). The clinical application of tirzepatide as a treatment for T2D has the potential to provide new insights into the disease and help unravel the role of GIP in its development.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9354517/.
- De Block, C., Bailey, C., Wysham, C., Hemmingway, A., Allen, S. E., & Peleshok, J. (2022). Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes, obesity & metabolism, 10.1111/dom.14831. Advance online publication. https://doi.org/10.1111/dom.14831.
Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective
Tirzepatide, a new medication, has been approved in the United States for adults with type 2 diabetes as an additional treatment to diet and exercise. It works by targeting the glucose-dependent insulinotropic polypeptide/glucagon-like peptide 1 (GLP-1) receptor. Furthermore, it is being investigated for its potential use in chronic weight management, reducing the risk of major adverse cardiovascular events, and managing other conditions such as heart failure with preserved ejection fraction, obesity, and non-cirrhotic non-alcoholic steatohepatitis.The Phase 3 SURPASS 1-5 clinical trial program was conducted to evaluate the safety and effectiveness of tirzepatide, which was administered once weekly through subcutaneous injections at different doses (5, 10, and 15 mg). The trials involved a wide range of individuals with type 2 diabetes, assessing both monotherapy and combination therapy approaches. Results from these clinical studies demonstrated significant improvements in glycaemic control, as indicated by notable reductions in glycated haemoglobin levels (-1.87% to -2.59%, -20 to -28 mmol/mol). Moreover, body weight was substantially reduced by tirzepatide use (-6.2 to -12.9 kg). Additionally, the medication led to decreases in various factors associated with an increased risk of heart and metabolic diseases, including blood pressure, visceral adiposity, and circulating triglycerides. Notably, the reductions observed in these parameters were greater than those seen with semaglutide 1 mg, another GLP-1 receptor agonist, in the SUPRASS-2 trial.Tirzepatide was generally well-tolerated, with a low risk of hypoglycaemia when used without insulin or insulin secretagogues. Its safety profile was comparable to that of other medications in the GLP-1 receptor agonist class. Consequently, the evidence from these clinical trials suggests that tirzepatide presents a new opportunity for effectively reducing glycated haemoglobin and body weight in adults with type 2 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10087310/.
- Garvey, W. T., Frias, J. P., Jastreboff, A. M., le Roux, C. W., Sattar, N., Aizenberg, D., Mao, H., Zhang, S., Ahmad, N. N., Bunck, M. C., Benabbad, I., Zhang, X. M., & SURMOUNT-2 investigators (2023). Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (London, England), S0140-6736(23)01200-X. Advance online publication. https://doi.org/10.1016/S0140-6736(23)01200-X.
Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial
The importance of weight reduction for improved health outcomes in individuals with obesity and type 2 diabetes is evident. The study aimed to evaluate the efficacy and safety of tirzepatide, a glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist, versus placebo for weight management in this population. A phase 3, double-blind, randomized, placebo-controlled trial was conducted across seven countries. Adults with a BMI of 27 kg/m² or higher and HbA1c levels between 7–10% were randomly assigned (1:1:1) to receive once-weekly subcutaneous tirzepatide (10 mg or 15 mg) or placebo for 72 weeks. Tirzepatide, at both doses, led to significant reductions in bodyweight compared to placebo at week 72. Adverse events with tirzepatide were mainly gastrointestinal-related and mostly mild to moderate in severity. The trial demonstrated that once-weekly tirzepatide 10 mg and 15 mg offered substantial and clinically meaningful bodyweight reduction, with a safety profile similar to other therapies for weight management using incretin-based approaches.
You can read the full article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01200-X/fulltext.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021 Aug 5;385(6):503-515. doi: 10.1056/NEJMoa2107519. Epub 2021 Jun 25. PMID: 34170647.
Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes
The SURPASS-2 trial evaluated the efficacy and safety of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist, for the treatment of type 2 diabetes. The trial involved 1879 patients with inadequate glycemic control on metformin monotherapy, who were randomly assigned to receive tirzepatide at doses of 5 mg, 10 mg, or 15 mg, or semaglutide at a dose of 1 mg.After 40 weeks of treatment, all doses of tirzepatide were found to be noninferior and superior to semaglutide in terms of reducing glycated hemoglobin (HbA1c) levels. The estimated mean reduction in HbA1c was -2.01 percentage points, -2.24 percentage points, and -2.30 percentage points with tirzepatide doses of 5 mg, 10 mg, and 15 mg, respectively, compared to -1.86 percentage points with semaglutide. Tirzepatide was also superior to semaglutide in terms of weight loss, with mean reductions in body weight of -7.6 kg, -9.3 kg, and -11.2 kg for tirzepatide doses of 5 mg, 10 mg, and 15 mg, respectively, compared to -5.7 kg with semaglutide.
The trial showed that a higher proportion of patients treated with tirzepatide achieved glycemic targets, with 82% to 86% reaching an HbA1c level of less than 7.0% and 27% to 46% reaching an HbA1c level of less than 5.7%. Tirzepatide also demonstrated favorable effects on lipid profiles, blood pressure, and biomarkers of insulin sensitivity and liver function.The most common adverse events reported with tirzepatide and semaglutide were gastrointestinal in nature, including nausea, diarrhea, and vomiting, which were mostly mild to moderate in severity. Serious adverse events were reported in a higher proportion of patients who received tirzepatide compared to semaglutide. Hypoglycemia events were rare, with the incidence ranging from 0.2% to 1.7% in the tirzepatide groups and 0.4% in the semaglutide group.In conclusion, tirzepatide demonstrated superior glycemic control and weight loss compared to semaglutide in patients with type 2 diabetes. The favorable effects on multiple metabolic parameters suggest that tirzepatide’s dual mechanism of action may provide additional benefits beyond GLP-1 receptor agonism alone. Further trials, including long-term cardiovascular outcome studies, are ongoing to assess the safety and efficacy of tirzepatide compared to other treatments for type 2 diabetes.
You can read the full article at https://www.nejm.org/doi/full/10.1056/NEJMoa2107519.
- Jung HN, Jung CH. The Upcoming Weekly Tides (Semaglutide vs. Tirzepatide) against Obesity: STEP or SURPASS? J ObesMetab Syndr. 2022 Mar 30;31(1):28-36. doi: 10.7570/jomes22012. PMID: 35314521; PMCID: PMC8987449.
The Upcoming Weekly Tides (Semaglutide vs. Tirzepatide) against Obesity: STEP or SURPASS?
The increasing prevalence of obesity and related health problems is becoming a growing global burden. Apart from lifestyle changes, there is increasing interest in using medication to help people lose weight and reduce cardiometabolic complications. Currently available anti-obesity medications have shown a modest weight reduction of approximately 5% compared to a placebo when used over the long term. However, two newly developed injectable drugs, semaglutide and tirzepatide, have demonstrated remarkable weight-loss effects in multinational randomized phase III trials. These peptides are considered the most promising contenders in the upcoming anti-obesity market. Therefore, it is crucial to compare their effectiveness and safety. This article provides a summary of the weight reduction effectiveness, glycemic control, and safety of semaglutide (up to a 2.4-mg dose) and tirzepatide (up to a 15-mg dose), with a focus on the Semaglutide Treatment Effect in People with Obesity (STEP) 2, SURPASS-1, and SURPASS-2 trials, which involved patients with type 2 diabetes mellitus.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8987449/.
- Lazzaroni E, Ben Nasr M, Loretelli C, Pastore I, Plebani L, Lunati ME, Vallone L, Bolla AM, Rossi A, Montefusco L, Ippolito E, Berra C, D’Addio F, Zuccotti GV, Fiorina P. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res. 2021 Sep;171:105782. doi: 10.1016/j.phrs.2021.105782. Epub 2021 Jul 22. PMID: 34302978.
Anti-diabetic drugs and weight loss in patients with type 2 diabetes
Type 2 diabetes often coexists with obesity. Even a small amount of weight loss can have a significant positive impact on glucose regulation and reduce cardiometabolic risk factors in patients with type 2 diabetes. However, lifestyle-based weight loss strategies are not effective in the long term. Therefore, there is a growing need to explore pharmacological approaches to aid weight loss in individuals with the “diabesity” syndrome. The objective of this review is to examine the effects of non-insulin glucose-lowering drugs on weight loss in patients with type 2 diabetes. We conducted a systematic analysis of the literature to evaluate the impact of non-insulin glucose-lowering drugs on weight loss in patients with type 2 diabetes. For each class of drugs, we analyzed the following parameters: average weight loss in kilograms, effects on body mass index (BMI), and changes in body composition.Our findings suggest that anti-diabetic drugs can be categorized into three groups based on their efficacy in promoting weight loss. Metformin, acarbose, empagliflozin, and exenatide were associated with mild weight loss (less than 3.2% of initial weight). Canagliflozin, ertugliflozin, dapagliflozin, and dulaglutide resulted in moderate weight loss (between 3.2% and 5%). Liraglutide, semaglutide, and tirzepatide demonstrated significant weight loss (greater than 5%).This study highlights that newer anti-diabetic drugs, particularly GLP1-RA (glucagon-like peptide-1 receptor agonists) and Tirzepatide, are highly effective in promoting weight loss among patients with type 2 diabetes. Notably, exenatide appears to be the only GLP1-RA that induces a mild weight loss.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1043661821003662?via%3Dihub.
- Available at https://www.prnewswire.com/news-releases/lillys-tirzepatide-delivered-up-to-22-5-weight-loss-in-adults-with-obesity-or-overweight-in-surmount-1–301534871.html.
Participants taking tirzepatide lost up to 52 lb. (24 kg) in this 72-week phase 3 study
Tirzepatide demonstrated significant weight loss superiority over placebo at 72 weeks in Eli Lilly and Company’s SURMOUNT-1 clinical trial, achieving up to 22.5% body weight reduction among participants without diabetes and with obesity or overweight plus comorbidities. This phase 3 global registration study involved 2,539 participants and met its primary endpoints for both mean percent change in body weight and the percentage of participants achieving ≥5% body weight reductions. Secondary endpoints were also achieved, with 10 mg and 15 mg doses leading to substantial weight loss (up to 63% achieving ≥20% reduction). Adverse events were generally mild to moderate gastrointestinal-related issues. Participants with pre-diabetes will continue for an additional 104 weeks to assess the impact on body weight and progression to type 2 diabetes. Lilly aims to further explore tirzepatide’s potential in treating obesity.
You can read the full article at https://www.prnewswire.com/news-releases/lillys-tirzepatide-delivered-up-to-22-5-weight-loss-in-adults-with-obesity-or-overweight-in-surmount-1–301534871.html.
- Hui, X., Lam, K. S., Vanhoutte, P. M., & Xu, A. (2012). Adiponectin and cardiovascular health: an update. British journal of pharmacology, 165(3), 574–590. https://doi.org/10.1111/j.1476-5381.2011.01395.x.
Adiponectin and cardiovascular health: an update
The worldwide obesity epidemic is accompanied by a higher incidence of cardiovascular disease (CVD), specifically stroke and heart attacks. The connection between obesity and CVD lies in dysfunctional adipose tissue, which releases various harmful bioactive lipids and pro-inflammatory factors (known as adipokines) that negatively affect the cardiovascular system. Among these adipokines, adiponectin stands out as one with multiple beneficial effects on insulin sensitivity and cardiovascular well-being. Clinical studies have identified a deficiency of adiponectin (known as hypoadiponectinemia) as an independent risk factor for CVD. In animal research, increasing plasma adiponectin levels through pharmacological or genetic means has shown to alleviate obesity-induced endothelial dysfunction, hypertension, atherosclerosis, myocardial infarction, and diabetic cardiomyopathy. Additionally, the therapeutic benefits of thiazolidinediones, a class of drugs that activate the peroxisome proliferator-activated receptor gamma, are largely attributed to their ability to induce adiponectin. Adiponectin safeguards cardiovascular health by exerting vasodilator, anti-apoptotic, anti-inflammatory, and anti-oxidative effects on both cardiac and vascular cells. This review provides a summary of recent discoveries regarding the physiological role and clinical significance of adiponectin in cardiovascular health. It also explores the identification of the receptor and post-receptor signaling events that mediate adiponectin’s cardiovascular actions, along with discussions on strategies for drug discovery targeting adiponectin to treat obesity, diabetes, and CVD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3315032/.
- Wilson, J. M., Nikooienejad, A., Robins, D. A., Roell, W. C., Riesmeyer, J. S., Haupt, A., Duffin, K. L., Taskinen, M. R., & Ruotolo, G. (2020). The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes, obesity & metabolism, 22(12), 2451–2459. https://doi.org/10.1111/dom.14174.
The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes
The aim of this study was to gain a better understanding of the significant reduction in serum triglycerides observed in patients with type 2 diabetes who were treated with tirzepatide. To achieve this, additional lipoprotein-related biomarkers were analyzed in available samples from the same study. Materials and Methods: The patients were randomly assigned to receive subcutaneous tirzepatide once a week at doses of 1, 5, 10, or 15 mg, dulaglutide at a dose of 1.5 mg, or a placebo. The serum lipoprotein profile, as well as apolipoprotein (apo) A-I, B, and C-III, and preheparin lipoprotein lipase (LPL) were measured at the beginning of the study and at 4, 12, and 26 weeks. The lipoprotein particle profile was assessed using nuclear magnetic resonance at the start of the study and at 26 weeks. The lipoprotein insulin resistance (LPIR) score was also calculated. After 26 weeks, tirzepatide demonstrated a dose-dependent decrease in apoB and apoC-III levels, along with an increase in serum preheparin LPL when compared to the placebo. The 10 and 15 mg doses of tirzepatide significantly reduced the number of large triglyceride-rich lipoprotein particles (TRLP), small low-density lipoprotein particles (LDLP), and the LPIR score compared to both the placebo and dulaglutide. Treatment with dulaglutide also led to reductions in apoB and apoC-III levels, but did not have an impact on serum LPL or large TRLP, small LDLP, and LPIR score. Tirzepatide at doses of 10 and 15 mg resulted in a decrease in the total number of LDLP compared to the placebo. Furthermore, a more pronounced reduction in apoC-III levels with tirzepatide was observed in patients with elevated baseline triglycerides compared to those with normal levels. At 26 weeks, changes in apoC-III, rather than body weight, were the most accurate predictors of changes in triglyceride levels with tirzepatide, accounting for up to 22.9% of the variability observed.Treatment with tirzepatide demonstrated a dose-dependent decrease in apoC-III and apoB levels, as well as a reduction in the number of large TRLP and small LDLP. These findings suggest an overall improvement in the lipoprotein profile associated with atherogenic risk.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7756479/.
- Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022 Mar;28(3):591-598. doi: 10.1038/s41591-022-01707-4. Epub 2022 Feb 24. PMID: 35210595; PMCID: PMC8938269.
Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis
Tirzepatide, a novel weekly dual GIP/GLP-1 receptor agonist in development for type 2 diabetes (T2D) and obesity treatment, was analyzed for cardiovascular outcomes. A meta-analysis of seven trials from the tirzepatide T2D program assessed time to first occurrence of major adverse cardiovascular events (MACE-4) in tirzepatide and control groups. The hazard ratios (HRs) comparing tirzepatide to controls were 0.80 (95% CI, 0.57–1.11) for MACE-4, 0.90 (95% CI, 0.50–1.61) for cardiovascular death, and 0.80 (95% CI, 0.51–1.25) for all-cause death. No increased cardiovascular risk was observed with tirzepatide in T2D participants versus controls.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8938269/.
- Wilson JM, Lin Y, Luo MJ, Considine G, Cox AL, Bowsman LM, Robins DA, Haupt A, Duffin KL, Ruotolo G. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes ObesMetab. 2022 Jan;24(1):148-153. doi: 10.1111/dom.14553. Epub 2021 Oct 11. PMID: 34542221.
Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis
Tirzepatide, a new dual GIP/GLP-1 receptor agonist administered once weekly, is currently being developed as a treatment for type 2 diabetes (T2D) and obesity. Its impact on cardiovascular outcomes needs to be assessed. This predetermined meta-analysis focused on cardiovascular factors and included all seven randomized controlled trials from the tirzepatide T2D clinical development program, known as SURPASS, with a minimum duration of 26 weeks. The primary objective of this analysis was to compare the time until the first occurrence of confirmed major adverse cardiovascular events with four components (MACE-4: cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina) between the combined tirzepatide groups and the control groups. A stratified Cox proportional hazards model was used, with treatment as a fixed effect and trial-level cardiovascular risk as the stratification factor, to estimate hazard ratios (HRs) and confidence intervals (CIs) when comparing tirzepatide to the control. Data from 4,887 participants treated with tirzepatide and 2,328 control participants were analyzed. Out of the total participants, 142 individuals experienced at least one MACE-4 event, with 109 from the high cardiovascular risk trial and 33 from the six trials with lower cardiovascular risk. The HRs comparing tirzepatide to the control were 0.80 (95% CI, 0.57-1.11) for MACE-4, 0.90 (95% CI, 0.50-1.61) for cardiovascular death, and 0.80 (95% CI, 0.51-1.25) for all-cause death. No evidence of effect modifications was observed for any subgroups, although the evidence was stronger for participants with high cardiovascular risk. In conclusion, compared to the control, tirzepatide did not increase the risk of major cardiovascular events in individuals with T2D.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8938269/.
- Tate, M., Chong, A., Robinson, E., Green, B. D., & Grieve, D. J. (2015). Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. British journal of pharmacology, 172(3), 721–736. https://doi.org/10.1111/bph.12943.
Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes
Glucagon-like peptide-1 (GLP-1), an incretin hormone, has been utilized as a novel strategy for managing glucose levels in type 2 diabetes. While the presence of GLP-1 receptors in the cardiovascular system (CVS) and their physiological functions have been known, recent focus has been on the specific cardiovascular effects of GLP-1 in diabetes. GLP-1 indirectly benefits cardiovascular disease (CVD) by addressing risk factors like hypertension, dyslipidemia, and obesity that are aggravated in diabetes. Moreover, GLP-1 has shown direct effects on diabetic CVD aspects including endothelial dysfunction, inflammation, angiogenesis, and cardiac remodeling. However, most research has used experimental models, with limited clinical data, although significant trials are ongoing. This review underscores the importance of comprehending GLP-1’s cardiovascular impact in diabetes, especially considering its increasing use in patients, discussing both risk factor mitigation and direct cardiac and vascular effects, and exploring GLP-1’s potential as a novel therapy for diabetic CVD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4301685/.
- Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, Karanikas CA, Duffin KL, Robins DA, Haupt A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care. 2020 Jun;43(6):1352-1355. doi: 10.2337/dc19-1892. Epub 2020 Apr 14. PMID: 32291277; PMCID: PMC7245348.
Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes
The impact of tirzepatide, a dual agonist targeting glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors, on biomarkers related to nonalcoholic steatohepatitis (NASH) and fibrosis in type 2 diabetes mellitus (T2DM) patients was investigated. Patients with T2DM received weekly doses of tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks. Notable reductions from baseline were observed in alanine aminotransferase (ALT), aspartate aminotransferase (AST), keratin-18 (K-18), procollagen III (Pro-C3), and increases in adiponectin. Tirzepatide demonstrated significant reductions in NASH-related biomarkers and elevated adiponectin levels compared to placebo or dulaglutide in patients with T2DM, as evidenced in post hoc analyses.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7245348/.
- Available at https://www.sciencedirect.com/science/article/abs/pii/S2213858722000705#.
Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial
Tirzepatide is a new medication being developed to treat type 2 diabetes. In this study, researchers wanted to see how tirzepatide affected the amount of fat in the liver (liver fat content or LFC), as well as the volume of fat in the belly (visceral adipose tissue or VAT) and under the skin in the abdomen (abdominal subcutaneous adipose tissue or ASAT). They compared tirzepatide to insulin degludec in a specific group of patients participating in the SURPASS-3 study.
The study involved adults with type 2 diabetes who were overweight and had been taking metformin alone or with another medication for at least 3 months. They had MRI scans to measure the amount of fat in their liver and were then randomly assigned to receive either tirzepatide (at doses of 5 mg, 10 mg, or 15 mg) or insulin degludec (taken once a day). The main goal was to see how LFC changed after 52 weeks.
Results showed that tirzepatide, at doses of 10 mg and 15 mg combined, led to a greater reduction in LFC compared to insulin degludec. The reduction in LFC was about 4.7% greater with tirzepatide than with insulin degludec. The researchers also found that the reduction in LFC was related to the amount of fat in the liver at the beginning of the study, as well as reductions in VAT, ASAT, and body weight in the tirzepatide groups.
These findings suggest that tirzepatide has a positive effect on reducing fat in the liver and the amount of fat in the belly compared to insulin degludec. This provides additional evidence on the metabolic effects of tirzepatide, which combines two different types of hormones to help control blood sugar levels in people with type 2 diabetes.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S2213858722000705#.
- Available at https://www.tandfonline.com/doi/full/10.1080/14656566.2021.1980538.
Considerations when prescribing pharmacotherapy for metabolic associated fatty liver disease
Currently, there is no approved drug for nonalcoholic fatty liver disease (NAFLD), affecting a significant portion of the global population. Trials focusing on advanced NAFLD stages have not met regulatory criteria for improving fibrosis and steatohepatitis. Obeticholic acid reduced fibrosis but didn’t resolve steatohepatitis. The shift to the metabolic (dysfunction)-associated fatty liver disease (MAFLD) concept from NAFLD underscores metabolic factors’ role and eliminates alcohol-related stigma. This positive term encourages preventive strategies to address limited awareness and adherence to guidelines. Metabolic factors and insulin resistance have been linked to NAFLD progression for over two decades. An international consensus now defines MAFLD through steatosis and diabetes/obesity or two metabolic abnormalities. The potential of pharmacologic strategies for MAFLD-related conditions like diabetes, obesity, dyslipidemia, and hypertension requires exploration, with emerging treatments like GLP-1RAs and SGLT-2Is showing promise. Extensive research is crucial to establish effective interventions, considering diverse factors.
You can read the full article at https://www.tandfonline.com/doi/full/10.1080/14656566.2021.1980538.
- Available at https://www.mdpi.com/2072-6643/14/1/103/htm.
Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota
Metabolic-associated fatty liver disease (MAFLD) is a prevalent condition linked with type 2 diabetes (T2DM) and cardiovascular disease (CVD). The revised term underscores the interplay between these conditions, prompting increased vigilance in diagnosing both MAFLD among T2DM/CVD patients and vice versa. While lifestyle changes, notably dietary fructose reduction, remain the primary treatment for MAFLD, emerging approaches including liver-gut axis targeting involving microbiome adjustments hold promise. This review offers insights into the connections among fructose intake, gut microbiota, diabetes, and CVD in NAFLD patients.
You can read the abstract of this article at https://www.mdpi.com/2072-6643/14/1/103/htm.
- Available at https://link.springer.com/article/10.1007/s00125-021-05442-2.
Liver-targeting drugs and their effect on blood glucose and hepatic lipids
The escalating global prevalence of non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH), particularly in individuals with type 2 diabetes, has captured the attention of liver disorder specialists. Numerous drugs, including glucose-lowering medications, are undergoing assessment for NAFLD/NASH treatment. These encompass nuclear hormone receptor agonists, fibroblast growth factors 19 and 21, incretins (single, dual, or triple), sodium-glucose cotransporter inhibitors, and agents modulating metabolic pathways. The review focuses on these drugs’ metabolic impacts, spanning diabetic hyperglycemia, fatty liver disease, peripheral metabolism, and insulin resistance enhancement.
You can read the abstract of this article at https://link.springer.com/article/10.1007/s00125-021-05442-2.
- Heerspink, H. J. L., Sattar, N., Pavo, I., Haupt, A., Duffin, K. L., Yang, Z., Wiese, R. J., Tuttle, K. R., & Cherney, D. Z. I. (2022). Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. The lancet. Diabetes & endocrinology, 10(11), 774–785. https://doi.org/10.1016/S2213-8587(22)00243-1.
Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial
In the SURPASS-4 trial, the dual GIP and GLP-1 receptor agonist tirzepatide demonstrated superior reductions in HbA1c concentrations, bodyweight, and blood pressure compared to titrated daily insulin glargine in individuals with type 2 diabetes and cardiovascular risk. Through a post-hoc analysis of this phase 3 study, we examined kidney parameters and outcomes. The study involved adults with type 2 diabetes and cardiovascular risk, who were randomized to receive tirzepatide (5 mg, 10 mg, or 15 mg) or insulin glargine (100 U/mL) over a treatment period of up to 104 weeks. The combined tirzepatide groups exhibited a more favorable decline in estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (UACR) compared to the insulin glargine group. Moreover, tirzepatide was associated with a notably lower occurrence of the composite kidney outcome compared to insulin glargine. This analysis indicates that in individuals with type 2 diabetes and high cardiovascular risk, tirzepatide may effectively mitigate the decline in eGFR and reduce UACR more effectively than insulin glargine.
You can read the abstract of this article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00243-1/fulltext.
- Bosch, C., Carriazo, S., Soler, M. J., Ortiz, A., & Fernandez-Fernandez, B. (2022). Tirzepatide and prevention of chronic kidney disease. Clinical kidney journal, 16(5), 797–808. https://doi.org/10.1093/ckj/sfac274.
Tirzepatide and prevention of chronic kidney disease
Tirzepatide is a twincretin recently approved to improve glycemic control in type 2 diabetes mellitus (T2DM). More specifically, tirzepatide is an agonist of both the glucose-dependent insulinotropic polypeptide (GIP) and the glucagon-like peptide-1 (GLP1) receptors. In recent clinical trials in persons with obesity or overweight with associated conditions, tirzepatide decreased body weight and other cardiorenal risk factors (blood pressure, low-density lipoprotein cholesterol, glycated hemoglobin and albuminuria). Moreover, in a post hoc analysis of the SURPASS-4 randomized clinical trial, tirzepatide decreased albuminuria and total estimated glomerular filtration rate (eGFR) slopes and nearly halved the risk of a pre-specified composite kidney endpoint (eGFR decline ≥40%, renal death, kidney failure or new-onset macroalbuminuria) in participants with T2DM and high cardiovascular risk when compared with insulin glargine. Similar to other kidney-protective drugs, tirzepatide, alone or combined with sodium-glucose co-transporter 2 inhibitors, caused an early dip in eGFR. Moreover, tirzepatide also decreased eGFR slopes in participants with eGFR >60 mL/min/1.73 m2 or with normoalbuminuria. We now review the potential kidney health implications of tirzepatide, addressing its structure and function, relationship to current GLP1 receptor agonists, impact of recent results for the treatment and prevention of kidney disease, and expectations for the future.
You can read the full article at https://academic.oup.com/ckj/article/16/5/797/6958830.
- Available from https://clinicaltrials.gov/ct2/show/study/NCT03987919.
- Available from https://auctoresonline.org/article/tirzepatide-a-useful-addition-for-treatment-of-type-2-diabetes-and-obesity.
Tirzepatide: A Useful Addition for Treatment of Type 2 Diabetes and Obesity
Tirzepatide, a dual receptor agonist of incretin hormones GIP and GLP-1, is being developed for type 2 diabetes and obesity treatment. Reviewed clinical trials showed tirzepatide (5-15 mg/week) led to -1.87% to -2.07% HbA1c reduction vs. +0.04% with placebo after 40 weeks. Another trial demonstrated -2.01% to -2.3% HbA1c decrease with tirzepatide compared to -1.86% with semaglutide (1.0 mg/week). Tirzepatide induced dose-related weight loss (-7.0 to -9.5 kg vs. -0.7 kg placebo, and -7.6 to -11.2 kg vs. -5.7 kg semaglutide) and improved lipid profile (triglycerides -19% to -24.8% vs. -11.5% semaglutide). Tirzepatide caused more GI adverse effects and hypoglycemia (1.7% vs. 0.4% for semaglutide) with discontinuation rates of 6-8.5% vs. 4.1% for semaglutide. While promising for HbA1c and weight reduction, tirzepatide exhibited a safety profile akin to GLP-1 agonists, though with increased GI issues and hypoglycemia.
You can read the full article at https://auctoresonline.org/article/tirzepatide-a-useful-addition-for-treatment-of-type-2-diabetes-and-obesity.
- Available from https://clinicaltrials.gov/ct2/show/results/NCT03987919.
- Vadher, K., Patel, H., Mody, R., Levine, J. A., Hoog, M., Cheng, A. Y., Pantalone, K. M., & Sapin, H. (2022). Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison. Diabetes, obesity & metabolism, 10.1111/dom.14775. Advance online publication. https://doi.org/10.1111/dom.14775.
Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison. Diabetes, obesity & metabolism
This study aimed to compare the efficacy of tirzepatide at different doses (5/10/15 mg) with semaglutide (2 mg) in treating type 2 diabetes. An adjusted indirect treatment comparison (aITC) was conducted using data from the SURPASS-2 and SUSTAIN FORTE trials. Tirzepatide at 10 and 15 mg significantly reduced HbA1c levels and body weight compared to semaglutide 2 mg, with greater reductions of -0.36% and -0.4% in HbA1c, and -3.15 kg and -5.15 kg in body weight, respectively. However, there were no significant differences between tirzepatide 5 mg and semaglutide 2 mg in terms of HbA1c and body weight reductions. These findings provide insights into the comparative effectiveness of tirzepatide and semaglutide without a direct head-to-head clinical trial.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546430/.
- Available from https://www.practiceupdate.com/content/tirzepatide-vs-semaglutide-in-patients-with-type-2-diabetes/120161.
Tirzepatide vs Semaglutide in Patients With Type 2 Diabetes
Achieving and maintaining glycemic targets is pivotal for managing type 2 diabetes. GLP-1 receptor agonists are effective in reducing glucose, enhancing satiety, promoting weight loss, and lowering cardiovascular risks in high-risk patients. Tirzepatide, acting on GIP and GLP-1 receptors, regulates glucose, insulin, and glucagon while boosting lipid regulation and reducing food intake. The SURPASS program evaluates tirzepatide’s efficacy and safety. In a 40-week SURPASS-2 trial, 1879 inadequately controlled type 2 diabetes patients were randomized to tirzepatide or semaglutide. Tirzepatide at all doses outperformed semaglutide in HbA1c reduction, weight loss, and lipid improvements. Its comparison with semaglutide and cardiovascular outcomes will define its benefits, potentially impacting long-term complications of type 2 diabetes.
You can read the full article at https://www.practiceupdate.com/content/tirzepatide-vs-semaglutide-in-patients-with-type-2-diabetes/120161.
- Available from https://diabetesjournals.org/diabetes/article/71/Supplement_1/732-P/145883.
Patients with Type 2 Diabetes Reach Glycemic Targets Faster with Tirzepatide Compared with Semaglutide and Titrated Insulin Degludec
Tirzepatide (TZP), a new dual GIP/GLP-1 receptor agonist for treating type 2 diabetes (T2D), displayed superior outcomes in HbA1c reduction and patients achieving target levels compared to semaglutide and titrated insulin degludec across 40-weeks (SURPASS-2) and 52-weeks (SURPASS-3) studies. Analyzing time to achieve glycemic goals using cox proportional-hazards model revealed TZP’s significantly quicker attainment of HbA1c <7%, ≤6.5%, and <5.7%, along with faster weight loss of 5% and 10%, compared to semaglutide. Gastrointestinal side effects were mostly mild to moderate and linked to TZP, occurring mainly during dose escalation. In summary, TZP proved to hasten the achievement of glycemic targets in T2D patients in comparison to semaglutide and titrated insulin degludec.
You can read the abstract of this article https://diabetesjournals.org/diabetes/article/71/Supplement_1/732-P/145883.
- Available from https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(22)00509-5/fulltext
- Available from https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1746270.
DUAL GIP-GLP-1 RECEPTOR AGONIST TIRZEPATIDE IMPROVES GLUCOSE CONTROL AND INSULIN SENSITIVITY IN MIXED MEAL TESTS IN PEOPLE WITH TYPE 2 DIABETES
In this Phase 1 trial, we investigated the impact of tirzepatide, a dual GIP and GLP-1 receptor agonist, on glucose control and insulin sensitivity in individuals with type 2 diabetes (T2D). Compared to the GLP-1 receptor agonist semaglutide and placebo, tirzepatide demonstrated superior reductions in HbA1c (2.05%) and body weight (11.2kg) over 28 weeks. Tirzepatide led to a significantly greater reduction in glucose total AUC (41%) compared to semaglutide (34%) and placebo (1% increase). This was accompanied by notable improvements in fasting glucose and insulin sensitivity indices derived from standardized mixed-meal tolerance testing (sMMTT). The findings suggest that tirzepatide’s substantial enhancements in glucose control and insulin sensitivity contribute to its effectiveness in T2D management.
You can read the abstract of this article at https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1746270.
- Available from https://diabetesjournals.org/diabetes/article/71/Supplement_1/337-OR/145168.
Tirzepatide Improved Markers of Islet-Cell Function (Fasting Glucagon and HOMA2-B) and Insulin Sensitivity (Fasting Insulin and HOMA2-IR) Compared with Semaglutide in People with Type 2 Diabetes
In a Phase 3 trial involving 1879 individuals with type 2 diabetes (T2D) on metformin, Tirzepatide (TZP) at doses of 5 and 15 mg demonstrated substantial improvements in HbA1c and weight reduction compared to 1 mg of semaglutide (SEMA). The trial, known as SURPASS-2, showcased significant enhancements in fasting markers of islet cell function and insulin sensitivity across the modified intent-to-treat population. Over the course of 40 weeks, TZP exhibited a remarkable average increase of 97-120% in HOMA2-B, calculated with C-peptide, compared to 84% with SEMA. Moreover, TZP induced a considerable average decrease of 53-55% in fasting glucagon levels (adjusted for fasting serum glucose), and a 16-24% decrease in HOMA2-IR, calculated with insulin, highlighting its superior effects on insulin sensitivity. Notably, fasting insulin levels also saw a substantial average reduction of 9-21% with all TZP doses in contrast to a minor 0.6% increase with SEMA. This dual GIP/GLP-1 receptor agonist, TZP, significantly outperformed the selective GLP-1 RA, SEMA, in enhancing markers of islet cell function and insulin sensitivity for individuals with T2D.
You can read the abstract of this article at https://diabetesjournals.org/diabetes/article/71/Supplement_1/337-OR/145168.
- Scheen A. J. (2022). Add-on value of tirzepatide versus semaglutide. The lancet. Diabetes & endocrinology, S2213-8587(22)00116-4. Advance online publication. https://doi.org/10.1016/S2213-8587(22)00116-4.
Add-on value of tirzepatide versus semaglutide
Type 2 diabetes is a multifaceted condition characterized by impaired insulin secretion, heightened glucagon secretion, and exacerbated insulin resistance, often linked to obesity. Effective treatment requires addressing these complexities, often achieved by combining drugs with complementary mechanisms. GLP-1 receptor agonists, known incretin hormone activators, offer a multifunctional approach. They enhance glucose-induced insulin release, inhibit glucagon secretion, and notably, foster substantial weight loss, potentially reducing insulin resistance. Additionally, these agents demonstrate remarkable cardiovascular risk reduction in high-risk type 2 diabetes patients. As a result, recent international guidelines emphasize the pivotal role of GLP-1 receptor agonists, particularly for individuals with atherosclerotic cardiovascular disease or obesity.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00116-4/fulltext.
- Gandhi G. Y. (2021). In type 2 diabetes, tirzepatide reduced HbA1c vs. semaglutide. Annals of internal medicine, 174(11), JC127. https://doi.org/10.7326/ACPJ202111160-127.
Success Stories
 before after
before after
At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health. I hav...
Nick Cassavetes ,60 yrs old
Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
 Before After
Before After
I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics.
Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old
Call 800-277-4041 for a Free Consultation
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
About
Genemedics® Health Institute is a global premier institute dedicated to revolutionizing health and medicine through healthy lifestyle education, guidance and accountability in harmony with functional medicine. Our physician-supervised health programs are personally customized to help you reach your health and fitness goals while looking and feeling better than ever.
Quick Links
Our Services
Our Locations
© Copyright Genemedics Health Institute. All Rights Reserved. Privacy Policy.

